Association between Dietary Anthocyanidins and Risk of Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
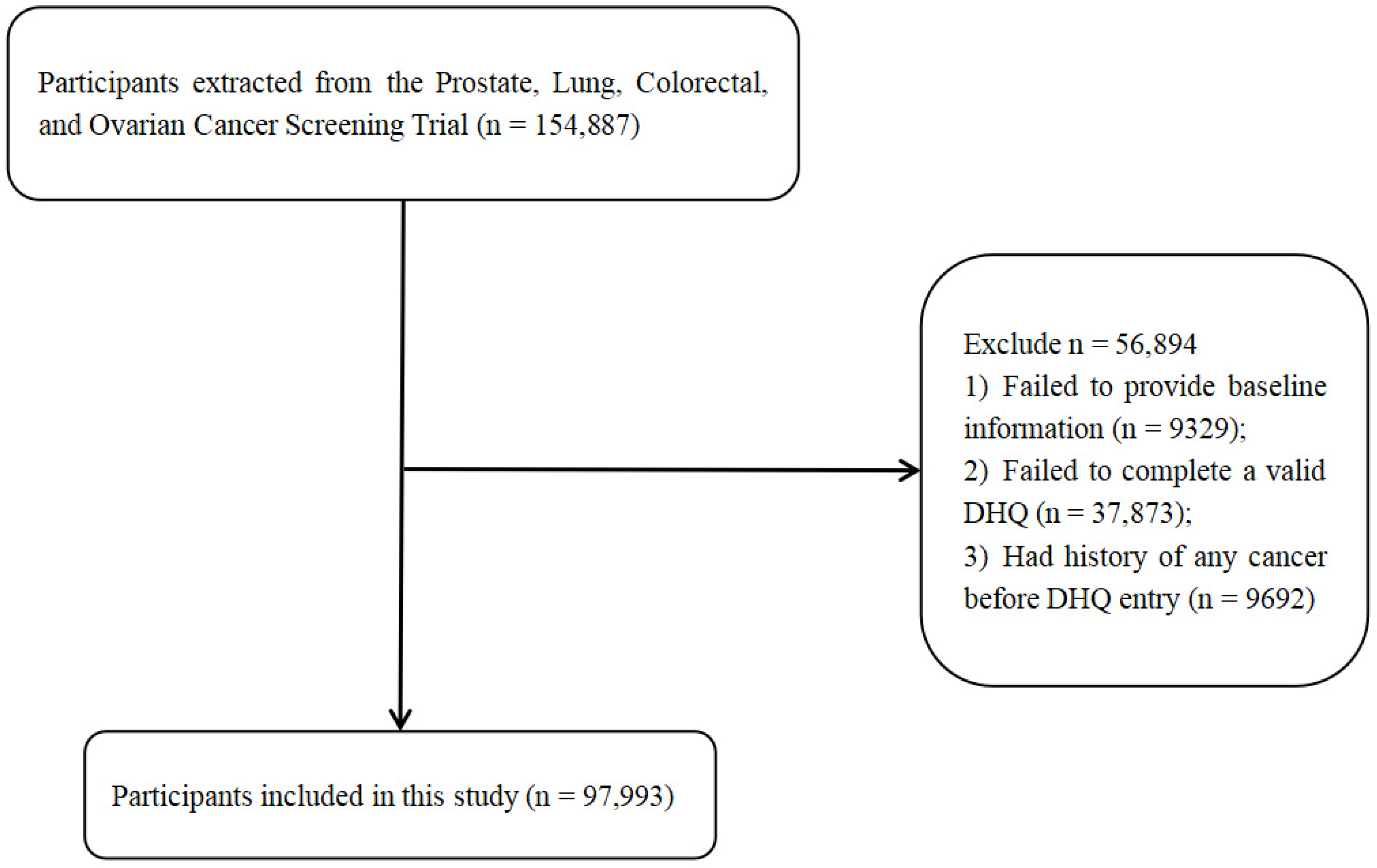
2.1. Study Population
2.2. Data Collection
2.3. Dietary Intake of Anthocyanidins
2.4. Lung Cancer Ascertainment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association between Anthocyanidins and Lung Cancer Risk
3.3. Additional Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhu, C.; Ji, M.; Fan, J.; Xie, J.; Huang, Y.; Jiang, X.; Xu, J.; Yin, R.; Du, L. Diet and Risk of Incident Lung Cancer: A Large Prospective Cohort Study in UK Biobank. Am. J. Clin. Nutr. 2021, 114, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Boushey, C.; Shvetsov, Y.; Wirth, M.; Shivappa, N.; Hébert, J.; Haiman, C.; Wilkens, L.; Le Marchand, L. Diet Quality and Risk of Lung Cancer in the Multiethnic Cohort Study. Nutrients 2021, 13, 1614. [Google Scholar] [CrossRef]
- Myneni, A.A.; Giovino, G.A.; Millen, A.E.; LaMonte, M.J.; Wactawski-Wende, J.; Neuhouser, M.L.; Zhao, J.; Shikany, J.M.; Mu, L. Indices of Diet Quality and Risk of Lung Cancer in the Women’s Health Initiative Observational Study. J. Nutr. 2021, 151, 1618–1627. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Furdak, P.; Sadowska-Bartosz, I. Delphinidin Increases the Sensitivity of Ovarian Cancer Cell Lines to 3-Bromopyruvate. Int. J. Mol. Sci. 2021, 22, 709. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, A.S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Park, M.; Sharma, A.; Lee, H.J. Anti-Adipogenic Effects of Delphinidin-3-O-β-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef] [Green Version]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Choi, H.K.; Kim, Y.K.; Lee, H.J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Bars-Cortina, D.; Sakhawat, A.; Piñol-Felis, C.; Motilva, M.J. Chemopreventive effects of anthocyanins on colorectal and breast cancer: A review. Semin. Cancer Biol. 2022, 81, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef]
- Cutler, G.J.; Nettleton, J.A.; Ross, J.A.; Harnack, L.J.; Jacobs, D.R., Jr.; Scrafford, C.G.; Barraj, L.M.; Mink, P.J.; Robien, K. Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Mursu, J.; Nurmi, T.; Tuomainen, T.P.; Salonen, J.T.; Pukkala, E.; Voutilainen, S. Intake of flavonoids and risk of cancer in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int. J. Cancer 2008, 123, 660–663. [Google Scholar] [CrossRef]
- Prorok, P.C.; Andriole, G.L.; Bresalier, R.; Buys, S.S.; Chia, D.; Crawford, E.D.; Fogel, R.; Gelmann, E.P.; Gilbert, F.; Hasson, M.A.; et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control. Clin. Trials 2000, 21 (Suppl. 1), 273S–309S. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F.; Brown, C.C.; Smith, A.F.; Sharbaugh, C.O.; Jobe, J.B.; Mittl, B.; Gibson, J.T.; Ziegler, R.G. Cognitive research enhances accuracy of food frequency questionnaire reports: Results of an experimental validation study. J. Am. Diet. Assoc. 2002, 102, 212–225. [Google Scholar] [CrossRef]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.G.; Berman, M.; Brasky, T.M.; Freudenheim, J.L.; Mathe, E.; McElroy, J.P.; Song, M.A.; Wewers, M.D. A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1175–1191. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Lee, K.W.; Hur, H.J.; Lee, H.J. Peonidin inhibits phorbol-ester-induced COX-2 expression and transformation in JB6 P+ cells by blocking phosphorylation of ERK-1 and -2. Ann. N. Y. Acad. Sci. 2007, 1095, 513–520. [Google Scholar] [CrossRef]
- Yang, M.; Koo, S.I.; Song, W.O.; Chun, O.K. Food matrix affecting anthocyanin bioavailability: Review. Curr. Med. Chem. 2011, 18, 291–300. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [Green Version]

| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p | |
|---|---|---|---|---|---|---|
| Number of participants | 97,993 | 24,533 | 24,467 | 24,516 | 24,477 | - |
| Number of cases | 1631 | 593 | 379 | 353 | 306 | - |
| Follow-up, years | 8.83 (1.96) | 8.68 (2.03) | 8.84 (1.97) | 8.89 (1.92) | 8.91 (1.90) | - |
| Person-years | 865,382.70 | 213,064.20 | 216,317.80 | 217,948.80 | 218,051.90 | - |
| Total energy intake, kcal/day | 1738.34 (734.92) | 1519.21 (712.76) | 1652.74 (686.51) | 1777.57 (698.68) | 2004.25 (751.94) | <0.001 |
| Total anthocyanidins, mg/d | 15.86 (14.01) | 4.34 (1.63) | 9.48 (1.50) | 15.74 (2.28) | 33.91 (16.61) | <0.001 |
| Cyanidin, mg/d | 3.58 (3.88) | 1.10 (0.68) | 2.13 (1.16) | 3.43 (1.85) | 7.66 (5.49) | <0.001 |
| Delphinidin, mg/d | 4.50 (3.68) | 1.32 (0.99) | 3.44 (1.93) | 5.09 (2.53) | 8.14 (4.27) | <0.001 |
| Malvidin, mg/d | 4.04 (5.93) | 0.86 (0.59) | 1.82 (1.23) | 3.62 (2.43) | 9.85 (9.16) | <0.001 |
| Pelargonidin, mg/d | 2.56 (3.86) | 0.77 (0.64) | 1.49 (1.26) | 2.53 (2.25) | 5.45 (6.30) | <0.001 |
| Peonidin, mg/d | 0.50 (0.65) | 0.11 (0.07) | 0.23 (0.13) | 0.44 (0.26) | 1.20 (0.95) | <0.001 |
| Petunidin, mg/d | 0.69 (0.84) | 0.19 (0.11) | 0.35 (0.19) | 0.63 (0.34) | 1.61 (1.20) | <0.001 |
| Trial arm | 0.379 | |||||
| Intervention | 50,218 (51.25%) | 12,485 (50.89%) | 12,492 (51.06%) | 12,639 (51.55%) | 12,602 (51.49%) | |
| Control | 47,775 (48.75%) | 12,048 (49.11%) | 11,975 (48.94%) | 11,877 (48.45%) | 11,875 (48.51%) | |
| Age, years | 65.50 (5.73) | 64.90 (5.61) | 65.58 (5.75) | 65.76 (5.75) | 65.76 (5.75) | <0.001 |
| Gender | <0.001 | |||||
| Female | 50,484 (51.52%) | 10,559 (43.04%) | 12,032 (49.18%) | 13,160 (53.68%) | 14,733 (60.19%) | |
| Male | 47,509 (48.48%) | 13,974 (56.96%) | 12,435 (50.82%) | 11,356 (46.32%) | 9744 (39.81%) | |
| Baseline body mass index, kg/m2 | 27.22 (4.81) | 27.59 (4.79) | 27.43 (4.80) | 27.06 (4.73) | 26.81 (4.89) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| White | 89,207 (91.03%) | 21,904 (89.28%) | 22,461 (91.80%) | 22,624 (92.28%) | 22,218 (90.77%) | |
| Non-white | 8786 (8.97%) | 2629 (10.72%) | 2006 (8.20%) | 1892 (7.72%) | 2259 (9.23%) | |
| Marital status | <0.001 | |||||
| Married | 76,847 (78.42%) | 18,870 (76.92%) | 19,395 (79.27%) | 19,515 (79.60%) | 19,067 (77.9%) | |
| Not married | 21,146 (21.58%) | 5663 (23.08%) | 5072 (20.73%) | 5001 (20.40%) | 5410 (22.10%) | |
| Cigarette pack-years | 17.85 (26.72) | 24.20 (30.71) | 17.58 (26.00) | 15.31 (24.45) | 14.31 (24.06) | <0.001 |
| Alcohol intake | <0.001 | |||||
| Never | 9910 (10.11%) | 2414 (9.84%) | 2587 (10.57%) | 2440 (9.95%) | 2469 (10.09%) | |
| Former | 14,188 (14.48%) | 4244 (17.30%) | 3492 (14.27%) | 3230 (13.18%) | 3222 (13.16%) | |
| Current | 71,151 (72.61%) | 17,152 (69.91%) | 17,684 (72.28%) | 18,249 (74.44%) | 18,066 (73.81%) | |
| Unknown | 2744 (2.80%) | 723 (2.95%) | 704 (2.88%) | 597 (2.44%) | 720 (2.94%) | |
| Family history of lung cancer | <0.001 | |||||
| Yes | 85,392 (87.14%) | 21,148 (86.2%) | 21,377 (87.37%) | 21,418 (87.36%) | 21,449 (87.63%) | |
| No | 10,252 (10.46%) | 2657 (10.83%) | 2522 (10.31%) | 2542 (10.37%) | 2531 (10.34%) | |
| Possible | 2349 (2.40%) | 728 (2.97%) | 568 (2.32%) | 556 (2.27%) | 497 (2.03%) | |
| Family history of any cancer | 0.002 | |||||
| Yes | 43,256 (44.14%) | 11,031 (44.96%) | 10,881 (44.47%) | 10,679 (43.56%) | 10,665 (43.57%) | |
| No | 54,737 (55.86%) | 13,502 (55.04%) | 13,586 (55.53%) | 13,837 (56.44%) | 13,812 (56.43%) |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Trend | p-Interaction Due to Sex | |
|---|---|---|---|---|---|---|
| Total anthocyanidins | ||||||
| Unadjusted | 1.00 (reference) | 0.63 (0.55,0.71) | 0.58 (0.51,0.66) | 0.50 (0.44,0.58) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.77 (0.67,0.88) | 0.74 (0.65,0.85) | 0.63 (0.55,0.73) | <0.001 | 0.691 |
| Multivariate for female | 1.00 (reference) | 0.74 (0.60,0.93) | 0.73 (0.59,0.91) | 0.62 (0.50,0.77) | <0.001 | |
| Multivariate for male | 1.00 (reference) | 0.79 (0.67,0.93) | 0.75 (0.63,0.89) | 0.63 (0.51,0.76) | <0.001 | |
| Cyanidin | ||||||
| Unadjusted | 1.00 (reference) | 0.67 (0.59,0.76) | 0.47 (0.41,0.55) | 0.55 (0.48,0.63) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.81 (0.71,0.92) | 0.62 (0.54,0.72) | 0.73 (0.63,0.84) | <0.001 | 0.634 |
| Multivariate for female | 1.00 (reference) | 0.76 (0.62,0.93) | 0.61 (0.48,0.76) | 0.70 (0.56,0.87) | <0.001 | |
| Multivariate for male | 1.00 (reference) | 0.83 (0.70,0.97) | 0.64 (0.53,0.77) | 0.74 (0.62,0.90) | <0.001 | |
| Delphinidin | ||||||
| Unadjusted | 1.00 (reference) | 0.69 (0.61,0.79) | 0.65 (0.57,0.75) | 0.60 (0.53,0.69) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.82 (0.72,0.94) | 0.81 (0.70,0.92) | 0.70 (0.60,0.80) | <0.001 | 0.214 |
| Multivariate for female | 1.00 (reference) | 0.96 (0.78,1.19) | 0.81 (0.65,1.01) | 0.77 (0.61,0.96) | 0.061 | |
| Multivariate for male | 1.00 (reference) | 0.74 (0.62,0.88) | 0.82 (0.69,0.97) | 0.65 (0.54,0.78) | <0.001 | |
| Malvidin | ||||||
| Unadjusted | 1.00 (reference) | 0.66 (0.58,0.75) | 0.56 (0.49,0.64) | 0.54 (0.47,0.62) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.78 (0.68,0.88) | 0.70 (0.61,0.81) | 0.65 (0.56,0.75) | <0.001 | 0.887 |
| Multivariate for female | 1.00 (reference) | 0.81 (0.65,1.01) | 0.79 (0.63,0.98) | 0.71 (0.57,0.89) | 0.02 | |
| Multivariate for male | 1.00 (reference) | 0.76 (0.65,0.90) | 0.66 (0.55,0.79) | 0.61 (0.50,0.73) | <0.001 | |
| Pelargonidin | ||||||
| Unadjusted | 1.00 (reference) | 0.67 (0.59,0.77) | 0.64 (0.56,0.72) | 0.52 (0.46,0.60) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.83 (0.73,0.95) | 0.86 (0.75,0.99) | 0.75 (0.65,0.87) | 0.001 | 0.935 |
| Multivariate for female | 1.00 (reference) | 0.91 (0.73,1.13) | 0.92 (0.74,1.14) | 0.80 (0.64,1.00) | 0.256 | |
| Multivariate for male | 1.00 (reference) | 0.79 (0.67,0.93) | 0.83 (0.70,0.99) | 0.73 (0.60,0.89) | 0.003 | |
| Peonidin | ||||||
| Unadjusted | 1.00 (reference) | 0.65 (0.57,0.73) | 0.57 (0.50,0.65) | 0.53 (0.47,0.61) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.76 (0.67,0.87) | 0.70 (0.61,0.80) | 0.63 (0.55,0.73) | <0.001 | 0.758 |
| Multivariate for female | 1.00 (reference) | 0.76 (0.61,0.95) | 0.77 (0.62,0.96) | 0.66 (0.53,0.82) | 0.002 | |
| Multivariate for male | 1.00 (reference) | 0.77 (0.65,0.90) | 0.66 (0.55,0.79) | 0.61 (0.50,0.74) | <0.001 | |
| Petunidin | ||||||
| Unadjusted | 1.00 (reference) | 0.77 (0.68,0.88) | 0.66 (0.58,0.76) | 0.60 (0.52,0.69) | <0.001 | |
| Multivariate * | 1.00 (reference) | 0.89 (0.78,1.01) | 0.78 (0.68,0.89) | 0.70 (0.60,0.81) | 0.001 | 0.491 |
| Multivariate for female | 1.00 (reference) | 0.89 (0.71,1.10) | 0.73 (0.58,0.92) | 0.73 (0.59,0.90) | 0.124 | |
| Multivariate for male | 1.00 (reference) | 0.89 (0.76,1.05) | 0.81 (0.68,0.96) | 0.66 (0.54,0.81) | 0.001 |
| Adenocarcinoma | Squamous Cell Carcinoma | Large Cell Carcinoma | Small Cell Carcinoma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Incidence Rate/10,000 Person-Years | Crude HR (95% CI) | Adjusted HR (95% CI) * | No. of Cases | Incidence Rate/10,000 Person-Years | Crude HR (95% CI) | Adjusted HR (95% CI) * | No. of Cases | Incidence Rate/10,000 Person-Years | Crude HR (95% CI) | Adjusted HR (95% CI) * | No. of Cases | Incidence Rate/10,000 Person-Years | Crude HR (95% CI) | Adjusted HR (95% CI) * | |
| Total anthocyanidins | ||||||||||||||||
| 179 | 8.40 | 1.00 (reference) | 1.00 (reference) | 130 | 6.10 | 1.00 (reference) | 1.00 (reference) | 22 | 1.03 | 1.00 (reference) | 1.00(reference) | 85 | 3.99 | 1.00 (reference) | 1.00 (reference) | |
| 154 | 7.12 | 0.85 (0.68,1.05) | 1.02 (0.82,1.26) | 65 | 3.00 | 0.49 (0.37,0.66) | 0.63 (0.47,0.85) | 11 | 0.51 | 0.50 (0.24,1.02) | 0.67 (0.32,1.39) | 56 | 2.59 | 0.65 (0.46,0.91) | 0.78 (0.55,1.10) | |
| 148 | 6.79 | 0.81 (0.65,1.00) | 1.01 (0.81,1.27) | 66 | 3.03 | 0.50 (0.37,0.67) | 0.69 (0.51,0.94) | 9 | 0.41 | 0.40 (0.19,0.88) | 0.58 (0.26,1.29) | 47 | 2.16 | 0.54 (0.38,0.77) | 0.67 (0.47,0.97) | |
| 111 | 5.09 | 0.60 (0.48,0.77) | 0.76 (0.59,0.97) | 58 | 2.66 | 0.44 (0.32,0.59) | 0.61 (0.44,0.84) | 7 | 0.32 | 0.31 (0.13,0.74) | 0.43 (0.18,1.05) | 36 | 1.65 | 0.41 (0.28,0.61) | 0.49 (0.32,0.73) | |
| p-trend | 0.001 | 0.072 | <0.001 | 0.003 | 0.015 | 0.246 | <0.001 | 0.005 | ||||||||
| Cyanidin | ||||||||||||||||
| 196 | 9.15 | 1.00 (reference) | 1.00 (reference) | 127 | 5.93 | 1.00 (reference) | 1.00 (reference) | 20 | 0.93 | 1.00 (reference) | 1.00 (reference) | 88 | 4.11 | 1.00 (reference) | 1.00 (reference) | |
| 151 | 6.96 | 0.76 (0.62,0.94) | 0.90 (0.72,1.11) | 69 | 3.18 | 0.54 (0.40,0.72) | 0.68 (0.50,0.91) | 12 | 0.55 | 0.60 (0.29,1.22) | 0.76 (0.37,1.56) | 57 | 2.63 | 0.64 (0.46,0.89) | 0.76 (0.54,1.07) | |
| 105 | 4.83 | 0.53 (0.42,0.67) | 0.68 (0.53,0.87) | 63 | 2.90 | 0.49 (0.36,0.66) | 0.69 (0.51,0.94) | 8 | 0.37 | 0.40 (0.18,0.90) | 0.58 (0.25,1.35) | 38 | 1.75 | 0.42 (0.29,0.62) | 0.54 (0.36,0.80) | |
| 140 | 6.46 | 0.71 (0.57,0.88) | 0.93 (0.74,1.17) | 60 | 2.77 | 0.47 (0.34,0.63) | 0.67 (0.49,0.93) | 9 | 0.42 | 0.45 (0.20,0.99) | 0.61 (0.27,1.39) | 41 | 1.89 | 0.46 (0.32,0.67) | 0.57 (0.39,0.84) | |
| p-trend | <0.001 | 0.016 | <0.001 | 0.016 | 0.072 | 0.519 | <0.001 | 0.004 | ||||||||
| Delphinidin | ||||||||||||||||
| 169 | 7.91 | 1.00 (reference) | 1.00 (reference) | 123 | 5.76 | 1.00 (reference) | 1.00 (reference) | 23 | 1.08 | 1.00 (reference) | 1.00 (reference) | 74 | 3.46 | 1.00 (reference) | 1.00 (reference) | |
| 149 | 6.90 | 0.87 (0.70,1.08) | 1.02 (0.82,1.28) | 66 | 3.06 | 0.53 (0.39,0.71) | 0.65 (0.48,0.89) | 10 | 0.46 | 0.43 (0.21,0.91) | 0.56 (0.26,1.18) | 48 | 2.22 | 0.64 (0.45,0.92) | 0.74 (0.51,1.08) | |
| 149 | 6.84 | 0.86 (0.69,1.07) | 1.05 (0.84,1.32) | 70 | 3.21 | 0.56 (0.42,0.75) | 0.73 (0.54,0.98) | 9 | 0.41 | 0.39 (0.18,0.84) | 0.51 (0.23,1.13) | 60 | 2.75 | 0.79 (0.56,1.11) | 0.97 (0.68,1.38) | |
| 125 | 5.74 | 0.72 (0.57,0.91) | 0.85 (0.67,1.08) | 60 | 2.75 | 0.48 (0.35,0.65) | 0.58 (0.42,0.79) | 7 | 0.32 | 0.30 (0.13,0.70) | 0.34 (0.14,0.82) | 42 | 1.93 | 0.55 (0.38,0.81) | 0.63 (0.43,0.93) | |
| p-trend | 0.054 | 0.316 | <0.001 | 0.003 | 0.007 | 0.066 | 0.01 | 0.061 | ||||||||
| Malvidin | ||||||||||||||||
| 191 | 8.81 | 1.00 (reference) | 1.00 (reference) | 122 | 5.63 | 1.00 (reference) | 1.00 (reference) | 25 | 1.15 | 1.00 (reference) | 1.00 (reference) | 84 | 3.88 | 1.00 (reference) | 1.00 (reference) | |
| 141 | 6.55 | 0.74 (0.60,0.92) | 0.86 (0.69,1.08) | 79 | 3.67 | 0.65 (0.49,0.86) | 0.79 (0.60,1.06) | 10 | 0.46 | 0.40 (0.19,0.84) | 0.50 (0.24,1.05) | 58 | 2.69 | 0.69 (0.50,0.97) | 0.77 (0.55,1.08) | |
| 145 | 6.71 | 0.76 (0.61,0.94) | 0.92 (0.74,1.15) | 54 | 2.50 | 0.44 (0.32,0.61) | 0.59 (0.42,0.82) | 10 | 0.46 | 0.40 (0.19,0.84) | 0.54 (0.26,1.15) | 42 | 1.94 | 0.50 (0.35,0.73) | 0.59 (0.40,0.86) | |
| 115 | 5.29 | 0.60 (0.48,0.76) | 0.70 (0.55,0.90) | 64 | 2.94 | 0.52 (0.39,0.71) | 0.69 (0.51,0.95) | 4 | 0.18 | 0.16 (0.06,0.46) | 0.21 (0.07,0.62) | 40 | 1.84 | 0.47 (0.33,0.69) | 0.53 (0.36,0.79) | |
| p-trend | <0.001 | 0.035 | <0.001 | 0.01 | 0.001 | 0.019 | <0.001 | 0.005 | ||||||||
| Pelargonidin | ||||||||||||||||
| 208 | 9.64 | 1.00 (reference) | 1.00 (reference) | 118 | 5.47 | 1.00 (reference) | 1.00 (reference) | 21 | 0.97 | 1.00 (reference) | 1.00 (reference) | 88 | 4.08 | 1.00 (reference) | 1.00 (reference) | |
| 140 | 6.49 | 0.67 (0.54,0.83) | 0.81 (0.65,1.00) | 62 | 2.87 | 0.52 (0.39,0.71) | 0.69 (0.51,0.94) | 11 | 0.51 | 0.53 (0.25,1.09) | 0.75 (0.36,1.59) | 47 | 2.18 | 0.53 (0.38,0.76) | 0.64 (0.44,0.91) | |
| 126 | 5.81 | 0.60 (0.48,0.75) | 0.78 (0.62,0.98) | 83 | 3.83 | 0.70 (0.53,0.93) | 1.06 (0.79,1.42) | 11 | 0.51 | 0.52 (0.25,1.08) | 0.87 (0.41,1.87) | 54 | 2.49 | 0.61 (0.44,0.86) | 0.79 (0.56,1.12) | |
| 118 | 5.43 | 0.56 (0.45,0.71) | 0.77 (0.60,0.97) | 56 | 2.58 | 0.47 (0.34,0.65) | 0.78 (0.56,1.09) | 6 | 0.28 | 0.29 (0.12,0.71) | 0.54 (0.21,1.38) | 35 | 1.61 | 0.40 (0.27,0.58) | 0.52 (0.34,0.78) | |
| p-trend | <0.001 | 0.064 | <0.001 | 0.03 | 0.029 | 0.607 | <0.001 | 0.006 | ||||||||
| Peonidin | ||||||||||||||||
| 203 | 9.12 | 1.00 (reference) | 1.00 (reference) | 126 | 5.66 | 1.00 (reference) | 1.00 (reference) | 23 | 1.03 | 1.00 (reference) | 1.00 (reference) | 88 | 3.95 | 1.00 (reference) | 1.00 (reference) | |
| 130 | 6.04 | 0.66 (0.53,0.83) | 0.77 (0.62,0.96) | 79 | 3.67 | 0.65 (0.49,0.86) | 0.79 (0.59,1.05) | 10 | 0.46 | 0.45 (0.22,0.95) | 0.56 (0.26,1.18) | 58 | 2.70 | 0.68 (0.49,0.95) | 0.77 (0.55,1.07) | |
| 142 | 6.75 | 0.74 (0.60,0.92) | 0.89 (0.71,1.11) | 50 | 2.38 | 0.42 (0.30,0.58) | 0.54 (0.39,0.76) | 10 | 0.48 | 0.46 (0.22,0.97) | 0.63 (0.29,1.35) | 41 | 1.95 | 0.49 (0.34,0.71) | 0.55 (0.38,0.81) | |
| 117 | 5.38 | 0.59 (0.47,0.74) | 0.69 (0.54,0.87) | 64 | 2.95 | 0.52 (0.38,0.70) | 0.68 (0.50,0.93) | 6 | 0.28 | 0.27 (0.11,0.66) | 0.35 (0.14,0.87) | 37 | 1.70 | 0.43 (0.29,0.63) | 0.47 (0.32,0.71) | |
| p-trend | <0.001 | 0.01 | <0.001 | 0.002 | 0.01 | 0.109 | <0.001 | 0.001 | ||||||||
| Petunidin | ||||||||||||||||
| 181 | 8.10 | 1.00 (reference) | 1.00 (reference) | 114 | 5.10 | 1.00 (reference) | 1.00 (reference) | 21 | 0.94 | 1.00 (reference) | 1.00 (reference) | 85 | 3.80 | 1.00 (reference) | 1.00 (reference) | |
| 128 | 6.11 | 0.75 (0.60,0.94) | 0.86 (0.68,1.08) | 89 | 4.25 | 0.83 (0.63,1.10) | 0.97 (0.73,1.28) | 12 | 0.57 | 0.61 (0.30,1.24) | 0.74 (0.36,1.52) | 57 | 2.72 | 0.71 (0.51,1.00) | 0.77 (0.55,1.08) | |
| 153 | 7.09 | 0.87 (0.70,1.08) | 1.03 (0.83,1.29) | 53 | 2.45 | 0.48 (0.35,0.67) | 0.58 (0.41,0.81) | 9 | 0.42 | 0.45 (0.20,0.97) | 0.57 (0.25,1.26) | 44 | 2.04 | 0.54 (0.37,0.77) | 0.56 (0.38,0.81) | |
| 130 | 6.00 | 0.74 (0.59,0.93) | 0.87 (0.68,1.10) | 63 | 2.91 | 0.57 (0.42,0.78) | 0.71 (0.51,0.98) | 7 | 0.32 | 0.35 (0.15,0.81) | 0.42 (0.17,1.02) | 38 | 1.76 | 0.46 (0.31,0.68) | 0.48 (0.32,0.72) | |
| p-trend | 0.026 | 0.283 | <0.001 | 0.004 | 0.046 | 0.225 | <0.001 | 0.001 | ||||||||
| Subgroup | Cases | Pearson-Years | HR (95% CI) * | p-Interaction |
|---|---|---|---|---|
| Quartile 4 vs. Quartile 1 | ||||
| Age, years | 0.035 | |||
| >65 | 1033 | 411,157 | 0.72 (0.60,0.87) | |
| ≤65 | 598 | 454,225.7 | 0.58 (0.46,0.74) | |
| BMI, kg/m2 | 0.895 | |||
| >25 | 995 | 564,941.1 | 0.61 (0.50,0.74) | |
| ≤25 | 636 | 300,441.6 | 0.66 (0.53,0.83) | |
| Race | 0.287 | |||
| White | 1510 | 788,776.1 | 0.62 (0.53,0.72) | |
| Non-White | 121 | 76,606.64 | 0.81 (0.48,1.37) | |
| Family history of lung cancer | 0.223 | |||
| Yes | 1285 | 755,559.2 | 0.51 (0.35,0.73) | |
| No/Possible | 346 | 109,823.5 | 0.66 (0.56,0.77) | |
| Smoking status | 0.004 | |||
| Never | 138 | 424,755.3 | 2.18 (1.25,3.78) | |
| 0–20 pack-years | 129 | 167,552.4 | 0.97 (0.58,1.61) | |
| ≥20 pack-years | 1364 | 273,075.1 | 0.55 (0.47,0.65) |
| Adjusted HR (95% CI) * (Q4 vs. Q1) | |||||||
|---|---|---|---|---|---|---|---|
| Total Anthocyanidins | Cyanidin | Delphinidin | Malvidin | Pelargonidin | Peonidin | Petunidin | |
| Primary analysis | 0.63 (0.55, 0.73) | 0.73 (0.63, 0.84) | 0.70 (0.60, 0.80) | 0.65 (0.56, 0.75) | 0.75 (0.65, 0.87) | 0.63 (0.55, 0.73) | 0.70 (0.60, 0.81) |
| Excluding participants with extreme energy intake | 0.66 (0.57, 0.76) | 0.75 (0.65, 0.87) | 0.72 (0.62, 0.83) | 0.67 (0.58, 0.77) | 0.78 (0.67, 0.90) | 0.66 (0.57, 0.76) | 0.72 (0.62, 0.84) |
| Excluding participants with the highest 1% intake of anthocyanidins | 0.64 (0.55, 0.74) | 0.74 (0.64, 0.85) | 0.71 (0.61, 0.81) | 0.66 (0.57, 0.76) | 0.76 (0.65, 0.88) | 0.64 (0.56, 0.74) | 0.71 (0.61, 0.82) |
| Excluding participants with a follow-up less than 2 years | 0.59 (0.50, 0.69) | 0.68 (0.58, 0.80) | 0.67 (0.58, 0.79) | 0.62 (0.53, 0.72) | 0.72 (0.62, 0.85) | 0.61 (0.52, 0.71) | 0.66 (0.57, 0.78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, M.; Wan, H.; Chen, L.; Luo, F. Association between Dietary Anthocyanidins and Risk of Lung Cancer. Nutrients 2022, 14, 2643. https://doi.org/10.3390/nu14132643
Zhang Y, Zhu M, Wan H, Chen L, Luo F. Association between Dietary Anthocyanidins and Risk of Lung Cancer. Nutrients. 2022; 14(13):2643. https://doi.org/10.3390/nu14132643
Chicago/Turabian StyleZhang, Yin, Min Zhu, Huajing Wan, Ling Chen, and Fengming Luo. 2022. "Association between Dietary Anthocyanidins and Risk of Lung Cancer" Nutrients 14, no. 13: 2643. https://doi.org/10.3390/nu14132643
APA StyleZhang, Y., Zhu, M., Wan, H., Chen, L., & Luo, F. (2022). Association between Dietary Anthocyanidins and Risk of Lung Cancer. Nutrients, 14(13), 2643. https://doi.org/10.3390/nu14132643






