Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan
Abstract
:1. Introduction
2. Methods
2.1. Participants and Procedures
2.2. Laboratory Analyses
2.3. Assessment of Excessive Drinking
2.4. Assessment of Metabolic Abnormalities
2.5. Statistical Analyses
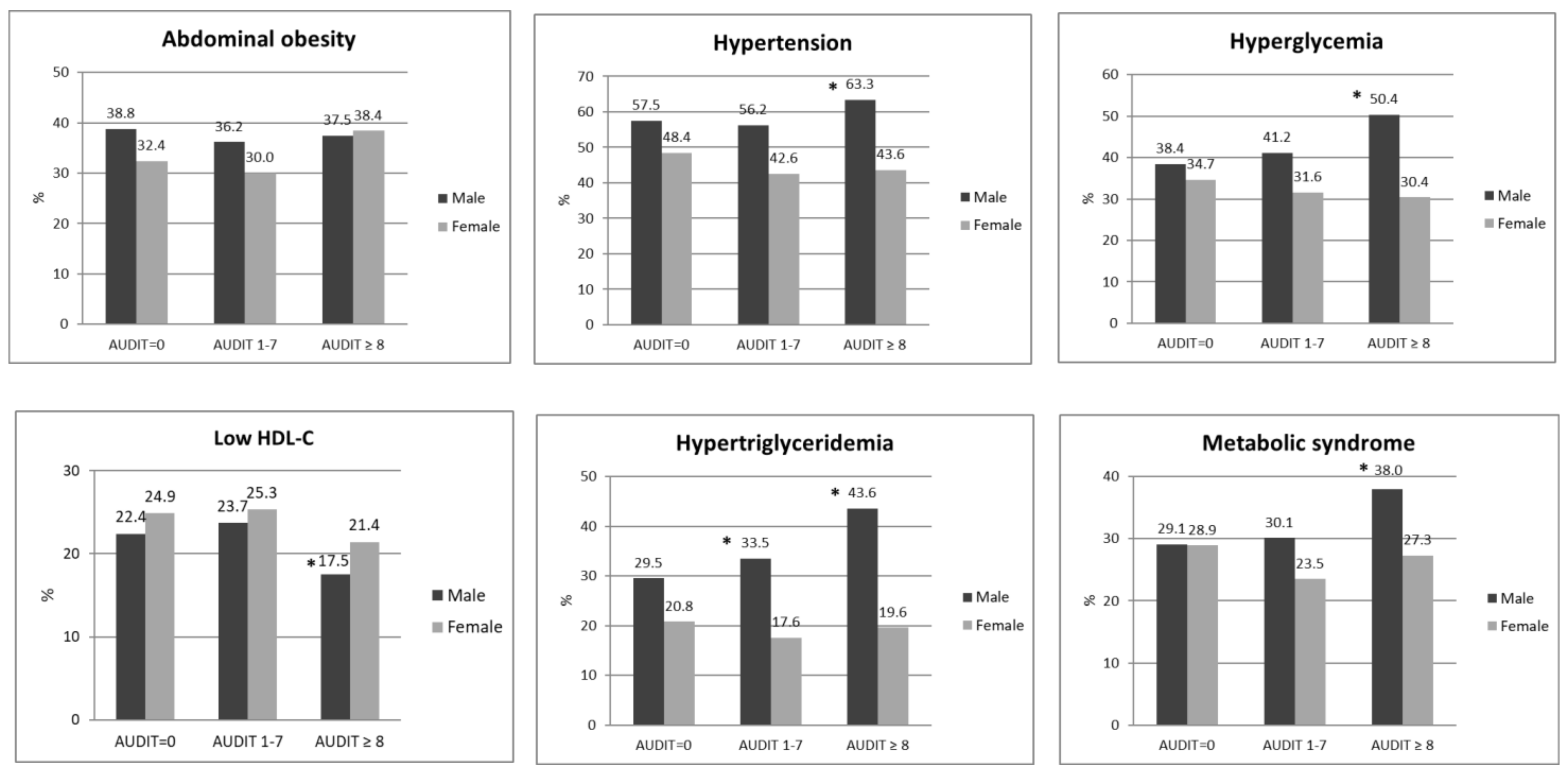
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemieux, I.; Despres, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Mukamal, K.J.; Ascherio, A.; Mittleman, M.; Conigrave, K.M.; Camargo, C.A.; Kawachi, I.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Alcohol and risk for ischemic stroke in men: The role of drinking patterns and usual beverage. Ann. Intern. Med. 2005, 142, 11–19. [Google Scholar] [CrossRef]
- Williams, T. Metabolic Syndrome: Nonalcoholic Fatty Liver Disease. FP Essent 2015, 435, 24–29. [Google Scholar]
- Fujita, N.; Takei, Y. Alcohol consumption and metabolic syndrome. Hepatol. Res. 2011, 41, 287–295. [Google Scholar] [CrossRef]
- Alkerwi, A.; Boutsen, M.; Vaillant, M.; Barre, J.; Lair, M.-L.; Albert, A.; Guillaume, M.; Dramaix, M. Alcohol consumption and the prevalence of metabolic syndrome: A meta-analysis of observational studies. Atherosclerosis 2009, 204, 624–635. [Google Scholar] [CrossRef]
- Sun, K.; Ren, M.; Liu, D.; Wang, C.; Yang, C.; Yan, L. Alcohol consumption and risk of metabolic syndrome: A meta-analysis of prospective studies. Clin. Nutr. 2014, 33, 596–602. [Google Scholar] [CrossRef]
- Vancampfort, D.; Hallgren, M.; Mugisha, J.; De Hert, M.; Probst, M.; Monsieur, D.; Stubbs, B. The Prevalence of Metabolic Syndrome in Alcohol Use Disorders: A Systematic Review and Meta-analysis. Alcohol Alcohol. 2016, 51, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Ying, Y.Y.; Li, S.X.; Wang, S.J.; Gong, Q.H.; Li, H. Association between alcohol consumption and metabolic syndrome among Chinese adults. Public Health Nutr. 2021, 24, 4582–4590. [Google Scholar] [CrossRef]
- Hirakawa, M.; Arase, Y.; Amakawa, K.; Ohmoto-Sekine, Y.; Ishihara, M.; Shiba, M.; Ogawa, K.; Okuda, C.; Jinno, T.; Kato, H.; et al. Relationship between Alcohol Intake and Risk Factors for Metabolic Syndrome in Men. Intern. Med. 2015, 54, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, I. Cross-sectional relationship between alcohol consumption and prevalence of metabolic syndrome in Japanese men and women. J. Atheroscler. Thromb. 2010, 17, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Kim, B.S.; Kang, J.H. Alcohol consumption and incidence of metabolic syndrome in korean men. A 3-year follow-up study. Circ. J. 2012, 76, 2363–2371. [Google Scholar] [CrossRef] [Green Version]
- Bhanushali, C.J.; Kumar, K.; Wutoh, A.K.; Karavatas, S.; Habib, M.J.; Daniel, M.; Lee, E. Association between Lifestyle Factors and Metabolic Syndrome among African Americans in the United States. J. Nutr. Metab. 2013, 2013, 516475. [Google Scholar] [CrossRef]
- Stoutenberg, M.; Lee, D.-C.; Sui, X.; Hooker, S.; Horigian, V.; Perrino, T.; Blair, S. Prospective study of alcohol consumption and the incidence of the metabolic syndrome in US men. Br. J. Nutr. 2013, 110, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Ou, Y.C.; Chuang, H.H.; Li, W.C.; Tzeng, I.S.; Chen, J.Y. Gender difference in the association between lower muscle mass and metabolic syndrome independent of insulin resistance in a middle-aged and elderly Taiwanese population. Arch. Gerontol. Geriatr. 2017, 72, 12–18. [Google Scholar] [CrossRef]
- Cuschieri, S.; Vassallo, J.; Calleja, N.; Pace, N.; Mamo, J. The effect of age, gender, TG/HDL-C ratio and behavioral lifestyles on the metabolic syndrome in the high risk Mediterranean Island population of Malta. Diabetes Metab. Syndr. 2017, 11, S321–S327. [Google Scholar] [CrossRef]
- Harriss, L.R.; English, D.R.; Hopper, J.L.; Powles, J.; Simpson, J.A.; O’Dea, K.; Giles, G.G.; Tonkin, A.M. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction 2007, 102, 1574–1585. [Google Scholar] [CrossRef]
- Agabio, R.; Pisanu, C.; Gessa, G.L.; Franconi, F. Sex Differences in Alcohol Use Disorder. Curr. Med. Chem 2017, 24, 2661–2670. [Google Scholar] [CrossRef]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef]
- Flores-Bonilla, A.; Richardson, H.N. Sex Differences in the Neurobiology of Alcohol Use Disorder. Alcohol Res. 2020, 40, 1–19. [Google Scholar] [CrossRef]
- Polsky, S.; Akturk, H.K. Alcohol Consumption, Diabetes Risk, and Cardiovascular Disease Within Diabetes. Curr. Diab. Rep. 2017, 17, 136. [Google Scholar] [CrossRef]
- Cho, K.I.; Kim, B.H.; Je, H.G.; Jang, J.S.; Park, Y.H. Gender-Specific Associations between Socioeconomic Status and Psychological Factors and Metabolic Syndrome in the Korean Population: Findings from the 2013 Korean National Health and Nutrition Examination Survey. Biomed. Res. Int. 2016, 2016, 3973197. [Google Scholar] [CrossRef]
- Pan, H.C.; Lee, C.C.; Chou, K.M.; Lu, S.C.; Sun, C.Y. Serum levels of uncoupling proteins in patients with differential insulin resistance: A community-based cohort study. Medicine (Baltimore) 2017, 96, e8053. [Google Scholar] [CrossRef]
- Huang, T.S.; Wu, I.W.; Lin, C.L.; Shyu, Y.C.; Chen, Y.C.; Chien, R.N. Prognosis of Chronic Kidney Disease in Patients with Non-Alcoholic Fatty Liver Disease: A Northeastern Taiwan Community Medicine Research Cohort. Biomed. J. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.J.; Babor, T.F.; Kranzler, H.R. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J. Stud. Alcohol 1995, 56, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.H.K.; Chung, R.Y.; Chung, V.C.H.; Kim, J.; Chan, I.W.T.; Wong, M.C.S.; Wong, S.Y.-S.; Griffiths, S.M. Is Alcohol Use Disorder Identification Test (AUDIT) or its shorter versions more useful to identify risky drinkers in a Chinese population? A diagnostic study. PLoS ONE 2015, 10, e0117721. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, Y.; Strauss, S.M.; Yin, L.; Liu, H.; Amico, K.R.; Zhang, C.; Shao, Y.; Qian, H.-Z.; Vermund, S.H. Alcohol misuse, risky sexual behaviors, and HIV or syphilis infections among Chinese men who have sex with men. Drug Alcohol Depend. 2016, 168, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar]
- Pose, E.; Pera, G.; Torán, P.; Gratacós-Ginès, J.; Avitabile, E.; Expósito, C.; Díaz, A.; Graupera, I.; Rubio, A.B.; Ginès, P.; et al. Interaction between metabolic syndrome and alcohol consumption, risk factors of liver fibrosis: A population-based study. Liver Int. 2021, 41, 1556–1564. [Google Scholar] [CrossRef]
- Jeong, J.; Yu, J. Prevalence and Influencing Factors of Metabolic Syndrome Among Persons with Physical Disabilities. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2018, 12, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Kahl, K.G.; Hillemacher, T. The metabolic syndrome in patients with alcohol dependency: Current research and clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 70, 49–56. [Google Scholar] [CrossRef]
- Katsiki, N.; Tziomalos, K.; Mikhailidis, D.P. Alcohol and the cardiovascular system: A double-edged sword. Curr. Pharm. Des. 2014, 20, 6276–6288. [Google Scholar] [CrossRef]
- Blum, K.; Thanos, P.K.; Wang, G.-J.; Febo, M.; Demetrovics, Z.; Modestino, E.; Braverman, E.R.; Baron, D.; Badgaiyan, R.D.; Gold, M.S. The Food and Drug Addiction Epidemic: Targeting Dopamine Homeostasis. Curr. Pharm. Des. 2018, 23, 6050–6061. [Google Scholar] [CrossRef]
- Cullmann, M.; Hilding, A.; Ostenson, C.G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet. Med. 2012, 29, 441–452. [Google Scholar] [CrossRef]
- Schrieks, I.C.; Heil, A.L.; Hendriks, H.F.; Mukamal, K.J.; Beulens, J.W. The effect of alcohol consumption on insulin sensitivity and glycemic status: A systematic review and meta-analysis of intervention studies. Diabetes Care 2015, 38, 723–732. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hung, H.J.; Chung, C.J.; Huang, C.T.; Wu, T.N.; Chen, C.Y. Ethnic disparity in metabolic syndrome and related obesity and health behavior: A community study in Taiwan. Diabetol. Metab. Syndr. 2021, 13, 134. [Google Scholar] [CrossRef]
- Yi, Y.; An, J. Sex Differences in Risk Factors for Metabolic Syndrome in the Korean Population. Int. J. Environ. Res. Public Health 2020, 17, 9513. [Google Scholar] [CrossRef]
- Kim, S.K.; Hong, S.H.; Chung, J.H.; Cho, K.B. Association Between Alcohol Consumption and Metabolic Syndrome in a Community-Based Cohort of Korean Adults. Med. Sci. Monit. 2017, 23, 2104–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Giovannucci, E.L.; Kim, J. The Effect of Smoking and Sex on the Association Between Long-term Alcohol Consumption and Metabolic Syndrome in a Middle-aged and Older Population. J. Epidemiol. 2021, 31, 249–258. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, H.-T.; Tseng, Y.-H.; Chen, H.-S.; Chiang, S.-C.; Chen, T.-J.; Hwang, S.-J. Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Sci. Rep. 2021, 11, 20267. [Google Scholar] [CrossRef]
- Kroll, D.S.; Feldman, D.E.; Biesecker, C.L.; McPherson, K.L.; Manza, P.; Joseph, P.V.; Volkow, N.D.; Wang, G.-J. Neuroimaging of Sex/Gender Differences in Obesity: A Review of Structure, Function, and Neurotransmission. Nutrients 2020, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Holst, C.; Becker, U.; Jorgensen, M.E.; Gronbaek, M.; Tolstrup, J.S. Alcohol drinking patterns and risk of diabetes: A cohort study of 70,551 men and women from the general Danish population. Diabetologia 2017, 60, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women. Arch. Intern. Med. 2010, 170, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef] [Green Version]
| Male (N = 1294) | Female (N = 2093) | X2 | p-Value | |
|---|---|---|---|---|
| Frequency of drinking | ||||
| Once per month or less | 825 (63.8%) | 1868 (89.2%) | 343.00 | <0.001 |
| 2–4 times per month | 166 (12.8%) | 122 (5.8%) | ||
| 2–3 times per week | 141 (10.9%) | 60 (2.9%) | ||
| 4 times per week or more | 162 (12.5%) | 43 (2.1%) | ||
| Types of alcoholic beverages | ||||
| Beer | 167 (12.9%) | 124 (5.9%) | 49.62 | <0.001 |
| Spirits | 186 (14.4%) | 85 (4.1%) | 115.54 | <0.001 |
| Cocktail | 37 (2.9%) | 184 (8.8%) | 46.13 | <0.001 |
| Medicinal liquor | 53 (4.1%) | 71 (3.4%) | 1.12 | 0.289 |
| Others | 221 (17.1%) | 161 (7.7%) | 70.41 | <0.001 |
| Alcohol amount consumed per occasion (standard alcohol units) | 238.67 | <0.001 | ||
| 0–2 | 1100 (75.5%) | 2152 (93.3%) | ||
| 3–4 | 191 (13.1%) | 92 (4%) | ||
| 5–6 | 82 (5.6%) | 29 (1.3%) | ||
| 7–9 | 39 (2.7%) | 14 (0.6%) | ||
| ≥10 | 42 (2.9%) | 20 (0.9%) | ||
| Risks of excessive drinking | ||||
| No drinking | 478 (36.9%) | 1385 (66.2%) | 405.75 | <0.001 |
| Low-risk drinking | 565 (43.7%) | 652 (31.2%) | ||
| Hazardous drinking | 251 (19.4%) | 56 (2.7%) |
| No Drinking N = 1863 (55%) | Low-Risk Drinking N = 1217 (35.9%) | Hazardous Drinking N = 307 (9.1%) | p-Value | |
|---|---|---|---|---|
| Demographics and lifestyle-related habits | ||||
| Male (%) | 478 (25.7%) | 565 (46.4%) | 251 (81.8%) | <0.001 |
| Age, years, mean ± SD | 59.3 ± 13.5 | 54 ± 13.2 | 54.6 ± 11.7 | <0.001 |
| Education levels (%) | <0.001 | |||
| Junior high school or lower | 999 (54.3%) | 466 (38.9%) | 124 (41.1%) | |
| Senior high school | 456 (24.8%) | 350 (29.2%) | 118 (39.1%) | |
| College or above | 386 (21.0%) | 382 (31.9%) | 60 (19.9%) | |
| Marital status (%) | <0.001 | |||
| Never married | 114 (6.3%) | 123 (10.4%) | 18 (6.0%) | |
| Married | 1446(79.5%) | 944 (79.7%) | 255 (84.7%) | |
| Divorced/Separated/Widowed | 260 (14.3%) | 117 (9.9%) | 28 (9.3%) | |
| Smoke (%) | ||||
| Never | 1554 (83.4%) | 799 (65.8%) | 76 (24.8%) | <0.001 |
| Current | 164 (8.8%) | 217 (17.9%) | 150 (48.9%) | |
| Previously | 145 (7.8%) | 199 (16.4%) | 81 (26.4%) | |
| Metabolic profiles (%) | ||||
| Obesity (BMI ≥ 27) | 766 (41.1%) | 553 (45.5%) | 159 (51.8%) | 0.001 |
| Abdominal obesity | 691 (37.2%) | 405 (33.3%) | 117 (38.2%) | 0.062 |
| Hypertension | 944 (50.8%) | 595 (48.9%) | 183 (59.8%) | 0.003 |
| Hyperglycemia | 662 (35.7%) | 439 (36.1%) | 143 (46.7%) | 0.001 |
| Low HDL-C | 452 (24.3%) | 299 (24.6%) | 56 (18.2%) | 0.053 |
| Hypertriglyceridemia | 428 (23%) | 304 (25%) | 120 (39.2%) | <0.001 |
| Metabolic syndrome | 538 (28.9%) | 323 (26.6%) | 110 (36.1%) | 0.004 |
| Dependent Variables | Independent Variables | Female | Male | ||
|---|---|---|---|---|---|
| Risks of Excessive Drinking | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| Obesity | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.25 (1.02–1.54) | 0.032 * | 0.98 (0.76–1.26) | 0.868 | |
| Hazardous drinking | 1.25 (0.70–2.26) | 0.452 | 0.96 (0.69–1.33) | 0.794 | |
| Abdominal obesity | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.19 (0.97–1.47) | 0.100 | 0.88(0.67–1.16) | 0.361 | |
| Hazardous drinking | 1.21 (0.66–2.22) | 0.546 | 1.20 (0.85–1.69) | 0.296 | |
| Hypertension | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.19 (0.96–1.47) | 0.106 | 1.14 (0.88–1.49) | 0.316 | |
| Hazardous drinking | 2.07 (1.11–3.88) | 0.022 | 1.69 (1.20–2.37) | 0.003 | |
| Hyperglycemia | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.26 (1.01–1.57) | 0.042 | 1.36 (1.03–1.78) | 0.028 | |
| Hazardous drinking | 1.66 (0.87–3.15) | 0.119 | 1.92 (1.36–2.69) | <0.000 * | |
| Low HDL-C | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.02 (0.81–1.28) | 0.869 | 0.93 (0.69–1.26) | 0.653 | |
| Hazardous drinking | 0.71 (0.35–1.42) | 0.327 | 0.63 (0.41–0.95) | 0.026 | |
| Hypertriglyceridemia | No drinking | 1 | 1 | ||
| Low-risk drinking | 0.95 (0.74–1.22) | 0.695 | 0.99 (0.75–1.30) | 0.943 | |
| Hazardous drinking | 0.91 (0.43–1.94) | 0.806 | 1.45 (1.03–2.03) | 0.033 | |
| Metabolic syndrome | No drinking | 1 | 1 | ||
| Low-risk drinking | 1.00 (0.79–1.26) | 0.975 | 0.98 (0.74–1.30) | 0.886 | |
| Hazardous drinking | 1.38 (0.71–2.68) | 0.347 | 1.31 (0.93–1.85) | 0.124 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-J.; Lin, C.-L.; Chen, Y.-C.; Lin, C.; Shyu, Y.-C.; Chen, C.-K. Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan. Nutrients 2022, 14, 2957. https://doi.org/10.3390/nu14142957
Wang L-J, Lin C-L, Chen Y-C, Lin C, Shyu Y-C, Chen C-K. Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan. Nutrients. 2022; 14(14):2957. https://doi.org/10.3390/nu14142957
Chicago/Turabian StyleWang, Liang-Jen, Chih-Lang Lin, Yi-Chih Chen, Chemin Lin, Yu-Chiau Shyu, and Chih-Ken Chen. 2022. "Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan" Nutrients 14, no. 14: 2957. https://doi.org/10.3390/nu14142957
APA StyleWang, L.-J., Lin, C.-L., Chen, Y.-C., Lin, C., Shyu, Y.-C., & Chen, C.-K. (2022). Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan. Nutrients, 14(14), 2957. https://doi.org/10.3390/nu14142957







