Honeys as Possible Sources of Cholinesterase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Determination of ChE Inhibitory Activity
2.4. Determination of the Total Polyphenolic Content
2.5. Statistical Analysis
3. Results and Discussion
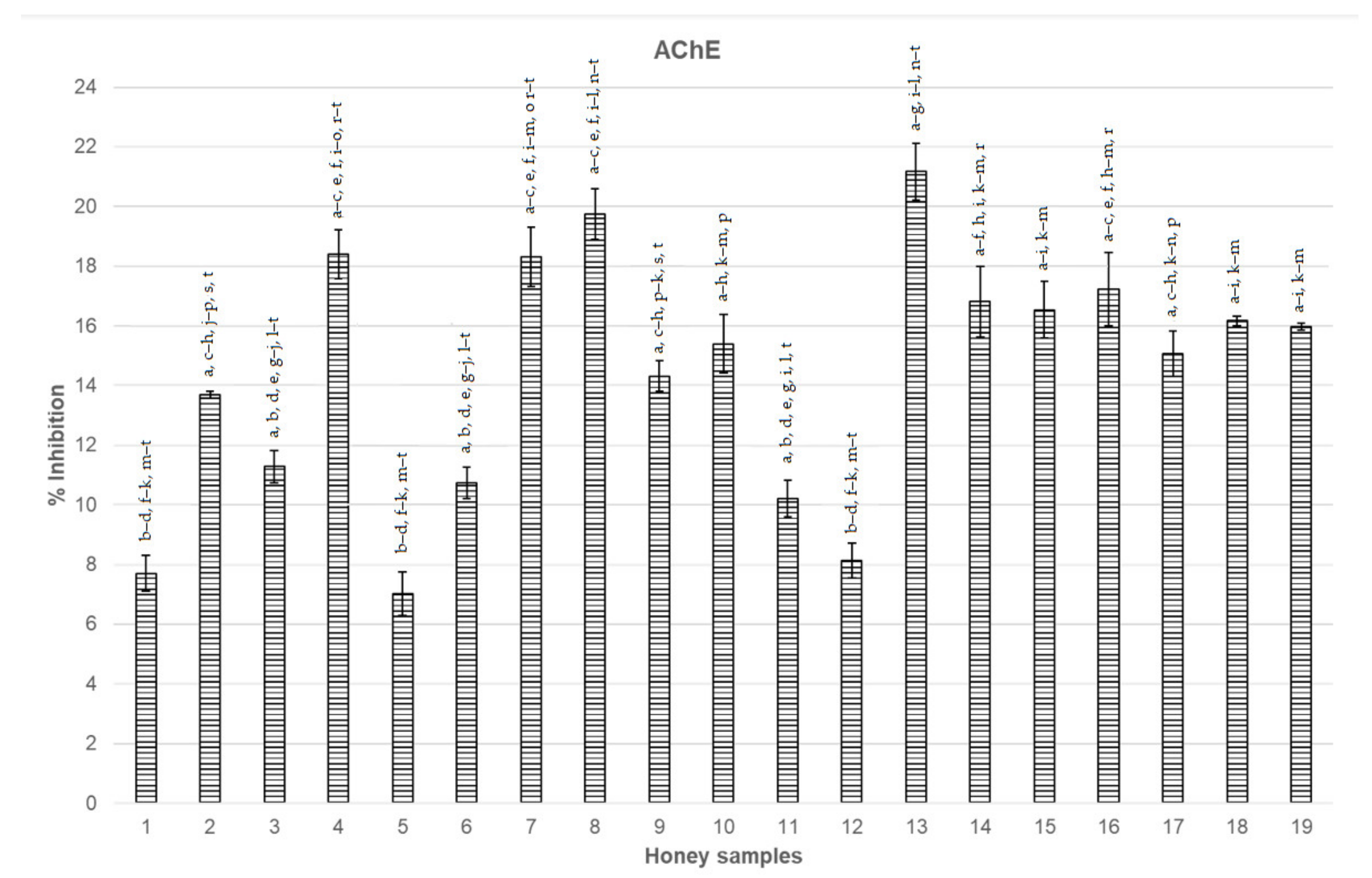
3.1. Inhibition of AChE and BChE
3.2. Total Phenolic Content (TPC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar]
- Prince, M.; Bryce, R.; Ferri, C. Alzheimer’s Disease International World Alzheimer Report 2011. The Benefits of Early Diagnosis and Intervention. Alz-heimer’s Disease International. 2011. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf (accessed on 25 May 2022).
- Adewusi, E.A.; Steenkamp, V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 2011, 4, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Langyanai, S.; Chaniad, P.; Puripattanavong, J. Acetylcholinesterase Inhibitory and Antioxidant Properties of Thai Vegetables. Int. J. Pharm. Med. Biol. Sci. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Zhao, T.; Ding, K.; Zhang, L.; Cheng, X.; Wang, C.; Wang, Z. Acetylcholinesterase and Butyrylcholinesterase inhibitory activities of ß-carboline and quinoline alkaloids derivatives from the plants of genus peganum. J. Chem. 2013, 2013, 717232. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent Knowledge on Medicinal Plants as Source of Cholinesterase Inhibitors for the Treatment of Dementia. Med. Chem. 2016, 16, 8. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, M.I. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorg. Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Korábečný, J.; Nepovimová, E.; Cikánková, T.; Špilovská, K.; Vašková, L.; Mezeiová, E.; Kucá, K.; Hroudova, J. Newly Developed Drugs for Alzheimer’s Disease in Relation to Energy Metabolism, Cholinergic and Monoaminergic Neurotransmission. Neuroscience 2018, 370, 191–206. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar]
- Nadri, H.; Pirali-Hamedani, M.; Moradi, A.; Sakhteman, A.; Vahidi, A.; Sheibani, V.; Asadipour, A.; Hosseinzadeh, N.; Abdollahi, M.; Shafiee, A.; et al. 5,6-Dimethoxybenzofuran-3-one derivatives: A novel series of dual Acetylcholinesterase/Butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety. J. Pharm. Sci. 2013, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [PubMed]
- Minden-Birkenmaier, B.A.; Cherukuri, K.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. Manuka honey modulates the inflammatory behavior of a dHL-60 neutrophil model under the cytotoxic limit. Int. J. Biomater. 2019, 2019, 6132581. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular mechanism-based therapeutic properties of honey. Biomed. Pharmacother. 2020, 130, 110590. [Google Scholar] [CrossRef] [PubMed]
- Al-Himyari, F.A. The use of honey as a natural preventive therapy of cognitive decline and dementia in the Middle East. Alzheimers Dement. 2009, 5, 247. [Google Scholar] [CrossRef]
- Othman, Z.; Zakaria, R.; Hussain, N.H.N.; Hassan, A.; Shafin, N.; Al-Rahbi, B.; Ahmad, A.H. Potential Role of Honey in Learning and Memory. Med. Sci. 2015, 3, 3–15. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Szwajgier, D. Anticholinesterase activities of selected polyphenols—A short report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids—Interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar]
- Szwajgier, D.; Baranowska-Wójcik, E.; Borowiec, K. Phenolic acids exert anticholinesterase and cognition-improving effects. Curr. Alzheimer Res. 2018, 15, 531–543. [Google Scholar]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Regardless of the brewing conditions, various types of tea are a source of acetylcholinesterase inhibitors. Nutrients 2020, 12, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Honey as the potential natural source of cholinesterase inhibitors in Alzheimer’s disease. Plant Food Hum. Nutr. 2020, 75, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Lourtney, D.K.; Andres, V.; Gmelin, G.A. New and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT J. Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Odunola, O.A.; Gbadegesin, M.A.; Sallau, A.B.; Ndidi, U.S.; Ibrahim, M.A. Inhibitory effects of sodium arsenite and acacia honey on acetylcholinesterase in rats. Int. J. Alzheimer’s Dis. 2015, 2015, 903603. [Google Scholar] [CrossRef] [Green Version]
- Philip, Y.; Mohd Fadzelly, A.B. Antioxidative and acetylcholinesterase inhibitor potential of selected honey of Sabah, Malaysian Borneo. Int. Food Res. J. 2015, 22, 1953–1960. [Google Scholar]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Araujo, M.E. Biological properties of phenolic compound extracts in selected Algerian honeys—The inhibition of acetylcholinesterase and α-glucosidase activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Şahin, B. Can sunflower honey have a protective effect against Alzheimer’s disease? J. Org. Chem. Res. 2021, 6, 6–9. [Google Scholar]
- Warad, V.B.; Shastri, R.; Habbu, P.; Katti, P.; Jagannath, A.B.; Kulkarni, V.H.; Chakraborty, M. Preparation and screening of Swarnaprashana for nootropic activity. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 170–178. [Google Scholar] [CrossRef]
- Ferreres, F.; Ortiz, A.; Silva, C.; Garcia-Viguera, C.; Tomás-Barberán, F.A.; Tomás-Lorente, F. Flavonoids of “La Alcarria” Honey, A study of their botanical origin. Z. Lebensm. Untersch. Forsch. 1992, 194, 139–143. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Ortiz, A.; Subra, E.; Tomas-Barberan, F.A. Plant phenolic metabolites and floral origin of rosemary honey. J. Agric. Food Chem. 1995, 43, 2833–2838. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomas-Barberan, F.A. Flavonoid composition of Tunisian honeys and propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of malaysian honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Belkhodja, H.; Belmimoun, A.; Meddah, B. Chemical characterization of polyphenols extracted from different honeys. Banat’s J. Biotechnol. 2017, 8, 78–82. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Passos, T.M.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]



| Sample No. | Type of Honey | Country of Origin |
|---|---|---|
| 1 | Acacia | Poland |
| 2 | Raspberry | Poland |
| 3 | Lavender | Spain |
| 4 | Bean | Poland |
| 5 | Buckwheat | Poland |
| 6 | Aloe | Poland |
| 7 | Heather | Spain |
| 8 | Linden | Poland |
| 9 | Eucalyptus | Uruguay |
| 10 | Sunflower | Ukraine |
| 11 | Goldenrod | Poland |
| 12 | Linden | Poland |
| 13 | Thyme | Spain |
| 14 | Rape | Poland |
| 15 | Rosemary | Spain |
| 16 | Hawthorn | Poland |
| 17 | Pomegranate juice | Bosnia |
| 18 | Acacia | Poland |
| 19 | Orange blossom | France |
| Honey Samples | TPC Content mg GAE/100 g |
|---|---|
| 1 | 46.49 ± 3.30 b–t |
| 2 | 146.34 ± 10.86 a, c–l, n–t |
| 3 | 222.01 ± 9.45 a, b, d–t |
| 4 | 166.59 ± 8.64 a–d, f, g, i, j, l–t |
| 5 | 177.93 ± 23.66 d–e, g–t |
| 6 | 81.56 ± 11.78 d–e, g–t |
| 7 | 326.23 ± 18.05 a–f, h–t |
| 8 | 176.51 ± 33.69 a–d, f, g, i–t |
| 9 | 278.66 ± 16.32 a–h, j–t |
| 10 | 212.26 ± 10.82 a–i, k–t |
| 11 | 178.97 ± 18.32 a–d, f–j, l–t |
| 12 | 102.44 ± 20.32 a–k, m–t |
| 13 | 146.58 ± 7.49 a, c–l, n–t |
| 14 | 96.99 ± 10.15 a–m, o–t |
| 15 | 133.48 ± 9.69 a–n, p–t |
| 16 | 161.11 ± 5.24 a–o, r–t |
| 17 | 153.34 ± 14.00 a–p, s, t |
| 18 | 245.26 ± 3.09 a–r, t |
| 19 | 184.08 ± 8.64 a–s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. https://doi.org/10.3390/nu14142969
Szwajgier D, Baranowska-Wójcik E, Winiarska-Mieczan A, Gajowniczek-Ałasa D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients. 2022; 14(14):2969. https://doi.org/10.3390/nu14142969
Chicago/Turabian StyleSzwajgier, Dominik, Ewa Baranowska-Wójcik, Anna Winiarska-Mieczan, and Dorota Gajowniczek-Ałasa. 2022. "Honeys as Possible Sources of Cholinesterase Inhibitors" Nutrients 14, no. 14: 2969. https://doi.org/10.3390/nu14142969
APA StyleSzwajgier, D., Baranowska-Wójcik, E., Winiarska-Mieczan, A., & Gajowniczek-Ałasa, D. (2022). Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients, 14(14), 2969. https://doi.org/10.3390/nu14142969







