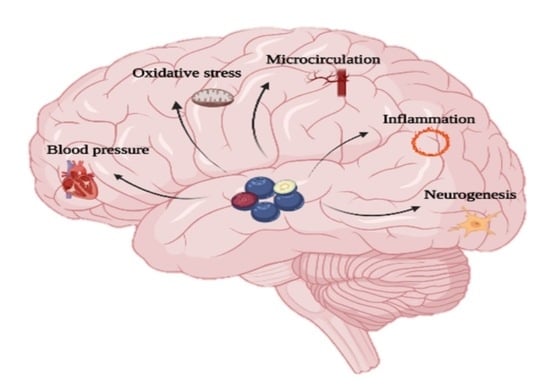
Systematic Review on the Potential Effect of Berry Intake in the Cognitive Functions of Healthy People
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Study Selection
3.2. Selected Studies Characteristics
3.3. Effects on Cognition
- −
- −
- one study [33] evaluated the platelet monoamine oxidase A and B (MAO-A and -B) activity in healthy young humans, observing a clinically significant inhibition of platelet MAO-B following blackcurrant supplementation;
- −
- one study evaluated modulation of pre-frontal cortex brain wave spectral activity measured by electroencephalogram (EEG) reporting an anxiolytic effect following blackcurrant juice drink somministration;
- −
- −
- one study [31] assessed the total polyphenols levels in urine, failing to find significant difference in neurotrofin levels (BDNF and NGF-R), but showing an improvement in executive function; and
- −
- one study [29] evaluated also mood and the cerebral blood flow with three different active beverages reporting an increased subjective energetic arousal and hemodynamic responses.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nouchi, R.; Kawashima, R. Improving Cognitive Function from Children to Old Age: A Systematic Review of Recent Smart Ageing Intervention Studies. Adv. Neurosci. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Kim, J.K.; Langa, K.M.; Weir, D.R. Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2011, 66 (Suppl. 1), i162-71. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global cognitive impairment prevalence and incidence in community dwelling older adults—a systematic review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimers. Dis. Other Demen. 2018, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, R.; Leone, A.; Foppiani, A.; Osio, D.; Lewandowski, L.; Giustizieri, V.; Cornelio, P.; Cornelio, F.; Fusari Imperatori, S.; Cappa, S.F.; et al. Mediterranean Diet and Cognitive Status in Free-Living Elderly: A Cross-Sectional Study in Northern Italy. J. Am. Coll. Nutr. 2018, 37, 494–500. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Businaro, R.; Vauzour, D. The role of diet in preventing and reducing cognitive decline. Curr. Opin. Psychiatry 2020, 33, 432–438. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers. Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Beck, T.; Bennett, D.A.; Schneider, J.A.; Hayden, K.M.; Shadyab, A.H.; Rajan, K.B.; Morris, M.C.; Cornelis, M.C. Adherence to MIND Diet, Genetic Susceptibility, and Incident Dementia in Three US Cohorts. Nutrients. 2022, 14, 2759. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Netzel, M.; Fanning, K. Food-based anthocyanin intake and cognitive outcomes in human intervention trials: A systematic review. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef]
- Martini, D.; Marino, M.; Angelino, D.; Del Bo’, C.; Del Rio, D.; Riso, P.; Porrini, M. Role of berries in vascular function: A systematic review of human intervention studies. Nutr. Rev. 2020, 78, 189–206. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vauzour, D.; Rendeiro, C. Flavonoids and cognition: The molecular mechanisms underlying their behavioural effects. Arch. Biochem. Biophys. 2009, 492, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.J.; Tapsell, L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Dworatzek, P.D.N. Food and dietary pattern-based recommendations: An emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can. J. Diabetes 2013, 37, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Pribis, P.; Shukitt-Hale, B. Cognition: The new frontier for nuts and berries. Am. J. Clin. Nutr. 2014, 100, 347S–352S. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Grames, E.M.; Stillman, A.N.; Tingley, M.W.; Elphick, C.S. An automated approach to identifying search terms for systematic reviews using keyword co-occurrence networks. Methods Ecol. Evol. 2019, 10, 1645–1654. [Google Scholar] [CrossRef]
- Eldridge, S.; Campbell, M.K.; Campbell, M.J.; Drahota, A.K.; Giraudeau, B.; Reeves, B.C.; Siegfried, N.; Higgins, J.P.T. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 18 January 2022).
- Amagase, H.; Nance, D.M. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (goji) juice, GoChiTM. J. Altern. Complement. Med. 2008, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Cheng, N.; Butler, L.T.; Lamport, D.J.; Williams, C.M. Flavonoid-Rich Mixed Berries Maintain and Improve Cognitive Function Over a 6 h Period in Young Healthy Adults. Nutrients 2019, 11, 2685. [Google Scholar] [CrossRef]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS ONE 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Schandry, R.; Duschek, S. The effect of Camphor-Crataegus berry extract combination on blood pressure and mental functions in chronic hypotension—A randomized placebo controlled double blind design. Phytomedicine 2008, 15, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.S.; Duschek, S.; Schandry, R. D-camphor-crataegus berry extract combination increases blood pressure and cognitive functioning in the elderly—A randomized, placebo controlled double blind study. Phytomedicine 2009, 16, 1077–1082. [Google Scholar] [CrossRef]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The effect of long-term aronia melanocarpa extract supplementation on cognitive performance, mood, and vascular function: A randomized controlled trial in healthy, middle-aged individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Jackson, P.A.; Wightman, E.L.; Veasey, R.; Forster, J.; Khan, J.; Saunders, C.; Mitchell, S.; Haskell-Ramsay, C.F.; Kennedy, D.O. A randomized, crossover study of the acute cognitive and cerebral blood flow effects of phenolic, nitrate and botanical beverages in young, healthy humans. Nutrients 2020, 12, 2254. [Google Scholar] [CrossRef]
- Whyte, A.R.; Rahman, S.; Bell, L.; Edirisinghe, I.; Krikorian, R.; Williams, C.M.; Burton-Freeman, B. Improved metabolic function and cognitive performance in middle-aged adults following a single dose of wild blueberry. Eur. J. Nutr. 2021, 60, 1521–1536. [Google Scholar] [CrossRef]
- García-Cordero, J.; Pino, A.; Cuevas, C.; Puertas-Martín, V.; Román, R.S.; de Pascual-Teresa, S. Neurocognitive effects of cocoa and red-berries consumption in healthy adults. Nutrients 2022, 14, 1. [Google Scholar] [CrossRef]
- Erfurt, L.; Schandry, R.; Rubenbauer, S.; Braun, U. The effects of repeated administration of camphor-crataegus berry extract combination on blood pressure and on attentional performance—A randomized, placebo-controlled, double-blind study. Phytomedicine 2014, 21, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef]
- Bell, L.; Williams, C.M. A pilot dose–response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults. Eur. J. Nutr. 2019, 58, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Okello, E.J.; Brooker, H.J.; Lester, S.; McDougall, G.J.; Wesnes, K.A. The impact of blackcurrant juice on attention, mood and brain wave spectral activity in young healthy volunteers. Nutr. Neurosci. 2019, 22, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.F.; Williams, C.M.; Butler, L.T.; Spencer, J.P.E. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr. Health Aging 2019, 5, 119–132. [Google Scholar] [CrossRef]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef]
- Novak, V.; Hajjar, I. The relationship between blood pressure and cognitive function. Nat. Rev. Cardiol. 2010, 7, 686–698. [Google Scholar] [CrossRef]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef]
- Dreiseitel, A.; Korte, G.; Schreier, P.; Oehme, A.; Locher, S.; Domani, M.; Hajak, G.; Sand, P.G. Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol. Res. 2009, 59, 306–311. [Google Scholar] [CrossRef]
- Schmitt, J.A.J. Nutrition and cognition: Meeting the challenge to obtain credible and evidence-based facts. Nutr. Rev. 2010, 68, S2–S5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Giau, V.; Bagyinszky, E.; An, S.S.A. Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 4149. [Google Scholar] [CrossRef]
- Levy, B.; Tsoy, E.; Gable, S. Developing Cognitive Markers of Alzheimer’s Disease for Primary Care: Implications for Behavioral and Global Prevention. J. Alzheimers. Dis. 2016, 54, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Toward a new philosophy of preventive nutrition: From a reductionist to a holistic paradigm to improve nutritional recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Lamport, D.J.; Williams, C.M. Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plast. 2021, 6, 139–153. [Google Scholar] [CrossRef]
| Study | Study Design | Sample Size 1 | Age (years) 1,2 | Berry | Daily Dose | Intervention Duration | Outcome Domain | Effect |
|---|---|---|---|---|---|---|---|---|
| Schandry and Duschek (2008) | Parallel RCT | 19 (21 controls) | 25.8 ± 5.0 | Crataegus berries | 25 drops = 0.97 g berry extract ~ 24 g fresh fruit | Acute effect | Processing speed (Attentional Performance Test—‘‘Test d2′’) | Significant and positive effect as assessed by the Number-Connection-Test |
| Attention and concentration (Alertness Test) | No significant differences found | |||||||
| 24 (24 controls) | 25.9 ± 5.0 | Crataegus berries | 25 drops = 0.97 g berry extract ~ 24 g fresh fruit | Acute effect | Processing speed (Number-Connection-Test-Wechsler Adult Intelligence Scale, Digit Symbol Subtest) | Significant and positive effect as assessed by the Number-Connection-Test and the Wechsler Adult Intelligence Scale (Digit Symbol subtest) | ||
| Werner et al. (2009) | Parallel RCT | 40 (40 controls) | 59.25 ± 8 | Crataegus berries | 25 drops = 0.97 g berry extract ~ 24 g fresh fruit | Acute effect | Processing speed (Number-Connection-Test, Digit-Symbol-Test) | Significant and positive effect as assessed by the Wechsler Adult Intelligence Scale (Digit Symbol subtest) |
| Erfurt et al. (2014) | Parallel RCT | 38 (15 controls) | 24.4 ± 4.4 | Crataegus berries | 4 × 20 drops = 3.1 g berry extract ~ 78 g fresh fruit | Acute effect | Attention and concentration (Digit Symbol Test) | Significant and positive effect as assessed by the Test d2 |
| Processing speed (Attentional Performance Test—‘‘Test d2′’) | No significant differences found | |||||||
| Watson et al. (2015) | Cross-over RCT | 36 | 24.8 ± 3.93, (18, 34) | Blackcurrant | 1.66 g berry extract ~ 525 ± 5 mg of polyphenols (values per 60 kg of bodyweight) 142 mL ~ 150 g fresh fruit ~ 499 mg of polyphenols (values per 60 kg of bodyweight) | Acute effect Acute effect | Attention and concentration (Digit Vigilance Task) | Significant and positive effect as assessed by the COMPASS test (Digit vigilance reaction time) |
| Processing speed (Rapid Visual Information Processing (RVIP)) | Significant and positive effect as assessed by the COMPASS test (Rapid visual information processing accuracy) | |||||||
| Executive functioning (Logical Reasoning) | No significant differences found | |||||||
| Attention and concentration (Stroop Task) | No significant differences found | |||||||
| Haskell-Ramsay et al. (2016) | Cross-over RCT | 20 | 21.05 ± 0.89 | Purple grape | 230 mL ~ 1681.7 μg/m of polyphenols | Acute effect | Memory (Immediate Word Recall) | No significant differences found |
| Attention and concentration (Bond-Lader Alert) | Significant and positive effect as assessed by the COMPASS test (Attention reaction time) | |||||||
| Bell and Williams (2018) | Cross-over RCT | 20 | 70.50 ± 5.49, (62, 81) | Haskap berry | 100 mg of anthocyanins | Acute effect | Memory (Auditory Verbal Learning Task) | No significant differences found |
| Executive functioning (Serial Subtraction) | Significant and positive effect as assessed by the serial subtraction test (7s errors) | |||||||
| Attention and concentration (Attention Network Task) | No significant differences found | |||||||
| Haskap berry | 200 mg of anthocyanins | Acute effect | Memory (Auditory Verbal Learning Task) | No significant differences found | ||||
| Executive functioning (Serial Subtraction, 3s and 7s) | Significant and positive effect as assessed by the serial subtraction test (3s errors) | |||||||
| Attention and concentration (Attention Network Test) | No significant differences found | |||||||
| Haskap berry | 400 mg of anthocyanins | Acute effect | Memory (Auditory Verbal Learning Task) | Significant and positive effect as assessed by the Auditory verbal learning task (recognition) | ||||
| Executive functioning (Serial Subtraction, 3s and 7s) | Significant and positive effect as assessed by the serial subtraction test (7s errors) | |||||||
| Attention and concentration (Attention Network Test) | No significant differences found | |||||||
| Watson et al. (2018) | Cross-over RCT | 9 | 23 (mean) | Blackcurrants, Ribes nigrum L. | 96.96 mL ~ 515.7 mg of polyphenols | Acute effect | Attention and concentration (CogTrack™) | No significant differences found |
| Processing speed (CogTrack™) | Significant and negative effect as assessed by the CogTrack™ test (Choice reaction time) | |||||||
| Attention and concentration (CogTrack™) | No significant differences found | |||||||
| Dodd et al. (2019) | Cross-over RCT | 18 | 68.72 ± 3.30, (62, 73) | Blueberry | 30 g ~ 200 g fresh fruit ~ 578.82 mg of polyphenols | Acute effect | Executive functioning (Go-NoGo, Correct Reaction Time, Digit Symbol Substitution Test, Total Correct) | No significant differences found |
| Processing speed (Stroop, Correct Reaction Time, Digit Switch, Switch Cost) | No significant differences found | |||||||
| Attention and concentration (Continuous Performance Task, Commission Errors) | No significant differences found | |||||||
| Memory (Random Word Generation, Total Correct, Three-Word Sets, Total Correct) | No significant differences found | |||||||
| Ahles et al. (2020) | Parallel RCT | 90 mg Aronia: 34 150 mg Aronia: 35 (32 controls) | 90 mg Aronia: 53 ± 1 150 mg Aronia: 53 ± 1 | Aronia melanocarpa | 90 mg ~ 16 mg anthocyanins 150 mg ~ 27 mg anthocyanins | 24 weeks | Motor skills and construction (Grooved Pegboard Test) | Significant and positive effect as assessed by the Grooved pegboard test (dominant hand) |
| Attention and concentration (Number Cross-Out Test) | No significant differences found | |||||||
| Processing speed (Stroop Color and Word Test) | No significant differences found | |||||||
| Jackson et al. (2020) | Cross-over RCT | 32 | 22.28 ± 4.27 | Blueberry | 2.49 g ~ 300 mg anthocyanins | Acute effect | Executive functioning (COMPASS) | No significant differences found |
| Processing speed (COMPASS) | No significant differences found | |||||||
| Whyte et al. (2021) | Cross-over RCT | 35 | 51 ± 8 | Blueberry (wild) | 25 g ~ 1 cup fresh fruit ~ 725 mg polyphenols | Acute effect | Memory (Auditory Verbal Learning Test) | Significant and positive effect as assessed by the Auditory verbal learning test (word rejection accuracy) |
| Executive functioning (Modified Attention Network Task) | No significant differences found | |||||||
| García-Cordero (2022) | Parallel RCT | 20 | 56.4 ± 4.14 | Red berry + blackcurrants + raspberries + blueberries | 1 tablespoon ~ 100 mg anthocyanins | 12 weeks | Memory (Memory Summary, Working Memory Summary) | No significant differences found |
| Processing speed/Attention and concentration | No significant differences found | |||||||
| 19 | 57.84 ± 6.76 | Red berry + blackcurrants + raspberries + blueberries | 1 tablespoon ~ 100 mg anthocyanins | 12 weeks | Memory (Memory Summary, Working Memory Summary) | No significant differences found | ||
| Processing speed/Attention and concentration (Processing Speed and Attention Summary) | No significant differences found |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Systematic Review on the Potential Effect of Berry Intake in the Cognitive Functions of Healthy People. Nutrients 2022, 14, 2977. https://doi.org/10.3390/nu14142977
De Amicis R, Mambrini SP, Pellizzari M, Foppiani A, Bertoli S, Battezzati A, Leone A. Systematic Review on the Potential Effect of Berry Intake in the Cognitive Functions of Healthy People. Nutrients. 2022; 14(14):2977. https://doi.org/10.3390/nu14142977
Chicago/Turabian StyleDe Amicis, Ramona, Sara Paola Mambrini, Marta Pellizzari, Andrea Foppiani, Simona Bertoli, Alberto Battezzati, and Alessandro Leone. 2022. "Systematic Review on the Potential Effect of Berry Intake in the Cognitive Functions of Healthy People" Nutrients 14, no. 14: 2977. https://doi.org/10.3390/nu14142977
APA StyleDe Amicis, R., Mambrini, S. P., Pellizzari, M., Foppiani, A., Bertoli, S., Battezzati, A., & Leone, A. (2022). Systematic Review on the Potential Effect of Berry Intake in the Cognitive Functions of Healthy People. Nutrients, 14(14), 2977. https://doi.org/10.3390/nu14142977