Abstract
Cancer-associated malnutrition, or cachexia, stemming from cancer or its treatments, is particularly prevalent in metastatic cancers, and is often interrelated with sarcopenia and frailty. Evidence suggests that dietary supplements play a role in managing these conditions. As metastatic cancer cells are associated with notable genomic and phenotypic alterations, response to dietary supplements may differ between metastatic and non-metastatic cancers. However, research in this area is lacking. This scoping review aims to identify the dietary supplements that have been studied in patients with metastatic cancers and malnutrition-related conditions, along with their proposed effects, mechanisms, outcome measures, and tools used. A systematic search was conducted across databases, including MEDLINE, EMBASE, CINAHL, and clinical trial registries. Of the initial 6535 records screened, a total of 48 studies were included, covering a range of dietary supplements—vitamins, minerals, antioxidants, proteins, amino acids, fatty acids, fiber, and others. While the types of dietary supplements included varied across cancer types, omega-3 and carnitine were investigated most often. Proposed relevant attributes of dietary supplements included their antioxidant, anti-inflammatory, anti-cancer, and immunomodulatory properties. Overall, there was a paucity of interventional studies, and more randomized controlled trials are warranted.
Keywords:
dietary supplements; metastatic cancers; malnutrition; cachexia; sarcopenia; frailty; weight loss 1. Introduction
Cancer treatments have significantly improved over time, leading to a prolonged survival time after a diagnosis of metastatic cancer, and consequently an increased number of advanced cancer survivors []. Advanced cancer survivors experience a range of complications arising from cancer, its treatments, and metabolic derangements [,,,,,]. Diet-related unmet care needs, including a lack of appetite and gastrointestinal symptoms, are commonly reported among people with metastatic cancers [].
Malnutrition, cachexia, and sarcopenia are highly prevalent in people with metastatic cancer. Approximately half of people with metastatic cancer are reported to be moderately to severely malnourished [,], and almost all require some form of nutritional intervention []. Cancer-associated malnutrition, or cachexia, is a weight loss syndrome involving systemic inflammation and complex metabolic processes, often due to reduced nutritional intake owing to cancer-related side effects [,]. Sarcopenia is defined by the presence of low muscle strength, low muscle quantity and quality, and poor physical performance []. Sarcopenia is frequently observed in people with metastatic cancer and is associated with poorer prognosis and outcomes, as compared to those without [,,]. Meanwhile, frailty is increasingly recognized as a critical health issue in people with metastatic cancer []. This state of vulnerability can be a culmination of aging, life prolonging cancer treatments, and cancer itself, and is an independent predictor of mortality [,]. Malnutrition, cachexia, sarcopenia, and frailty exhibit phenotypically similar features that are interrelated [].
Modifications to diet, such as the use of dietary supplements, can alleviate cancer-associated symptoms [] and, at times, enhance the efficacy of cancer treatments []. Dietary supplements are defined as concentrated sources of nutrients or other ingredients with a nutritional or physiological effect []. These include vitamins, minerals, botanicals or herb extracts, amino acids, essential fatty acids, and fiber [,]. Although there are a lack of clinical guidelines or consistent recommendations for the use of dietary supplements in people with metastatic cancer, dietary supplements are reportedly used by cancer survivors with the intention of improving symptoms and outcomes [,]. A previous systematic review investigating the role of vitamins, minerals, proteins, and other supplements for the treatment of cancer cachexia reported insufficient evidence advocating for the use of dietary supplements in people with cancer []. However, the review did not include younger people or people with malnutrition-related conditions other than cachexia (e.g., sarcopenia and frailty), and inclusion criteria were limited to cancer survivors with cachexia who may not necessarily have metastatic cancer. As metastatic cancer cells are associated with significant genomic and phenotypic alterations, responses of people with metastatic cancer to dietary supplements may differ from people with non-metastatic cancer and earlier stages of disease []. There is currently a lack of evidence synthesis that has systematically explored different dietary supplements that have been used in studies conducted in people with metastatic cancer and malnutrition-related syndromes (e.g., cachexia, sarcopenia, and frailty).
To address this gap, the primary aim of this scoping review was to systematically map out the body of evidence regarding dietary supplements administered orally or enterally in patients with metastatic cancers and malnutrition-related syndromes, including their hypothesized effects, proposed mechanisms, as well as outcome measures (and corresponding tools) used to evaluate their effects.
2. Materials and Methods
2.1. Protocol and Registration
The scoping review was conducted using a systematic approach, following the JBI methodology for scoping reviews [], and is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews (PRISMA-ScR) Checklist [] (Supplementary File S1). A preliminary search for previous scoping reviews and systematic reviews on the topic was conducted in Medline via PubMed on 5 December 2021.
2.2. Aims and Methodology
The aims and method for this review were prospectively documented in the Open Science Framework Registry (https://osf.io/g483m, accessed on 5 April 2022). The search strategy was developed by two authors (JJ and CH) and a review, consisting of keywords and controlled vocabulary terms, was conducted by a research librarian (Supplementary File S2). A search was conducted across seven included electronic databases (MEDLINE, EMBASE, CINAHL, Cochrane CENTRAL, JBI Evidence Synthesis, Scopus, and Web of Science) and clinical trial registries (ISRCTN registry, clinicaltrials.gov, and World Health Organization International Clinical Trials Registry) on 5 February 2022. Sources that were published in English were included. To provide a comprehensive map of the literature, no restrictions were placed on the publication dates of sources. The sources yielded from the search were imported into the Covidence software [], where duplicates were removed by the software and confirmed by two researchers in the study. Screening and selection of articles were conducted independently by two authors via Covidence [] using the study inclusion and exclusion criteria (Table 1). Discrepancies regarding the inclusion of articles were resolved via consensus. In this study, dietary supplements were defined as vitamins, minerals, proteins/amino acids, fatty acids, prebiotics/fiber, probiotics, and plant/herbal extracts, while oral nutrition supplements (ONS) were defined as liquid-based unenriched or unfortified energy-protein formulations. In line with the definition of ‘dietary supplements’ [], only supplements which were delivered orally were considered. This was extended to include enteral routes to account for people who require tube feeding. While it was recognized that people with metastatic cancers may require alternate routes of delivery (e.g., intravenous or intramuscular), these were not included, as these absorption pathways (and subsequently, required doses) differ from that of oral administration.

Table 1.
List of inclusion and exclusion criteria.
Where there was missing information that precluded a decision on the inclusion or exclusion of the article, corresponding authors of the article were emailed to retrieve more information. Data extraction was performed jointly by two authors (JJ and CH) using a data extraction form (Supplementary File S3) that was developed by the team and pilot tested by the same two authors (JJ and CH) prior to use to ensure all relevant results were extracted. Extracted data were subsequently reviewed by another author (RJ). Data extraction included information on the dietary supplements that have been investigated in the target population, as well as their hypothesized effects and proposed mechanisms, and outcome measures that have been assessed. In line with the purpose of scoping reviews outlined in the literature, the present scoping review was intended to map and summarize available evidence, without investigating effectiveness or formulating recommendations for clinical practice []. Hence, findings on the actual outcomes of the included studies were not analyzed. Findings of interest to the present scoping review (supplement types, hypothesized effects, proposed mechanisms, and outcome measures used) were narratively synthesized.
3. Results
3.1. Search Results
The initial search yielded 8070 records from the databases, 227 records from the clinical trial registries, and 3 records from other sources (Figure 1). After removal of duplicates, 6535 records remained, of which 6286 were excluded following title and abstract screening. Of the 248 full-text articles assessed for eligibility, 200 were excluded. A total of 48 articles met the criteria and were included in narrative synthesis.
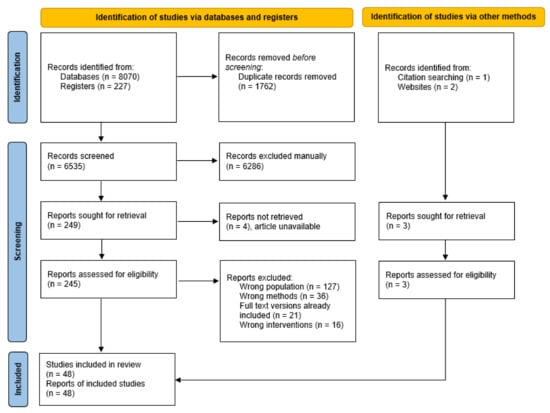
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020) flow diagram.
3.2. Study Characteristics
The 48 articles were classified into the following categories: full text peer-reviewed manuscripts (n = 38), conference abstracts (n = 8), and clinical trial registrations (n = 2). Of the full text peer-reviewed manuscripts, the majority reported on randomized controlled trials (RCTs) (n = 18) [,,,,,,,,,,,,,,,,,], followed by quasi-experimental trials (n = 15) [,,,,,,,,,,,,,,], retrospective cohort observational studies (n = 2) [,], and case studies (n = 3) [,,]. Conference abstracts reported on RCTs (n = 3) [,,], quasi-experimental trials (n = 4) [,,,], and a case study (n = 1) []. The two clinical trial registrations consisted of one RCT [], which was terminated as sample size could not be reached, and one quasi-experimental trial [], which has not commenced recruitment. It was noted that two articles [,] may have included the same subset of patients with gynecological cancers, though one of the trials also included additional patients with mixed cancer types []. The number of studies and the corresponding range of sample sizes across the different study types, cancer types, and countries are all included in Table 2.

Table 2.
Number of completed studies (excluding one clinical trial registration which has not started recruitment) and number of participants included, according to study type, cancer type, and country.
3.3. Types of Dietary Supplements
A variety of dietary supplements were investigated across studies and were grouped into the following categories: vitamins (n = 13), minerals (n = 5), antioxidants (apart from vitamins and minerals) (n = 7), proteins (n = 3), amino acids (n = 14), fatty acids (n = 18), fiber (n = 1), and others (n = 5). Dietary supplements were provided orally in all except three studies [,,], where they were administered enterally. Table 3 shows the types of dietary supplements, cancers, and malnutrition-related conditions in each of the different studies.

Table 3.
Types of dietary supplements, cancers, and malnutrition-related conditions included in each of the studies.
3.4. Forms and Dosages
3.4.1. Vitamins, Minerals, and Other Antioxidants
Vitamins included in studies were vitamin A (n = 2) [,], B1 (n = 1) [], B6 (n = 2) [,], B9 (n = 4) [,,,], B12 (n = 1) [], C (n = 2) [,], D (n = 4) [,,,], and E (n = 3) [,,]. Minerals included were calcium (n = 2) [,], iron (n = 2) [,], and selenium (n = 1) []. Antioxidants (other than vitamins and minerals) included carbocysteine (n = 4) [,,,], lipoic acid (n = 4) [,,,], quercetin (n = 2) [,], curcumin (n = 2) [,], and lycopene (n = 1) [].
The three antioxidant vitamins—Vitamins A, C, and E— were administered concomitantly as part of antioxidant treatments with the same dosages (Vitamin A 30,000 IU daily; Vitamin C 500 mg daily; Vitamin E 400 mg daily) in two studies (an RCT [] and a quasi-experimental single-group trial []) that investigated the efficacy of combined treatments and also included other dietary supplements, such as quercetin, carbocysteine, lipioic acid, eicosapentaenoic acid (EPA), and/or carnitine. In the RCT, all five study arms were given the vitamins []. Vitamin E was additionally included in one other RCT, where it was given to the intervention group which received fish oil as the main intervention [].
Vitamins B1 (thiamine) and B6 (pyridoxine) were included in a quasi-experimental trial, in the form of Aminotrofic® sachets, which mainly consisted of amino acids []. Vitamin B6 was additionally included in a case study as a replacement therapy in doses of 150 mg daily []. Vitamin B9 (folate) was administered in an RCT [], two quasi-experimental trials [,], and an observational study [], of which all also involved concomitant vitamin B12 administration and pemetrexed therapy. In the RCT, where the main aim was to test the efficacy of different doses of pemetrexed, Vitamin B9 was administered to both study groups via a daily multivitamin containing 500 mg folic acid, along with regular intramuscular vitamin B12 []. In one of the quasi-experimental trials, where the aim of the study was to test if the lead-in time for vitamin B supplementation prior to cisplatin-pemetrexed therapy could be shortened, vitamin B9 supplementation in doses of 350–500 µg daily were administered, along with intramuscular vitamin B12 []. In the other quasi-experimental trial, where the aim was to evaluate the safety of oral administration of vitamin B12, vitamin B9 (500 µg daily) was administered along with vitamin B12 in patients receiving pemetrexed []. In the observational study, vitamin B9 was administered in doses of either 400 µg, 700 µg, or 1000 µg once daily depending on the individual’s baseline total plasma homocysteine level, along with regular oral iron and intramuscular vitamin B12 []. The latter study aimed to assess the prevalence of elevated total plasma homocysteine levels at baseline and following pemetrexed treatment, as well as the association between folic acid supplementation and hematological toxicity [].
One study (a quasi-experimental trial) included vitamin B12 administered orally. The first six participants received 500 µg vitamin B12 daily (along with vitamin B9), while subsequent participants were treated under the updated protocol (following the findings of a study, published then, which showed a lack of efficacy of 1000 µg vitamin B12), where they received 2000 µg daily of vitamin B12 for seven days followed by a dose of 500 µg daily thereafter instead [].
Vitamin D was administered in one RCT [] and three quasi-experimental trials [,,]. Vitamin D was the sole dietary supplement investigated and was provided in the form of cholecalciferol to participants with vitamin D insufficiency, at a dose of 2000 IU daily in the RCT [] and 50,000 IU weekly in one of the quasi-experimental trials []. In the remaining two quasi-experimental trials, Vitamin D was administered concomitantly with calcium, either in the form of 2000 units ergocalciferol (commercially available liquid vitamin D analogue) daily along with 500 mg calcium daily among participants with insufficient vitamin D [], or in the form of 0.5 µg 1α-OH vitamin D3 (1α-OHD3) daily along with 1 g calcium carbonate daily [].
In an observational study, iron was administered in the form of ferrous sulphate 200 mg twice daily at the initiation of chemotherapy as per clinic protocol, along with oral vitamin B9 and vitamin B12 injection []. In a case report, iron was administered in the form of 30 mg sucrosomial iron daily as a supportive intervention to radiation therapy in a patient with sideropenic anemia []. Selenium was investigated in only one study (a quasi-experimental trial) and was given in the form of seleno-L-methionine in doses of 2500, 3000, or 4000 µg twice daily for 14 days followed by once daily, in combination with axitinib [].
In terms of antioxidants other than vitamins and minerals, carbocysteine and lipoic acid were administered concomitantly in three RCTs [,,] and one quasi-experimental trial []. A combination of carbocysteine and lipoic acid was administered in two RCTs (that likely shared overlapping participants) as part of an antioxidant treatment (dosage specified as 2.7 g carbocysteine daily and 600 mg lipoic acid daily in one of the RCTs) along with dietary supplement carnitine [,]. In the remaining RCT and quasi-experimental trial, carbocysteine (2.7 g daily) and lipoic acid (300 mg daily) were administered in combination with quercetin, as well as EPA and the antioxidant vitamins A, C, E [,], and either with or without additional carnitine in the RCT [].
Curcumin was investigated as the sole dietary supplement in a quasi-experimental trial in doses of 2 g daily (equivalent to 400 mg daily of active curcuminoids extract) []. In an RCT, curcumin was administered as part of a combined treatment including other dietary supplements (carnitine and lactoferrin) at a dose of 4 g daily []. Lycopene was investigated in only one study (quasi-experimental trial), where it was given at a dose of 30 mg daily in people receiving concomitant docetaxel therapy [].
3.4.2. Proteins and Amino Acids
Protein supplements were investigated in three RCTs. Types of protein supplements included in studies were whey protein isolate (n = 1) [] and lactoferrin (n = 2) [,]. Whey protein isolate was administered in the form of two sachets of cysteine-rich lipid- and lactose-free cow milk whey protein (Prother®) consisting of 20 g protein []. Lactoferrin was administered as two tablets daily (equivalent to 200 mg daily), along with recombinant human erythropoietin, to people with anemia []. In another RCT, the same dose of lactoferrin (200 mg daily) was administered along with carnitine and curcumin, as part of a combined treatment, in people with cancer-related anemia and cachexia [].
For amino acids, carnitine, an amino acid derivative, was the most investigated and was included in seven studies, including five RCTs [,,,,] and two quasi-experimental trials [,]. It was the sole dietary supplement investigated in two studies [,] and part of a combined treatment with other dietary supplements (EPA, branched chain amino acids, coenzyme Q10, lipoic acid, carbocysteine, curcumin, lactoferrin, quercetin, and/or vitamins A, C, and E) in five studies [,,,,]. Carnitine was given as L-carnitine and in doses of 50 mg, 2 g, 4 g, or 6 g daily in included studies.
Arginine was the second most commonly investigated amino acid. Arginine was the main dietary supplement investigated in an RCT where it was administered in the form of a specially formulated enteral formula and replaced 41% of casein [], as well as in an observational study where it was given in the form of an immunonutrition enteral formula (Impact®) containing 12.5 g/L L-arginine, dietary nucleotides, EPA, and docosahexaenoic acid (DHA) []. Arginine was investigated as part of a combined treatment with glutamine (amino acid) and β-hydroxyl β-methyl butyrate (HMB) (amino acid metabolite) in three studies (two RCTs [,] and one quasi-experimental trial []), which were also the only studies where glutamine or HMB were included. Daily doses were in the following ranges: 14–28 g arginine, 14–28 g glutamine, and 2.4–6 g HMB daily [,,].
Branched chain amino acids (BCAA) were investigated in a quasi-experimental trial, where they were administered in the form of an enriched ONS (Inner Power®), which consists of 2500 mg BCAA per pack, along with coenzyme Q10 and carnitine, with one pack given daily []. Two studies, an RCT [] and a quasi-experimental trial [], included all the essential amino acids. In the RCT, a 4 g essential amino acid powder was the sole intervention and was given thrice daily (equivalent to 12 g amino acids daily) []. In the quasi-experimental trial, essential amino acids were given in the form of two sachets of Aminotrific® supplement, which also consisted of vitamins B1 and B6 [].
3.4.3. Fatty Acids
Omega-3 fatty acids, EPA and DHA, were the most commonly investigated dietary supplement overall (n = 18), and were studied in nine RCTs [,,,,,,,,], six quasi-experimental trials [,,,,,], one observational study [], and two case studies [,]. Omega-3 fatty acids were administered in the form of free EPA acids [], purified EPA + DHA capsules [], krill oil capsules [], fish oil capsules [,,,], fish oil liquid [], marine phospholipids [], or in fortified ONS [,,,,,,,,,]. Marine phospholipids and krill oil consisted of omega-3 fatty acids that were bound to phospholipids, which were suggested by study authors to have a different uptake and metabolism from those bound to triacylglycerols (such as those in fish oil) [,]. With the exception of one study where omega-3 was administered enterally [], omega-3 was given orally for all. Reported doses ranged from 1.1 g to 6 g EPA daily and 0.2 to 2.7 g DHA daily [,,,,,,,,,,,,]. Omega-3 fatty acids were the only dietary supplement included in 13 studies [,,,,,,,,,,,,], while additional dietary supplements (e.g., carnitine, arginine, dietary nucleotides, fiber, quercetin, lipoic acid, carbocysteine, and vitamins A, C, and E) were included in five studies [,,,,]. Although DHA was not explicitly mentioned in some studies, it was present along with EPA by virtue of the forms in which EPA was administered [,,,,,,].
3.4.4. Fiber
Fiber was only included in one case study where omega-3 fatty acids were the main dietary supplement of interest []. The participant consumed one serving of fortified ONS (Forticare Nutricia) daily, which contains both EPA and fiber and provides 2.6 g fiber daily.
3.4.5. Others
Other dietary supplements that were investigated were β-hydroxybutyrate (BHB) [], coenzyme Q10 [], muscadine grape extract [], dietary nucleotides [], and royal jelly []. The supplement BHB was included in a quasi-experimental trial that has not started recruitment yet and will be administered in the form of liquid ketone supplement, two tablespoons three times daily (providing 1 g/kg body weight daily of BHB) []. Coenzyme Q10 was administered in a quasi-experimental trial in the form of one pack of enriched ONS (Inner Power® which contains BCAA, carnitine, and 30 mg coenzyme Q10 per pack) daily []. Muscadine grape extract was investigated in a quasi-experimental trial in the form of capsules that were taken twice daily (each capsule containing ~160 mg phenolics) in five dose levels of 320 to 1600 mg total phenolics []. Royal jelly was administered in the form of 800 mg capsules three times daily (equivalent to 2400 mg daily), as per figures published in the erratum [].
3.5. Concomitant Interventions
Of the 48 studies, single dietary supplements were administered on their own, without concomitant interventions, in 19 studies (e.g., omega-3 only) [,,,,,,,,,,,,,,,,,,]. The remaining 29 studies had single dietary supplements administered with other dietary supplements (e.g., omega-3 and arginine), ONS, counseling, exercise, and/or non-cancer specific drugs. Non-cancer specific drugs prescribed concomitantly in those studies were celecoxib [,,,,,], medroxyprogesterone acetate or megestrol acetate [,,,], thalidomide [], or recombinant human erythropoietin [].
In studies with concomitant interventions, dietary supplements were administered with ONS in 12 studies and were used to fortify/enrich ONS in some studies. Oral nutritional supplements were the only other intervention in three studies [,,], while different types of dietary supplements were used as a combination (i.e., omega-3 and arginine) in addition to ONS in two studies [,]. In the remaining studies with ONS, dietary supplements were also used in conjunction with nutritional counseling [,], prescribed diet [], drug [], or a combination of drug, nutritional counseling, and exercise (home-based aerobic and resistance training) [], or were part of a combination of dietary supplements and drugs [,]. In the 17 studies with concomitant interventions but without ONS, dietary supplements were delivered concurrently with drugs [] or nutritional counseling [], or as part of a combination with other dietary supplements [,,,,,,,] that were accompanied by drugs [,,], or in-person structured nutrition, exercise, and symptom advice via the Macmillan Durham Cachexia Pack [], or in-person individualized nutritional and exercise counseling along with prescription of individualized home-based resistance training [].
3.6. Malnutrition-Related Conditions
The malnutrition-related conditions investigated in the included studies included cachexia (n = 14) [,,,,,,,,,,,,,], weight loss (n = 10) [,,,,,,,,,], malnutrition (n = 6) [,,,,,], vitamin D deficiency (n = 3) [,,], anorexia (n = 2) [,] vitamin B6 deficiency (n = 1) [], muscle depletion (n = 1) [], and cancer-related anemia (n = 1) []. The specific malnutrition-related conditions were not specified in other studies (n = 15) [,,,,,,,,,,,,,,]. Cachexia was most commonly investigated, and where reported, definitions were variable and included the following: >2% weight loss in patients with BMI <20 kg/m2 [], >5% weight loss according to ideal or pre-illness body weight in the past three months [], >5% weight loss in the past six months [,], >5% weight loss since first diagnosis [], >10% weight loss [], or the presence of muscle depletion []. No study investigated frailty or sarcopenia (both in accordance with published definitions), and only one study included patients with muscle depletion [], defined by lumbar skeletal muscle index cut-offs [].
3.7. Cancer Types
Dietary supplements that were investigated in the included studies varied across different cancer types, as illustrated in Figure 2. Omega-3 fatty acids (EPA and/or DHA) showed the most consistency, where they were among the most ubiquitous dietary supplements for mixed or unspecified cancer types (n = 7) [,,,,,,], as well as for the individual cancer types—lung (n = 3) [,,], head and neck (n = 2) [,], pancreatic (n = 3) [,,], and colorectal cancers (n = 2) [,].
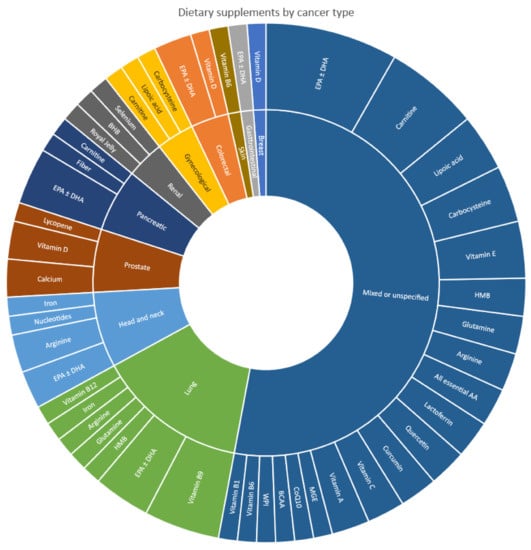
Figure 2.
Types of dietary supplements included in studies, according to cancer type.
In terms of individual cancer types, and beyond omega-3, the predominant dietary supplements investigated were vitamin B9 in lung cancer (n = 4) [,,,], arginine in head and neck cancer (n = 2) [,], and vitamin D plus calcium in prostate cancer (n = 2) [,]. All remaining dietary supplements investigated in individual cancer types were present only in one study in each of the cancer types. For mixed or unspecified cancer types, carnitine (n = 5) [,,,,] was most commonly prescribed after omega-3, followed by vitamin E (n = 3) [,,], carbocysteine (n = 3) [,,], and lipoic acid (n = 3) [,,]. The remaining dietary supplements investigated in mixed or unspecified cancer types were only present in one or two studies each.
3.8. Proposed Effects, Mechanisms/Rationale, and Outcome Measures
As shown in Table 4, dietary supplements were hypothesized to improve malnutrition-related conditions in a majority of the studies [,,,,,,,,,,,,,,,,,,,,,,,,,,]. In some studies, the hypothesized main effects of dietary supplements were related to benefits that were not directly relevant to malnutrition-related conditions. These were improvements in survival [], symptoms of cancer metastasis (e.g., pain) [], antitumor efficacy of chemotherapy [], and treatment response []; counteracting adverse effects of chemotherapy, (e.g., anti-folate effects of pemetrexed) [,,,], bone loss from complete androgenic blockade use [], muscle and joint pain from estrogen blockade use [], and inflammation; oxidative stress immune system dysfunction induced by tyrosine kinase inhibitors []; and counteracting adverse effects of other dietary supplements that are concomitantly administered (such as compensating for the oxidative effect of omega-3 supplements) [].

Table 4.
Hypothesized effects of dietary supplements, mechanisms/rationale, and outcomes measures assessed.
The intended attributes of dietary supplements included antioxidant [,,,,,,,,,,], anti-inflammatory [,,,,,,,,,,,,,], anti-cancer [,,,,,], and immunomodulatory [,,,,,,,,] properties; ability to reduce infections [,], facilitate wound healing [,], improve physical function [], improve muscle strength [], mitigate muscle loss and reduce muscle damage [,,], improve muscle trophism [,], and regulate protein synthesis or turnover [,]; and having a role in energy and amino acid metabolism [,,,].
3.9. Tools Used in Outcome Measurements
The tools that were used to assess each of the different outcome measures, where specified, are presented in Table 5.

Table 5.
Outcome measures and corresponding tools used in included studies.
4. Discussion
This scoping review summarized the types of dietary supplements used in studies involving metastatic cancer patients with malnutrition-related conditions. Thirty-one supplements were identified, which varied across different cancer types. Dietary supplements were investigated as part of combined treatments in most of the studies, where they could be administered as single (i.e., omega-3) or multiple types (i.e., omega-3 + arginine), or along with ONS, other dietary supplements, counseling, exercise, and/or drugs. Omega-3 and L-carnitine were the top two most predominantly investigated supplements—omega-3 for its anti-inflammatory [,,,,,], antioxidant [,,], and immunomodulatory [,,,] properties, and carnitine for its suggested benefits in the modulation of inflammatory response mechanisms that have been associated with cancer cachexia [] and role in metabolism [,,,]. To combat oxidative stress and manage the complexity of cancer-related malnutrition, multimodal treatments were considered necessary to reduce proinflammatory cytokines [,]. Metastasis itself is a complex challenge that necessitates multimodal therapeutic agents for effective inhibition [] and managing its associated syndromes. While multimodal interventions may confer benefits over single interventions, it is challenging to identify the individual contribution of dietary supplements to any beneficial effects seen. Hence, it might be worthwhile investigating this with multi-arm RCTs, including both single and combined interventions which are compared to controls in future studies.
Overall, the included studies consistently reported positive effects for multimodal treatments as well as omega-3 supplements. Evidence for vitamins, minerals, and amino acids was less consistent. While antioxidants and other dietary supplements were reported by studies to exert positive effects, the number of studies that they have been included in were scant. As the present scoping review was, however, not designed to investigate effectiveness, critical appraisal and synthesis of outcome findings of the included studies were not carried out, and recommendations regarding their use cannot be made within the scope of this review.
Cancer-related malnutrition is a complex condition attributable to the imbalance of in vivo redox systems (including antioxidant enzymes and antioxidants) and upregulation of proinflammatory cytokines [,]. Omega-3 has the ability to inhibit the production of proinflammatory cytokines [,,,,], and thus holds promise in its potential to manage this syndrome [,,,]. Two previous systematic reviews (one in adults with cancer undergoing chemotherapy and/or radiotherapy [] and the other in adults with cancer cachexia who were not undergoing cancer treatment during the study period []) indicated beneficial effects (e.g., improvements in body composition, weight, appetite, QoL) of omega-3 fatty acid supplements (EPA; DHA). However, a systematic review in patients with advanced cancer (which included locally recurrent cancers in definition) [] did not find sufficient evidence to support the superiority of omega-3 fatty acid supplements (specifically EPA) over placebo. As the Cochrane review was conducted over 15 years ago, it may be useful to conduct an updated review focusing on patients with metastatic cancer, as more primary studies become available. This can also help identify the effectiveness of dietary supplements in managing cancer-related malnutrition and address the lack of Level 1 evidence in this population.
Overall, more primary studies are warranted for the dietary supplements elucidated in this review. For example, carnitine was investigated in seven studies, of which only two studies [,] investigated it as the sole intervention. Additionally, the present review has mapped the types of dietary supplements to their proposed usefulness for particular cancer types (i.e., vitamin D for prostate cancer). This may provide some indication to researchers regarding the potentially efficacious dietary supplements that can be investigated in studies for specific cancer types.
The strengths of this review include its methodological rigor in line with standards and guidance for scoping review conduct and reporting, the dual approach to screening and extraction to reduce error, and the comprehensive search strategy. Additionally, a wide scope of all available evidence at varying levels on the evidence hierarchy was included. The limitations of the present review include the exclusion of studies where dietary factors (e.g., vitamins, minerals, fatty acids) were administered via the intramuscular and intravenous routes. These were excluded as they were technically not dietary supplements []; however, they might have otherwise provided additional useful information. Additionally, while efforts were made to contact study authors where missing information precludes the inclusion of a paper, only a small number of replies (5/38) were received. Lastly, as none of the included studies specified the inclusion of children, sarcopenic, or frail populations, the present review is unable to provide information on these population groups.
Implications for Research
With the identified dietary supplements and their noteworthy mechanisms and rationale for use in patients with metastatic cancers, particularly for omega-3, vitamin D, and amino acids (arginine, carnitine, glutamine, and HMB), future research in this area is required to assess efficacy on patient outcomes. Future studies should consider conducting fully powered RCTs to increase the reliability of the results. With most of the existing studies having been conducted among mixed or unspecified cancer populations, it would be worthwhile to investigate the efficacy of dietary supplements in specific cancer types, particularly in cancers where malnutrition-related issues are more prevalent (e.g., head and neck cancers). As the forms of dietary supplements were considered to make a difference in some cases (i.e., purported superiority of phospholipids-bound omega-3 over triacylglycerols-bound), researchers may consider the merit of the different forms of dietary supplements when designing future studies. There is also a need to report malnutrition with validated nutritional assessment tools (e.g., PG-SGA) in specific cancer cohorts (e.g., breast, lung, brain).
5. Conclusions
Dietary supplements investigated in studies conducted among patients with metastatic cancers were multifarious and differed across cancer types. With plausible effects and mechanisms proposed in relation to their role in managing malnutrition-related conditions in this population group, future studies assessing the efficacy of dietary supplements on patient outcomes are needed. As primary trials are still lacking for most of the dietary supplements, future RCTs can be considered, along with a consideration of the forms of dietary supplements to be tested, concomitant interventions to be employed (if any), and the relevance of dietary supplements to the specific cancer type of interest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14132642/s1, File S1: PRISMA-ScR Checklist; File S2: Search Strategy; File S3: Data Extraction Form Template.
Author Contributions
Conceptualization, N.H.H., J.J., C.Y.H. and Z.M.; methodology, Z.M., R.J.C., J.J. and C.Y.H.; data extraction, J.J., C.Y.H. and R.J.; formal analysis, N.H.H., J.J. and C.Y.H.; writing—original draft preparation, J.J. and C.Y.H.; writing—review and editing, N.H.H., R.J., Z.M., O.A.A., F.C.-W., M.P.W. and R.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data are available upon request. Details of the excluded papers are available from the corresponding author on request.
Acknowledgments
The authors would like to thank Josephine McGill (Research Librarian) at Flinders University, for her support with the search strategies used in this study, and Amardeep Johal for his graphical support.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Siemens, W.; Schönsteiner, S.S.; Orellana-Rios, C.L.; Schaekel, U.; Kessler, J.; Eschbach, C.; Viehrig, M.; Mayer-Steinacker, R.; Becker, G.; Gaertner, J. Severe symptoms and very low quality-of-life among outpatients newly diagnosed with advanced cancer: Data from a multicenter cohort study. Support. Care Cancer 2020, 28, 5547. [Google Scholar] [CrossRef]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-term effects of breast cancer surgery, treatment, and survivor care. J. Midwifery Womens Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
- Moffat, G.T.; Epstein, A.S.; O’Reilly, E.M. Pancreatic cancer—A disease in need: Optimizing and integrating supportive care. Cancer 2019, 125, 3927–3935. [Google Scholar] [CrossRef]
- Brose, M.S.; Bible, K.C.; Chow, L.Q.; Gilbert, J.; Grande, C.; Worden, F.; Haddad, R. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat. Rev. 2018, 66, 64–73. [Google Scholar] [CrossRef]
- Chan, K.K.; Bass, A.R. Autoimmune complications of immunotherapy: Pathophysiology and management. BMJ 2020, 369, m376. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Molassiotis, A.; Chung, B.P.M.; Tan, J.-Y. Unmet care needs of advanced cancer patients and their informal caregivers: A systematic review. BMC Palliat. Care 2018, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Pardo, J.; Jara, C.; Zugazabeitia, L.; Carulla, J.; de Las Peñas, R.; García-Cabrera, E.; Azuara, M.L.; Casadó, J.; Gómez-Candela, C. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin. Nutr. 2005, 24, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884. [Google Scholar] [PubMed]
- Omlin, A.; Blum, D.; Wierecky, J.; Haile, S.R.; Ottery, F.D.; Strasser, F. Nutrition impact symptoms in advanced cancer patients: Frequency and specific interventions, a case–control study. J. Cachexia Sarcopenia Muscle 2013, 4, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2018, 33, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.S.; Kim, E.Y.; Jin, W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS ONE 2018, 13, e0202700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Dou, Q.-L.; Zeng, Y.; Yang, Y.; Cheng, A.S.; Zhang, W.-W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. The prognostic importance of frailty in cancer survivors. J. Am. Geriatr. Soc. 2015, 63, 2538–2543. [Google Scholar] [CrossRef]
- Balducci, L.; Stanta, G. Cancer in the frail patient: A coming epidemic. Hematol. Oncol. Clin. N. Am. 2000, 14, 235–250. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 7 April 2022).
- U.S. Food & Drug Administration. Dietary Supplement Products & Ingredients. Available online: https://www.fda.gov/food/dietary-supplements/dietary-supplement-products-ingredients (accessed on 7 April 2022).
- Velicer, C.M.; Ulrich, C.M. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Asher, G.N. Use of dietary supplements at a comprehensive cancer center. J. Altern. Complement. Med. 2018, 24, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Mochamat; Cuhls, H.; Marinova, M.; Kaasa, S.; Stieber, C.; Conrad, R.; Radbruch, L.; Mücke, M. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: A European Palliative Care Research Centre cachexia project. J. Cachexia Sarcopenia Muscle 2017, 8, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H.J. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation. Melbourne, Australia. Available online: www.covidence.org (accessed on 17 May 2022).
- National Institutes of Health Office of Dietary Supplements. Background Information: Dietary Supplements. Available online: https://ods.od.nih.gov/factsheets/DietarySupplements-Consumer/ (accessed on 17 May 2022).
- von Haehling, S.; Anker, S.D. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachexia Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef]
- Pollock, D.; Davies, E.L.; Peters, M.D.J.; Tricco, A.C.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H.; Munn, Z. Undertaking a scoping review: A practical guide for nursing and midwifery students, clinicians, researchers, and academics. J. Adv. Nurs. 2021, 77, 2102–2113. [Google Scholar] [CrossRef]
- Araki, K.; Miyata, Y.; Ohba, K.; Nakamura, Y.; Matsuo, T.; Mochizuki, Y.; Sakai, H. Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines 2018, 6, 2. [Google Scholar] [CrossRef]
- Berk, L.; James, J.; Schwartz, A.; Hug, E.; Mahadevan, A.; Samuels, M.; Kachnic, L. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support. Care Cancer 2008, 16, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.; van Bokhorst-de van der Schueren, M.A.; Langius, J.A.; Leemans, C.R.; Kuik, D.J.; Vermeulen, M.A.; van Leeuwen, P.A. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am. J. Clin. Nutr. 2010, 92, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Cerchietti, L.C.; Navigante, A.H.; Castro, M.A. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr. Cancer 2007, 59, 14–20. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Gogos, C.A.; Ginopoulos, P.; Salsa, B.; Apostolidou, E.; Zoumbos, N.C.; Kalfarentzos, F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: A randomized control trial. Cancer 1998, 82, 395–402. [Google Scholar] [CrossRef]
- Antunac Golubić, Z.; Baršić, I.; Librenjak, N.; Pleština, S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer. Nutr. Cancer 2018, 70, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)--a randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Floris, C.; Sanna, E.; Cau, M.C.; Panzone, F.; Mantovani, G. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: Evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol. Oncol. 2012, 124, 417–425. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Sanna, E.; Mantovani, G. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO-beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: Open-label, randomized controlled study. Oncologist 2010, 15, 894–902. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Serpe, R.; Massa, E.; Dessì, M.; Panzone, F.; Contu, P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010, 15, 200–211. [Google Scholar] [CrossRef]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
- Ohe, Y.; Ichinose, Y.; Nakagawa, K.; Tamura, T.; Kubota, K.; Yamamoto, N.; Adachi, S.; Nambu, Y.; Fujimoto, T.; Nishiwaki, Y.; et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Pratt, V.C.; Watanabe, S.; Bruera, E.; Mackey, J.; Clandinin, M.T.; Baracos, V.E.; Field, C.J. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementation. Br. J. Cancer 2002, 87, 1370–1378. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 4826. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Ueno, M.; Sugimori, K.; Taguri, M.; Ohkawa, S.; Kobayashi, S.; Miwa, H.; Kaneko, T.; Morimoto, M.; Yamanaka, T. Randomized Phase II Study of Gemcitabine Monotherapy vs. Gemcitabine with an EPA-Enriched Oral Supplement in Advanced Pancreatic Cancer. Nutr. Cancer 2022, 74, 122–130. [Google Scholar] [CrossRef]
- Bitting, R.L.; Tooze, J.A.; Isom, S.; Petty, W.J.; Grant, S.C.; Desnoyers, R.J.; Thomas, A.; Thomas, C.Y.; Alistar, A.T.; Golden, S.L.; et al. Phase I Study of Muscadine Grape Extract for Patients With Advanced Cancer. Am. J. Clin. Oncol. 2021, 44, 239–246. [Google Scholar] [CrossRef]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L.; Lamonica, G.; Dessì, M.; Spiga, C.; Astara, G.; et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Gramignano, G.; Lusso, M.R.; Serpe, R.; Massa, E.; Astara, G.; Deiana, L. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1030–1034. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef]
- Naito, T.; Mitsunaga, S.; Miura, S.; Tatematsu, N.; Inano, T.; Mouri, T.; Tsuji, T.; Higashiguchi, T.; Inui, A.; Okayama, T.; et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, J.; Jackson, A.; Gaskell, C.; Gaunt, C.; Thompson, J.; Billingham, L.; Steven, N. Beta-hydroxy beta-methylbutyrate/arginine/glutamine (HMB/Arg/Gln) supplementation to improve the management of cachexia in patients with advanced lung cancer: An open-label, multicentre, randomised, controlled phase II trial (NOURISH). BMC Cancer 2021, 21, 800. [Google Scholar] [CrossRef] [PubMed]
- Read, J.A.; Beale, P.J.; Volker, D.H.; Smith, N.; Childs, A.; Clarke, S.J. Nutrition intervention using an eicosapentaenoic acid (EPA)-containing supplement in patients with advanced colorectal cancer. Effects on nutritional and inflammatory status: A phase II trial. Support. Care Cancer 2007, 15, 301–307. [Google Scholar] [CrossRef]
- Maureen Sheean, P.; Robinson, P.; Bartolotta, M.B.; Joyce, C.; Adams, W.; Penckofer, S. Associations Between Cholecalciferol Supplementation and Self-Reported Symptoms Among Women With Metastatic Breast Cancer and Vitamin D Deficiency: A Pilot Study. Oncol. Nurs. Forum 2021, 48, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Hosomi, Y.; Nagamata, M.; Watanabe, K.; Takahashi, S.; Nakahara, Y.; Yomota, M.; Sunami, K.; Okuma, Y.; Shimokawa, T.; et al. Phase II study of oral vitamin B12 supplementation as an alternative to intramuscular injection for patients with non-small cell lung cancer undergoing pemetrexed therapy. Cancer Chemother. Pharmacol. 2016, 77, 559–564. [Google Scholar] [CrossRef]
- Takagi, Y.; Hosomi, Y.; Sunami, K.; Nakahara, Y.; Okuma, Y.; Yomota, M.; Shimokawa, T.; Nagamata, M.; Iguchi, M.; Okamoto, H.; et al. A prospective study of shortened vitamin supplementation prior to cisplatin-pemetrexed therapy for non-small cell lung cancer. Oncologist 2014, 19, 1194–1199. [Google Scholar] [CrossRef][Green Version]
- Tałalaj, M.; Kapitan-Malinowska, B.; Debski, K.; Nowakowski, R.; Marcinowska-Suchowierska, E.; Witeska, A. Administration of 1 alpha-OH vitamin D3 and calcium prevents bone mass loss in patients with advanced prostatic carcinoma after orchidectomy treated with complete androgenic blockade. Endokrynol. Pol. 2005, 56, 225–232. [Google Scholar]
- Taylor, L.A.; Pletschen, L.; Arends, J.; Unger, C.; Massing, U. Marine phospholipids--a promising new dietary approach to tumor-associated weight loss. Support. Care Cancer 2010, 18, 159–170. [Google Scholar] [CrossRef]
- Van Veldhuizen, P.J.; Taylor, S.A.; Williamson, S.; Drees, B.M. Treatment of vitamin D deficiency in patients with metastatic prostate cancer may improve bone pain and muscle strength. J. Urol. 2000, 163, 187–190. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Barber, M.D.; Ross, J.A.; Tisdale, M.J.; Fearon, K.C. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr. Cancer 2000, 36, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, E.; Uchio, E.; Lilly, M.; Zi, X.; Fruehauf, J.P. A phase II study of docetaxel plus lycopene in metastatic castrate resistant prostate cancer. Biomed. Pharmacother. 2021, 143, 112226. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Galindo, D.E.; Vidal-Casariego, A.; Pintor-de la Maza, B.; Fernández-Martínez, P.; Ramos-Martínez, T.; García-Arias, S.; Hernández-Moreno, A.; Urioste-Fondo, A.; Cano-Rodríguez, I.; Ballesteros-Pomar, M.D. Postoperative enteral immunonutrition in head and neck cancer patients: Impact on clinical outcomes. Endocrinol Diabetes Nutr. (Engl. Ed.) 2020, 67, 13–19. [Google Scholar] [PubMed]
- Singh, N.; Aggarwal, A.N.; Kaur, J.; Behera, D. Association of Graded Folic Acid Supplementation and Total Plasma Homocysteine Levels With Hematological Toxicity During First-line Treatment of Nonsquamous NSCLC Patients With Pemetrexed-based Chemotherapy. Am. J. Clin. Oncol. 2017, 40, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, R.; Ramalho, P.; Couto, N.; Pereira, P. Omega-3 therapeutic supplementation in a patient with metastatic adenocarcinoma of the pancreas with muscle mass depletion. Eur. J. Clin. Nutr. 2017, 71, 795–797. [Google Scholar] [CrossRef]
- Rauf, M.; Gleason, C.; Nooka, A.K.; Husman, A.; Waller, E.K. Treatment of severe neutropenia with high-dose pyridoxine in a patient with chronic graft versus host disease and squamous cell carcinoma: A case report. J. Med. Case Rep. 2011, 5, 372. [Google Scholar] [CrossRef]
- Yoshii, R.; Yokoyama, J.; Ohba, S.; Fujimaki, M.; Kojima, M.; Ikeda, K. Impact of EPA nutritional approach on cachexic patients with advanced hypopharyngeal cancer treated by induction chemotherapy. Head Neck Oncol. 2014, 6, 18. [Google Scholar]
- Von Haehling, S. Novel treatment approaches to cancer cachexia: Results from two recent randomized controlled trials using essential amino acids and espindolol. J. Cachexia Sarcopenia Muscle 2017, 8, 151. [Google Scholar]
- Madeddu, C.; Gramignano, G.; Tanca, L.; Cherchi, M.C.; Floris, C.A.; Macciò, A. A combined treatment approach for cachexia and cancer-related anemia in advanced cancer patients: A randomized placebo-controlled trial. J. Clin. Oncol. 2014, 32, 189. [Google Scholar] [CrossRef]
- Madeddu, C.; Maccio, A.; Gramignano, G.; Mulas, C.; Floris, C.; Sanna, E.; Cau, M.C.; Panzone, F.; Dessi, M.; Serpe, R.; et al. A randomized phase III clinical trial of a combined treatment with megestrol acetate+carnitine+celecoxib+antioxidants vs. Megestrol acetate alone for patients with cancer cachexia syndrome. Support. Care Cancer 2012, 20, S57–S58. [Google Scholar]
- Garje, R.; Brown, J.A.; Nepple, K.G.; Dahmoush, L.; Bellizzi, A.; Bonner, J.; Mott, S.L.; Zamba, G.; Laux, D.E.; Milhem, M.M.; et al. Preliminary results of phase I clinical trial of high doses of seleno-L-methionine (SLM) in sequential combination with axitinib in previously treated and relapsed clear cell renal cell carcinoma (ccRCC) patients. J. Clin. Oncol. 2019, 37, 660. [Google Scholar] [CrossRef]
- Lugini, A. Phase II clinical trial using aminotrofic in the prevention of anorexia and cachexia in cancer patients receiving chemotherapy: Evaluation of the efficacy and safety. Support. Care Cancer 2013, 21, 46. [Google Scholar]
- Mantovani, G.; Madeddu, C.; Panzone, F.; Cau, M.C.; Antoni, G.; Leo, F.d.; Macciò, A.; Serpe, R. Curcuma Longa extract is effective in improving inflammatory status and redox balance in patients with cancer-related cachexia and oxidative stress. Ann. Oncol. 2012, 23, 463. [Google Scholar] [CrossRef]
- Serpe, R.; Madeddu, C.; Panzone, F.; Antoni, G.; Cau, M.C.; Macciò, A.; Mantovani, G.; Banni, S. Krill oil to improve blood lipids status in advanced cancer patient with cachexia. J. Clin. Oncol. 2012, 30, e19634. [Google Scholar] [CrossRef]
- Ricottone, N. Preventive Surcosomial® iron supplementation in an elder patient undergoing exclusive radiation therapy treatment for nasopharyngeal cancer with laterocervical lymph node metastasis: Clinical case. Expert Rev. Hematol. 2017, 10, 25. [Google Scholar]
- NCT00398333. Study to Assess the Effectiveness of a Omega-3 Enriched Supplement on Chemotherapy Tolerance in Colon Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT00398333 (accessed on 17 May 2022).
- NCT05119010. A Pilot Study Evaluating a Ketogenic Diet Concomitant to Nivolumab and Ipilimumab in Patients with Metastatic Renal Cell Carcinoma (KETOREIN). Available online: https://clinicaltrials.gov/ct2/show/NCT05119010 (accessed on 17 May 2022).
- Miyata, Y.; Araki, K.; Ohba, K.; Mastuo, T.; Nakamura, Y.; Yuno, T.; Mukai, Y.; Otsubo, A.; Mitsunari, K.; Mochizuki, Y.; et al. Oral intake of royal jelly improves anti-cancer effects and suppresses adverse events of molecular targeted therapy by regulating TNF-α and TGF-β in renal cell carcinoma: A preliminary study based on a randomized double-blind clinical trial. Mol. Clin. Oncol. 2020, 13, 29. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- de Aguiar Pastore Silva, J.; Emilia de Souza Fabre, M.; Waitzberg, D.L. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin. Nutr. 2015, 34, 359–366. [Google Scholar] [CrossRef]
- Colomer, R.; Moreno-Nogueira, J.M.; García-Luna, P.P.; García-Peris, P.; García-de-Lorenzo, A.; Zarazaga, A.; Quecedo, L.; del Llano, J.; Usán, L.; Casimiro, C. n-3 Fatty acids, cancer and cachexia: A systematic review of the literature. Br. J. Nutr. 2007, 97, 823–831. [Google Scholar] [CrossRef]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007, 2007, CD004597. [Google Scholar] [CrossRef]
- Dickinson, A. History and overview of DSHEA. Fitoterapia 2011, 82, 5–10. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).