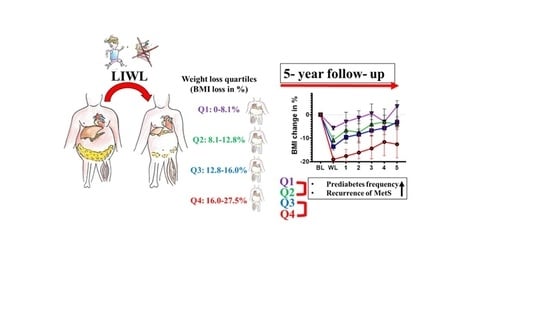
The Extent of Lifestyle-Induced Weight Loss Determines the Risk of Prediabetes and Metabolic Syndrome Recurrence during a 5-Year Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Study Population
2.2. Clinical and Laboratory Parameters
2.3. Statistical Analysis
3. Results
3.1. Long-Term Effects of Lifestyle-Induced Weight Loss on BMI
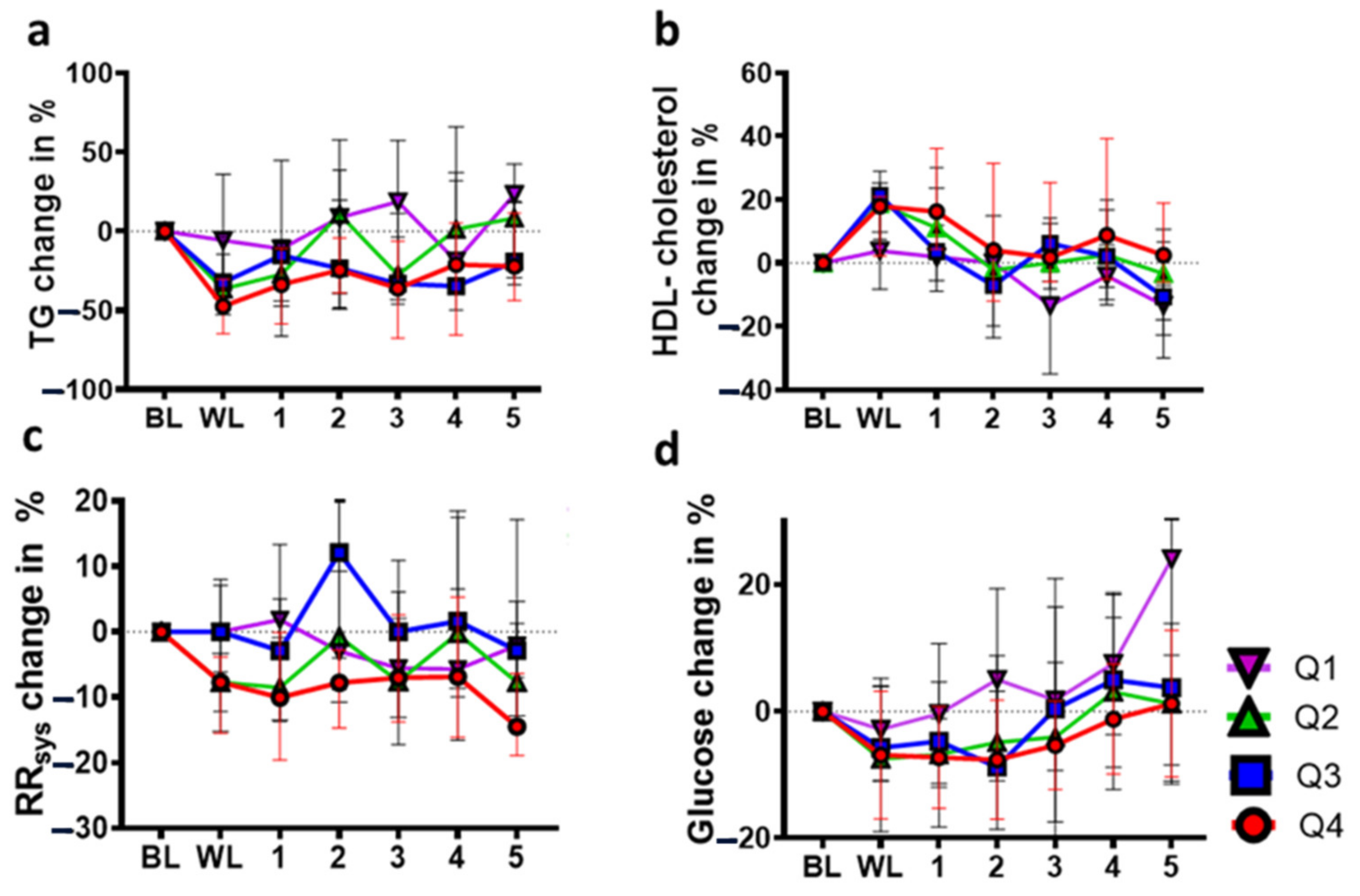
3.2. Long-Term Effects of Lifestyle-Induced Weight Loss on TGs
3.3. Long-Term Effects of Lifestyle-Induced Weight Loss on HDL Cholesterol
3.4. Long-Term Effects of Lifestyle-Induced Weight Loss on Systolic Blood Pressure (RRsys)
3.5. Long-Term Effects of Lifestyle-Induced Weight Loss on Fasting Plasma Glucose
3.6. Frequency of Prediabetes after Lifestyle-Induced Weight Loss
3.7. Long-Term Effect of Lifestyle-Induced Weight Loss on Metabolic Syndrome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grave, R.D.; Calugi, S.; Centis, E.; Marzocchi, R.; Ghoch, M.E.; Marchesini, G. Lifestyle modification in the management of the metabolic syndrome: Achievements and challenges. Diabetes Metab. Syndr. Obes. 2010, 3, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Ciccone, G.; Baldi, C.; Benini, L.; Dusio, F.; Forastiere, G.; Lucia, C.; Nuti, C.; Durazzo, M.; Cassader, M.; et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J. Gen. Intern. Med. 2007, 22, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, A.M.; Foreyt, J.P.; Mann, D.L. Treatment of the metabolic syndrome: The impact of lifestyle modification. Curr. Atheroscler. Rep. 2005, 7, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of lost weight and long-term management of obesity. Med. Clin. North Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.-M.; Samuels, B.; Chatman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Proietto, J. The defence of body weight: A physiological basis for weight regain after weight loss. Clin. Sci. 2013, 124, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef]
- Pasquet, P.; Apfelbaum, M. Recovery of initial body weight and composition after long-term massive overfeeding in men. Am. J. Clin. Nutr. 1994, 60, 861–863. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, I.L.; Lotter, E.C.; Kulkosky, P.J.; Porte, D.; Woods, S.C. Effect of force-feeding upon basal insulin levels of rats. Proc. Soc. Exp. Biol. Med. 1975, 150, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Haubenreiser, M.; Johnson, M.; Nordby, K.; Aggarwal, S.; Myer, S.; Thomas, C. Mindfulness Approaches and Weight Loss, Weight Maintenance, and Weight Regain. Curr. Obes. Rep. 2018, 7, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Hoogeveen, R.C.; McNeill, A.M.; Heiss, G.; Schmidt, M.I.; Duncan, B.B.; Pankow, J.S. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int. J. Obes. 2008, 32 (Suppl. 2), S21–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.W.F.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Penner, M.; Borucki, K.; Westphal, S.; Luley, C.; Rönicke, R.; Biemann, K.; Weikert, C.; Lux, A.; Goncharenko, N.; et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: Results of a randomized controlled trial. Sci. Rep. 2016, 6, 30173. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Costa, M.B.W.; Mathew, A.; Krishnan, S.; Schneider, J.G.; Roomp, K.; Isermann, B.; Biemann, R. Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome. Metabolites 2021, 11, 526. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Biemann, R.; Roomp, K.; Noor, F.; Krishnan, S.; Li, Z.; Shahzad, K.; Borucki, K.; Luley, C.; Schneider, J.G.; Isermann, B. Gene expression profile of CD14+ blood monocytes following lifestyle-induced weight loss in individuals with metabolic syndrome. Sci. Rep. 2020, 10, 17855. [Google Scholar] [CrossRef]
- Luley, C.; Blaik, A.; Götz, A.; Kicherer, F.; Kropf, S.; Isermann, B.; Stumm, G.; Westphal, S. Weight loss by telemonitoring of nutrition and physical activity in patients with metabolic syndrome for 1 year. J. Am. Coll. Nutr. 2014, 33, 363–374. [Google Scholar] [CrossRef]
- R. Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.R-project.org (accessed on 20 June 2022).
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [CrossRef]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the Metabolic Syndrome and Type 2 Diabetes Through Lifestyle Modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Hill, J.O. Successful weight loss maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef]
- Boden, G.; Jadali, F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes 1991, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Szuszkiewicz-Garcia, M.; Browning, J.D.; Baxter, J.D.; Abate, N.; Malloy, C.R. Influence of Liver Triglycerides on Suppression of Glucose Production by Insulin in Men. J. Clin. Endocrinol. Metab. 2014, 100, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mitrakou, A.; Kelley, D.; Mokan, M.; Veneman, T.; Pangburn, T.; Reilly, J.; Gerich, J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N. Engl. J. Med. 1992, 326, 22–29. [Google Scholar] [CrossRef]
- Picarel-Blanchot, F.; Berthelier, C.; Bailbé, D.; Portha, B. Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am. J. Physiol. 1996, 271, E755–E762. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Investig. 1995, 96, 1261–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saloranta, C.; Groop, L.; Ekstrand, A.; Franssila-Kallunki, A.; Taskinen, M.R. The effect of an antilipolytic agent (acipimox) on the insulin resistance of lipid and glucose metabolism in hypertriglyceridaemic patients. Acta Diabetol. 1994, 31, 6–13. [Google Scholar] [CrossRef]
- Barrett, J.S.; Whytock, K.L.; Strauss, J.A.; Wagenmakers, A.J.M.; Shepherd, S.O. High intramuscular triglyceride turnover rates and the link to insulin sensitivity: Influence of obesity, type 2 diabetes and physical activity. Appl. Physiol. Nutr. Metab. 2022, 47, 343–356. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Mani, P.; Ren, H.-Y.; Neeland, I.J.; McGuire, D.K.; Ayers, C.R.; Khera, A.; Rohatgi, A. The association between HDL particle concentration and incident metabolic syndrome in the multi-ethnic Dallas Heart Study. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S175–S179. [Google Scholar] [CrossRef]
- Santos, H.O.; Lavie, C.J. Weight loss and its influence on high-density lipoprotein cholesterol (HDL-C) concentrations: A noble clinical hesitation. Clin. Nutr. ESPEN 2021, 42, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Stene, M.C.A.; Sethi, A.A.; Remaley, A.T.; Schnohr, P.; Grande, P.; Tybjaerg-Hansen, A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008, 299, 2524–2532. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, N.; Li, Y.; Kuxhaus, O.; Stefan, N.; Hu, F.B.; Schulze, M.B. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 714–724. [Google Scholar] [CrossRef]
- Yárnoz-Esquiroz, P.; Olazarán, L.; Aguas-Ayesa, M.; Perdomo, C.M.; García-Goñi, M.; Silva, C.; Fernández-Formoso, J.A.; Escalada, J.; Montecucco, F.; Portincasa, P.; et al. ‘Obesities’: Position statement on a complex disease entity with multifaceted drivers. Eur. J. Clin. Investig. 2022, 52, e13811. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Avilés-Olmos, I.; Rodríguez, A.; Becerril, S.; Fernández-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Time to Consider the “Exposome Hypothesis” in the Development of the Obesity Pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Hogan, P.E.; Neiberg, R.H.; Cheskin, L.J.; Dutton, G.R.; Evans-Hudnall, G.; Jeffery, R.; Kitabchi, A.E.; Nelson, J.A.; Pi-Sunyer, F.X.; et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity 2014, 22, 1608–1616. [Google Scholar] [CrossRef]
- Unick, J.L.; Neiberg, R.H.; Hogan, P.E.; Cheskin, L.J.; Dutton, G.R.; Jeffery, R.; Nelson, J.A.; Pi-Sunyer, X.; West, D.S.; Wing, R.R. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity 2015, 23, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Beavers, D.; Jakicic, J.M.; Kitabchi, A.E.; Knowler, W.C.; Wadden, T.A.; Wing, R.R. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: Results from the Look AHEAD trial. Diabetes Care 2011, 34, 2152–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
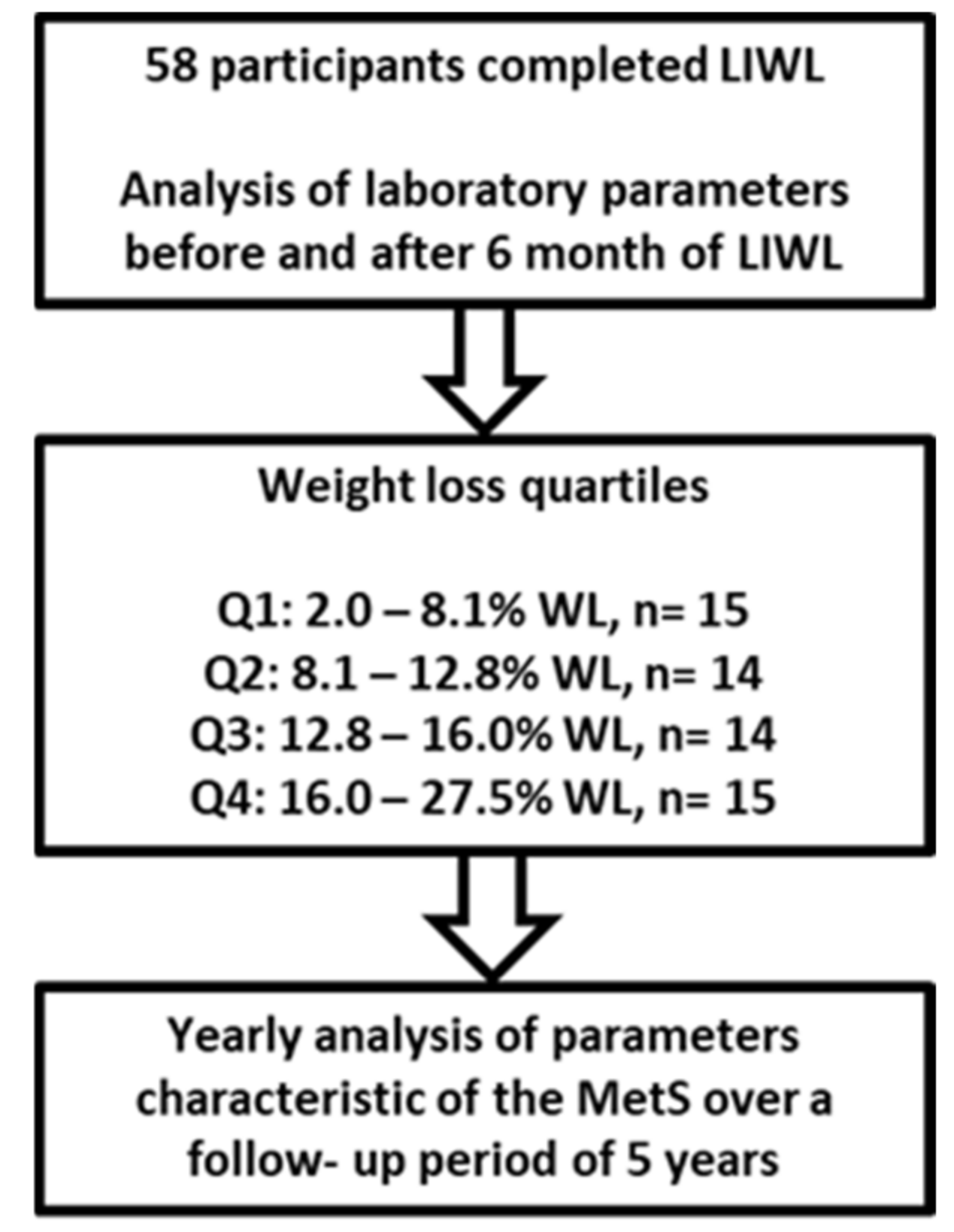


| Q1 Total (N)/Relative (%) | Q2 Total (N)/Relative (%) | Q3 Total (N)/Relative (%) | Q4 Total (N)/Relative (%) | p | |
|---|---|---|---|---|---|
| BL | 15/100 | 14/100 | 14/100 | 15/100 | |
| LIWL | 15/100 | 14/100 | 14/100 | 15/100 | |
| FU1 | 11/73 | 13/80 | 12/73 | 15/93 | n.s. |
| FU2 | 8/53 | 12/73 | 12/73 | 13/80 | n.s. |
| FU3 | 8/53 | 9/53 | 11/67 | 12/73 | n.s. |
| FU4 | 6/40 | 11/67 | 12/73 | 13/80 | 0.02 |
| FU5 | 7/47 | 10/60 | 12/73 | 14/87 | 0.01 |
| BMI (kg/m2) | BL | Q1 | p | BL | Q2 | p | BL | Q3 | p | BL | Q4 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWL | 32.9 ± 6.6 | 30.9 ± 7.3 | <0.01 | 33.0 ± 5.7 | 28.8 ± 4.9 | <0.01 | 35.4 ± 3.4 | 30.0 ± 3.5 | <0.01 | 35.2 ± 3.2 | 27.5 ± 5.1 | <0.01 |
| FU1 | 32.6 ± 6.2 | n.s. | 30.7 ± 7.2 | <0.01 | 29.8 ± 3.2 | <0.01 | 28.2 ± 5.1 | <0.01 | ||||
| FU2 | 32.2 ± 4.1 | n.s. | 30.4 ± 6.7 | <0.01 | 31.3 ± 4.4 | <0.01 | 29.8 ± 6.4 | <0.01 | ||||
| FU3 | 31.9 ± 5.4 | n.s. | 30.1 ± 4.1 | n.s. | 31.2 ± 3.7 | <0.01 | 30.2 ± 7.8 | <0.01 | ||||
| FU4 | 32.3 ± 5.0 | n.s. | 32.1 ± 4.7 | 0.03 | 32.4 ± 3.6 | <0.01 | 31.2 ± 8.7 | <0.01 | ||||
| FU5 | 33.8 ± 3.7 | n.s. | 33.6 ± 3.9 | n.s. | 33.6 ± 4.0 | 0.03 | 31.1 ± 8.7 | <0.01 |
| TG (mmol/L) | BL | Q1 | p | BL | Q2 | p | BL | Q3 | p | BL | Q4 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWL | 2.19 ± 2.2 | 2.16 ± 2.8 | n.s. | 2.0 ± 2.6 | 1.36 ± 0.8 | <0.01 | 2.03 ± 1.9 | 1.4 ± 0.8 | <0.01 | 1.83 ± 1.5 | 0.98 ± 0.4 | <0.01 |
| FU1 | 1.93 ± 1.8 | n.s. | 1.42 ± 2.1 | 0.03 | 1.37 ± 1.4 | <0.01 | 1.10 ± 1.5 | <0.01 | ||||
| FU2 | 2.31 ± 1.0 | n.s. | 1.46 ± 2.3 | n.s. | 2.01 ± 1.7 | n.s. | 1.4 ± 1.3 | 0.01 | ||||
| FU3 | 2.76 ± 1.8 | n.s. | 1.5 ± 1.4 | n.s. | 1.97 ± 1.2 | n.s. | 1.23 ± 1.6 | 0.02 | ||||
| FU4 | 2.46 ± 1.3 | n.s. | 1.8 ± 3.2 | n.s. | 1.95 ± 1.7 | n.s. | 1.25 ± 1.1 | 0.04 | ||||
| FU5 | 2.9 ± 1.7 | n.s. | 1.87 ± 2.4 | n.s. | 2.18 ± 2.3 | n.s. | 1.55 ± 1.0 | n.s. |
| HDL (mmol/L) | BL | Q1 | p | BL | Q2 | p | BL | Q3 | p | BL | Q4 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWL | 1.41 ± 0.7 | 1.53 ± 0.8 | n.s. | 1.2 ± 0.4 | 1.33 ± 0.4 | <0.01 | 1.23 ± 0.4 | 1.45 ± 0.6 | <0.01 | 1.21 ± 0.4 | 1.46 ± 0.4 | <0.01 |
| FU1 | 1.6 ± 0.4 | n.s. | 1.26 ± 0.5 | 0.03 | 1.19 ± 0.7 | n.s. | 1.45 ± 0.4 | <0.01 | ||||
| FU2 | 1.34 ± 0.7 | n.s. | 1.28 ± 0.5 | n.s. | 1.13 ± 0.7 | n.s. | 1.28 ± 0.6 | n.s. | ||||
| FU3 | 1.23 ± 0.5 | 0.02 | 1.28 ± 0.5 | n.s. | 1.24 ± 0.5 | n.s. | 1.21 ± 0.5 | n.s. | ||||
| FU4 | 1.38 ± 0.5 | n.s. | 1.32 ± 0.7 | n.s. | 1.13 ± 0.7 | n.s. | 1.27 ± 0.6 | n.s. | ||||
| FU5 | 1.24 ± 0.7 | 0.02 | 1.16 ± 0.5 | n.s. | 1.25 ± 0.4 | n.s. | 1.25 ± 0.4 | n.s. |
| RRsys (mmHg) | BL | Q1 | p | BL | Q2 | p | BL | Q3 | p | BL | Q4 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWL | 140 ± 12 | 138 ± 16.0 | n.s. | 138 ± 14.0 | 122 ± 13.0 | <0.01 | 140 ± 28.5 | 130 ± 14.0 | n.s. | 149.0 ± 18.0 | 130 ± 18.0 | 0.02 |
| FU1 | 140 ± 23.8 | n.s. | 123 ± 18.8 | 0.03 | 125 ± 28.0 | n.s. | 135 ± 22.5 | 0.02 | ||||
| FU2 | 148 ± 57.3 | n.s. | 138 ± 20.0 | n.s. | 145 ± 25.0 | n.s. | 131 ± 23.3 | n.s. | ||||
| FU3 | 137 ± 35.0 | n.s. | 131.0 ± 10.0 | n.s. | 138 ± 25.0 | n.s. | 135 ± 25.0 | n.s. | ||||
| FU4 | 128 ± 27.3 | n.s. | 144.5 ± 33.3 | n.s. | 141 ± 30.0 | n.s. | 129.5 ± 31.3 | n.s. | ||||
| FU5 | 137 ± 12.3 | n.s. | 134 ± 16.0 | n.s. | 130 ± 18.0 | n.s. | 123 ± 17.5 | <0.01 |
| Glucose (mmol/L) | BL | Q1 | p | BL | Q2 | p | BL | Q3 | p | BL | Q4 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWL | 5.9 ± 0.8 | 5.8 ± 0.7 | n.s. | 6.2 ± 1.2 | 5.7 ± 0.6 | 0.02 | 5.9 ± 1.2 | 5.4 ± 0.6 | n.s. | 6.2 ± 0.9 | 5.6 ± 0.7 | 0.02 |
| FU1 | 5.9 ± 0.2 | n.s. | 5.6 ± 0.9 | n.s. | 5.6 ± 0.7 | n.s. | 5.6 ± 0.5 | 0.02 | ||||
| FU2 | 6.3 ± 0.7 | n.s. | 5.7 ± 1.0 | n.s. | 5.4 ± 0.7 | n.s. | 5.4 ± 0.9 | 0.03 | ||||
| FU3 | 6.1 ± 1.8 | n.s. | 5.7 ± 1.1 | n.s. | 5.7 ± 1.4 | n.s. | 5.7 ± 0.9 | 0.02 | ||||
| FU4 | 6.4 ± 1.0 | n.s. | 6.4 ± 1.2 | n.s. | 6.3 ±1.5 | n.s. | 5.8 ± 1.3 | n.s. | ||||
| FU5 | 6.8 ± 1.5 | 0.05 | 6.2 ± 1.4 | n.s. | 6.0 ± 1.2 | n.s. | 5.9 ± 0.8 | n.s. |
| Q1 PD/Total | p | Q2 PD/Total | p | Q3 PD/Total | p | Q4 PD/Total | p | |
|---|---|---|---|---|---|---|---|---|
| BL | 7/15 | 7/14 | 5/14 | 7/15 | ||||
| LIWL | 2/15 | 2/14 | 2/14 | 1/15 | ||||
| FU1 | 4/11 | n.s. | 3/12 | n.s. | 1/11 | n.s. | 1/14 | n.s. |
| FU2 | 2/8 | n.s. | 3/11 | n.s. | 2/11 | n.s. | 1/12 | n.s. |
| FU3 | 3/8 | n.s. | 4/8 | 0.05 | 3/10 | n.s. | 3/11 | n.s. |
| FU4 | 2/6 | 0.03 | 4/10 | n.s. | 3/11 | n.s. | 1/12 | n.s. |
| FU5 | 3/7 | 0.03 | 3/9 | n.s. | 3/11 | n.s. | 1/13 | n.s. |
| Q1 MetS/Total | p | Q2 MetS/Total | p | Q3 MetS/Total | p | Q4 MetS/Total | p | |
|---|---|---|---|---|---|---|---|---|
| BL | 15/15 | 14/14 | 14/14 | 15/15 | ||||
| LIWL | 11/15 | 0.05 | 6/14 | <0.01 | 4/14 | <0.01 | 5/15 | <0.01 |
| FU1 | 6/11 | 0.03 | 5/12 | <0.01 | 4/11 | <0.01 | 4/14 | <0.01 |
| FU2 | 7/8 | n.s. | 7/11 | 0.05 | 7/11 | 0.05 | 7/12 | 0.03 |
| FU3 | 6/8 | n.s. | 6/8 | n.s. | 8/10 | n.s. | 8/11 | n.s. |
| FU4 | 5/6 | n.s. | 10/10 | n.s. | 9/11 | n.s. | 9/12 | n.s. |
| FU5 | 6/7 | n.s. | 9/9 | n.s. | 8/11 | n.s. | 9/13 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, S.; Vogel, M.; Mathew, A.; Ebert, T.; Rana, R.; Jiang, S.; Isermann, B.; Biemann, R. The Extent of Lifestyle-Induced Weight Loss Determines the Risk of Prediabetes and Metabolic Syndrome Recurrence during a 5-Year Follow-Up. Nutrients 2022, 14, 3060. https://doi.org/10.3390/nu14153060
Zimmermann S, Vogel M, Mathew A, Ebert T, Rana R, Jiang S, Isermann B, Biemann R. The Extent of Lifestyle-Induced Weight Loss Determines the Risk of Prediabetes and Metabolic Syndrome Recurrence during a 5-Year Follow-Up. Nutrients. 2022; 14(15):3060. https://doi.org/10.3390/nu14153060
Chicago/Turabian StyleZimmermann, Silke, Mandy Vogel, Akash Mathew, Thomas Ebert, Rajiv Rana, Shihai Jiang, Berend Isermann, and Ronald Biemann. 2022. "The Extent of Lifestyle-Induced Weight Loss Determines the Risk of Prediabetes and Metabolic Syndrome Recurrence during a 5-Year Follow-Up" Nutrients 14, no. 15: 3060. https://doi.org/10.3390/nu14153060
APA StyleZimmermann, S., Vogel, M., Mathew, A., Ebert, T., Rana, R., Jiang, S., Isermann, B., & Biemann, R. (2022). The Extent of Lifestyle-Induced Weight Loss Determines the Risk of Prediabetes and Metabolic Syndrome Recurrence during a 5-Year Follow-Up. Nutrients, 14(15), 3060. https://doi.org/10.3390/nu14153060