Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
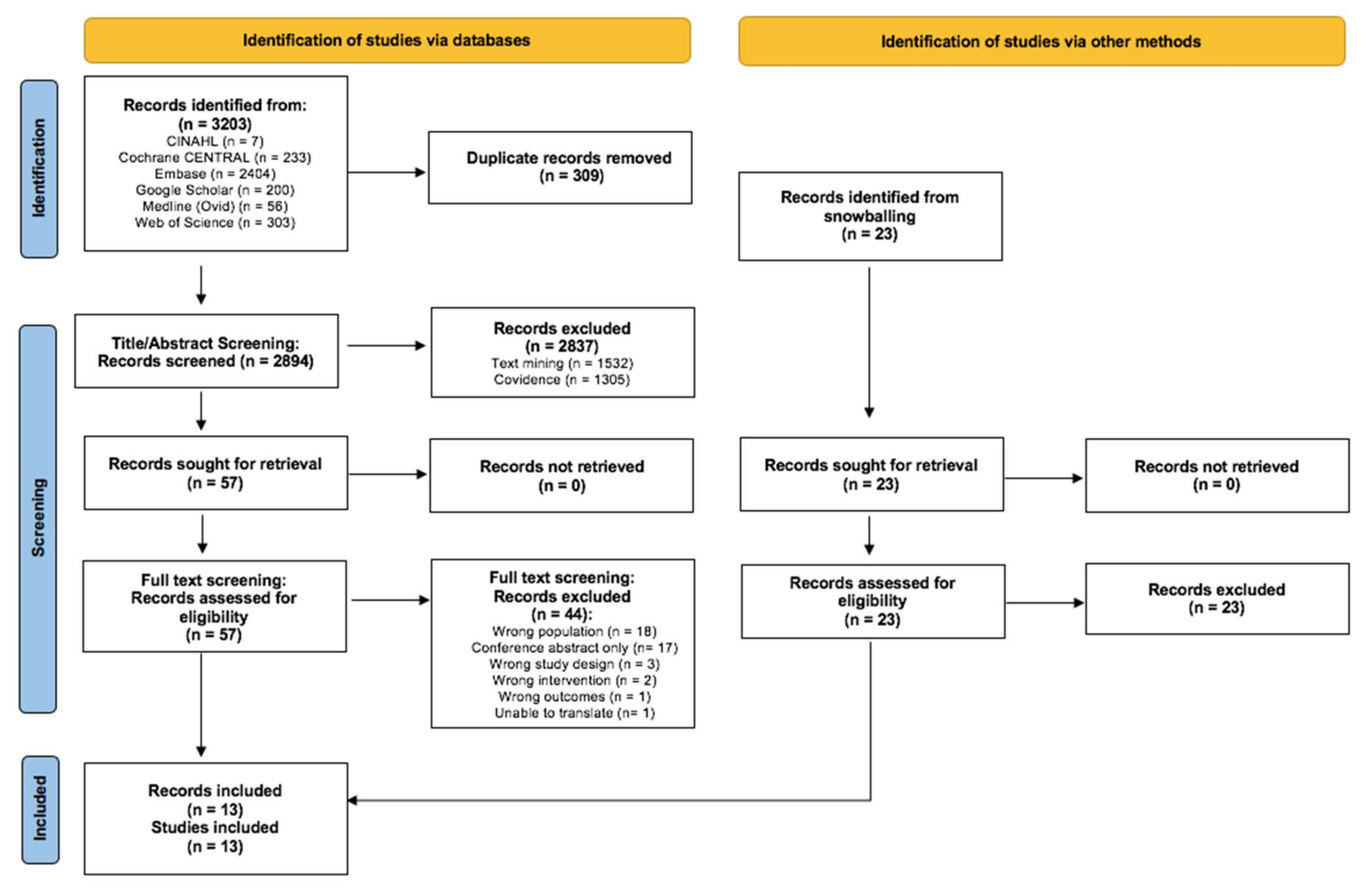
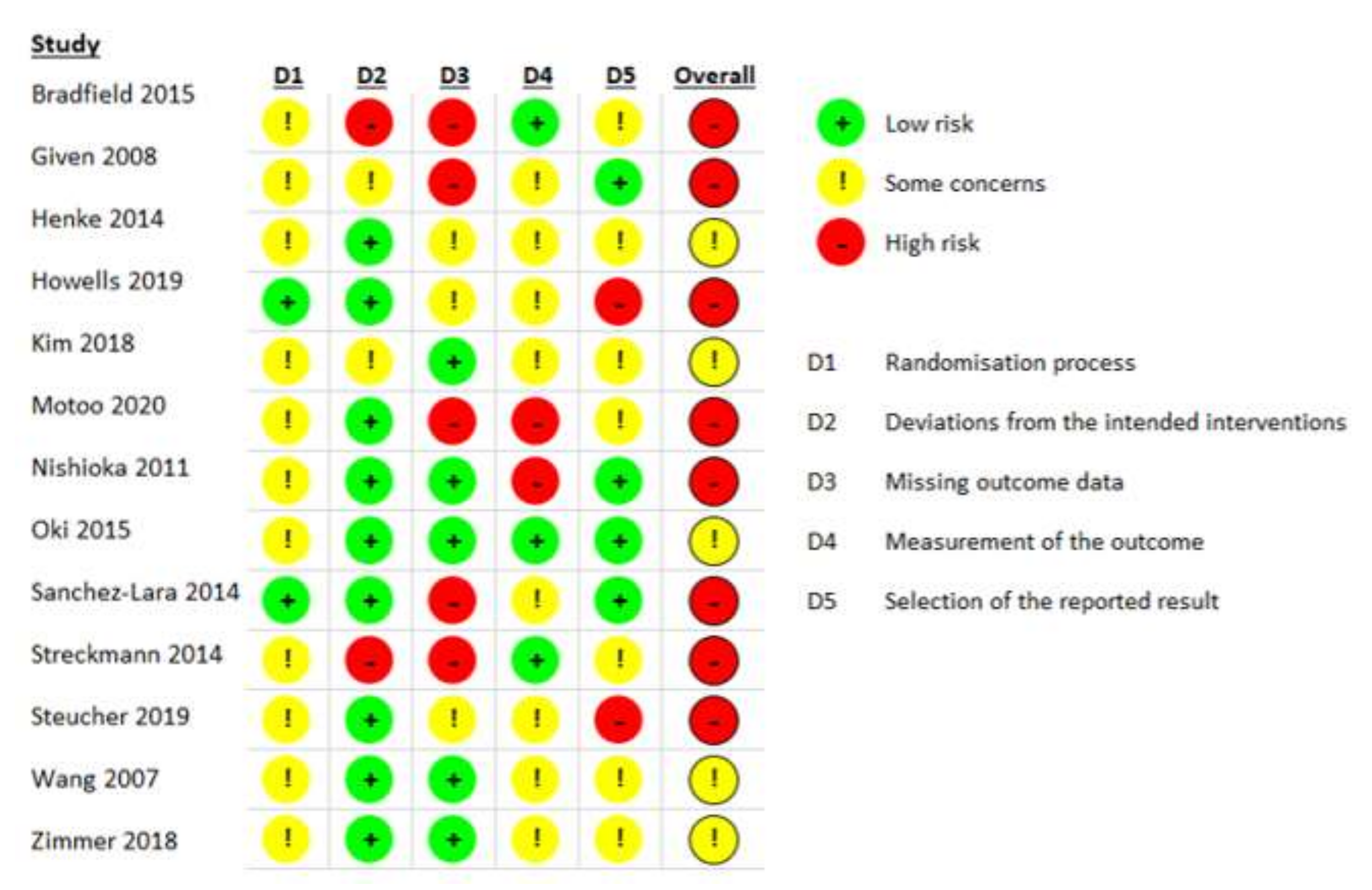
3.1. Search Results and Study Quality
3.2. Study Samples
3.3. Intervention Characteristics
3.4. Effect on CIPN Symptoms and Related Outcomes
3.4.1. Physical Exercise
3.4.2. Nutrition Supplements
3.4.3. Japanese Herbal Medicine
3.4.4. Technology-Facilitated Education for Symptom Self-Management
4. Discussion
Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rand, K.L.; Banno, D.A.; Shea, A.M.; Cripe, L.D. Life and treatment goals of patients with advanced, incurable cancer. Supportive Care Cancer 2016, 24, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- DeMartini, J.; Fenton, J.J.; Epstein, R.; Duberstein, P.; Cipri, C.; Tancredi, D.; Xing, G.; Kaesberg, P.; Kravitz, R.L. Patients’ Hopes for Advanced Cancer Treatment. J. Pain Symptom Manag. 2018, 57, 57–63.e2. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, H.L.; Lopez, V.; Sundar, R.; Yorke, J.; Molassiotis, A. Redefining chemotherapy-induced peripheral neuropathy through symptom cluster analysis and patient-reported outcome data over time. BMC Cancer 2019, 19, 1151. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Cohen, J.; Egger, S.; Blinman, P.L.; Vardy, J.L. Systematic review of long-term chemotherapy-induced peripheral neuropathy (CIPN) following adjuvant oxaliplatin for colorectal cancer. Supportive Care Cancer 2022, 30, 33–47. [Google Scholar] [CrossRef]
- Selvy, M.; Kerckhove, N.; Pereira, B.; Barreau, F.; Nguyen, D.; Busserolles, J.; Giraudet, F.; Cabrespine, A.; Chaleteix, C.; Soubrier, M.; et al. Prevalence of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma Patients and its Impact on Quality of Life: A Single Center Cross-Sectional Study. Front. Pharmacol. 2021, 12, 637593. [Google Scholar] [CrossRef]
- Hung, H.-W.; Liu, C.-Y.; Chen, H.-F.; Chang, C.-C.; Chen, S.-C. Impact of Chemotherapy-Induced Peripheral Neuropathy on Quality of Life in Patients with Advanced Lung Cancer Receiving Platinum-Based Chemotherapy. Int. J. Environ. Res. Public Health 2021, 18, 5677. [Google Scholar] [CrossRef]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef]
- Song, X.; Wilson, K.L.; Kagan, J.; Panjabi, S. Cost of peripheral neuropathy in patients receiving treatment for multiple myeloma: A US administrative claims analysis. Ther. Adv. Hematol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.J.; Yap, D.W.; Teo, C.B.; Tan, B.K.; Molassiotis, A.; Ishiguro, H.; Fan, S.W.; Sundar, R.; Soon, Y.Y.; Bandla, A. A Systematic Review and Meta-Analysis of the Effectiveness of Neuroprotectants for Paclitaxel-Induced Peripheral Neuropathy. Front. Oncol. 2022, 11, 763229. [Google Scholar] [CrossRef]
- Maihöfner, C.; Diel, I.; Tesch, H.; Quandel, T.; Baron, R. Chemotherapy-induced peripheral neuropathy (CIPN): Current therapies and topical treatment option with high-concentration capsaicin. Supportive Care Cancer 2021, 29, 4223–4238. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, X.; Bensoussan, A. Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses. Integr. Cancer Ther. 2020, 19. [Google Scholar] [CrossRef]
- Van Dongen, S.I.; De Nooijer, K.; Cramm, J.M.; Francke, A.L.; Oldenmenger, W.H.; Korfage, I.J.; Witkamp, F.E.; Stoevelaar, R.; Van Der Heide, A.; Rietjens, J.A. Self-management of patients with advanced cancer: A systematic review of experiences and attitudes. Palliat. Med. 2020, 34, 160–178. [Google Scholar] [CrossRef]
- Agbejule, O.A.; Hart, N.H.; Ekberg, S.; Crichton, M.; Chan, R.J. Self-management support for cancer-related fatigue: A systematic review. Int. J. Nurs. Stud. 2022, 129, 104206. [Google Scholar] [CrossRef]
- Boland, L.; Bennett, K.; Connolly, D. Self-management interventions for cancer survivors: A systematic review. Supportive Care Cancer 2018, 26, 1585–1595. [Google Scholar] [CrossRef]
- Ogle, T.; Alexander, K.; Miaskowski, C.; Yates, P. Systematic review of the effectiveness of self-initiated interventions to decrease pain and sensory disturbances associated with peripheral neuropathy. J. Cancer Surviv. 2020, 14, 444–463. [Google Scholar] [CrossRef]
- Khalighinejad, N.; Schurger, A.; Desantis, A.; Zmigrod, L.; Haggard, P. Precursor processes of human self-initiated action. NeuroImage 2017, 165, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- O’Mara-Eves, A.; Thomas, J.; McNaught, J.; Miwa, M.; Ananiadou, S. Using text mining for study identification in systematic reviews: A systematic review of current approaches. Syst. Rev. 2015, 4, 5. [Google Scholar] [CrossRef]
- Bertero, L.; Massa, F.; Metovic, J.; Zanetti, R.; Castellano, I.; Ricardi, U.; Papotti, M.; Cassoni, P. Eighth Edition of the UICC Classification of Malignant Tumours: An overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch. 2018, 472, 519–531. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schuenemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Higgins, J.P.T.T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021): Cochrane; John Wiley & Sons: Hoboken, NJ, USA, 2021; Available online: https://www.training.cochrane.org/handbook (accessed on 20 March 2022).
- Ryan, R. Heterogeneity and Subgroup Analyses in Cochrane Consumers and Communication Group Reviews: Planning the Analysis at Protocol Stage; Cochrane Consumers and Communication Review Group: Melbourne, Australia, 2016. [Google Scholar]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; De Wit, M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support. Care Cancer 2013, 22, 95–101. [Google Scholar] [CrossRef]
- Stuecher, K.; Bolling, C.; Vogt, L.; Niederer, D.; Schmidt, K.; Dignaß, A.; Banzer, W. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: a single-blind RCT. Support. Care Cancer 2018, 27, 2159–2169. [Google Scholar] [CrossRef]
- Streckmann, F.; Kneis, S.; Leifert, J.A.; Baumann, F.T.; Kleber, M.; Ihorst, G.; Herich, L.; Grüssinger, V.; Gollhofer, A.; Bertz, H. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann. Oncol. 2014, 25, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support. Care Cancer 2017, 26, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, S.M.; Sandler, E.; Geller, T.; Tamura, R.N.; Krischer, J.P. Glutamic acid not beneficial for the prevention of vincristine neurotoxicity in children with cancer. Pediatr. Blood Cancer 2014, 62, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Iwuji, C.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: RANDOMISED trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Wang, W.S.; Lin, J.K.; Lin, T.C.; Chen, W.S.; Jiang, J.K.; Wang, H.S.; Chiou, T.J.; Liu, J.H.; Yen, C.C.; Chen, P.M. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 2007, 12, 312–319. [Google Scholar] [CrossRef]
- Motoo, Y.; Tomita, Y.; Fujita, H. Prophylactic efficacy of ninjin’yoeito for oxaliplatin-induced cumulative peripheral neuropathy in patients with colorectal cancer receiving postoperative adjuvant chemotherapy: A randomized, open-label, phase 2 trial (HOPE-2). Int. J. Clin. Oncol. 2020, 25, 1123–1129. [Google Scholar] [CrossRef]
- Nishioka, M.; Shimada, M.; Kurita, N.; Iwata, T.; Morimoto, S.; Yoshikawa, K.; Higashijima, J.; Miyatani, T.; Kono, T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int. J. Clin. Oncol. 2011, 16, 322–327. [Google Scholar] [CrossRef]
- Oki, E.; Emi, Y.; Kojima, H.; Higashijima, J.; Kato, T.; Miyake, Y.; Kon, M.; Ogata, Y.; Takahashi, K.; Ishida, H.; et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): A placebo-controlled, double-blind, randomized phase III study. Int. J. Clin. Oncol. 2015, 20, 767–775. [Google Scholar] [CrossRef]
- Given, C.W.; Sikorskii, A.; Tamkus, D.; Given, B.; You, M.; McCorkle, R.; Champion, V.; Decker, D. Managing Symptoms Among Patients with Breast Cancer During Chemotherapy: Results of a Two-Arm Behavioral Trial. J. Clin. Oncol. 2008, 26, 5855–5862. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Kim, S.M.; Shin, H.; Jang, J.-S.; Kim, Y.I.; Han, D.H. A Mobile Game for Patients with Breast Cancer for Chemotherapy Self-Management and Quality-of-Life Improvement: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e273. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z. Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. Int. J. Endocrinol. Metab. 2021, 19, e108170. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Han, W.; Wang, P.; Wang, X.; Fang, X. Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review and meta-analysis. J. Cancer Surviv. 2022, 11. E-pub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Park, S.B.; Streckmann, F.; Wiskemann, J.; Mohile, N.; Kleckner, A.S.; Colloca, L.; Dorsey, S.G.; Kleckner, I.R. Mechanisms, Mediators, and Moderators of the Effects of Exercise on Chemotherapy-Induced Peripheral Neuropathy. Cancers 2022, 14, 1224. [Google Scholar] [CrossRef] [PubMed]
- Brett Whalen, L.; Zachary Wright, W.; Kundur, P.; Angadi, S.; Modesitt, S.C. Beneficial effects of exercise on chemotherapy-induced peripheral neuropathy and sleep disturbance: A review of literature and proposed mechanisms. Gynecol. Oncol. Rep. 2022, 39, 100927. [Google Scholar] [CrossRef] [PubMed]
- Heywood, R.; McCarthy, A.L.; Skinner, T. Efficacy of Exercise Interventions in Patients with Advanced Cancer: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.N.; Watt, A.E.; Isenring, E.A.; De Van Der Schueren, M.A.E.; Van Der Meij, B.S. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3815–3826. [Google Scholar] [CrossRef]
- Chen, J.; Shan, H.; Yang, W.; Zhang, J.; Dai, H.; Ye, Z. Vitamin E for the Prevention of Chemotherapy-Induced Peripheral Neuropathy: A meta-Analysis. Front. Pharmacol. 2021, 12, 684550. [Google Scholar] [CrossRef]
- Schloss, J.M.; Colosimo, M.; Airey, C.; Masci, P.; Linnane, A.W.; Vitetta, L. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support. Care Cancer 2016, 25, 195–204. [Google Scholar] [CrossRef]
- Wesselink, E.; Winkels, R.M.; van Baar, H.; Geijsen, A.J.M.R.; van Zutphen, M.; Van Halteren, H.K.; Hansson, B.M.E.; Radema, S.A.; De Wilt, J.H.W.; Kampman, E.; et al. Dietary Intake of Magnesium or Calcium and Chemotherapy-Induced Peripheral Neuropathy in Colorectal Cancer Patients. Nutrients 2018, 10, 39. [Google Scholar] [CrossRef]
- Sun, W.-N.; Su, J.-W.; Shen, Z.-P.; Hsu, H.-T. Effect of Oral Glutamine on Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: An Evidence-Based Appraisal. Hu li za zhi J. Nurs. 2018, 65, 61–69. [Google Scholar]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D deficiency increases severity of paclitaxel-induced peripheral neuropathy. Breast Cancer Res. Treat. 2020, 180, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C.; Tanay, M.; Perlman, A.; Starr, J.; Advani, P.; Sheffield, K.; Brigham, T. A Systematic Review of Nutritional Lab Correlates with Chemotherapy Induced Peripheral Neuropathy. J. Clin. Med. 2022, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Silva, E.; Lawler, S.; Langbecker, D. The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: A systematic review. J. Cancer Surviv. 2019, 13, 97–107. [Google Scholar] [CrossRef]
- Azizoddin, D.R.; Adam, R.; Kessler, D.; Wright, A.A.; Kematick, B.; Sullivan, C.; Zhang, H.; Hassett, M.J.; Cooley, M.E.; Ehrlich, O.; et al. Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Supportive Care Cancer 2021, 29, 5741–5751. [Google Scholar] [CrossRef]
- Molassiotis, A.; Cheng, H.L.; Lopez, V.; Au, J.S.K.; Chan, A.; Bandla, A.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Suen, L.K.P.; et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 2019, 19, 132. [Google Scholar] [CrossRef]
- Oh, P.-J.; Kim, Y.L. Effectiveness of Non-Pharmacologic Interventions in Chemotherapy Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Korean Acad. Nurs. 2018, 48, 123–142. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparator |
| |
| Outcomes |
| |
| Study design |
|
|
| Language |
|
| Study and Population Characteristics | Intervention Characteristics | Findings | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIPN | CIPN-Related Outcomes | |||||||||||||||||||
| Citation and Country | Population | Cancer | CTX | Intervention | Control | Tool and Time Point | Incidence | Severity | Quality of Life | Physical function | Pain | Sleep | Fatigue | GI symptoms | Nutrition status | Psychological | Social | Treatment | Financial | Adverse events |
| Physical exercise | ||||||||||||||||||||
| Henke 2014 [31]; Germany | N: 46 Age (yrs): NR Males: NR | Type: lung Stage: ≥3 Existing CIPN: NR | Type: Platinum-based Frequency: NR Duration: NR Status: ongoing | Strategy: Strength and endurance training (n = 25) Regimen: 8 min endurance or 3 sets of 4 strength exercises daily Duration: 3 CTX cycles, from C1D1 | Standard care (n = 21) | EORTC QLQ-LC13; pre and post (C3) | + | + | + | + | o | o | o | o | o | o | ||||
| Stuecher 2019 [32]; Germany | N: 44 Age (yrs): 67 ± 8 Males: 67% | Type: gastrointestinal Stage: ≥3 Existing CIPN: no | Type: NR Frequency: NR Duration: NR Status: ongoing | Strategy: Walking (n = 22) Regimen: 150 min per wk Duration: 12 wks, from C1D1 | Standard care (n = 22) | Tuning fork test; pre and post (6 and 12 wks) | o | + | + | o | ||||||||||
| Streckmann 2014 [33]; Germany | N: 61 Age (yrs): 46 (19–73) Males: 77% | Type: lymphoma Stage: progressive Existing CIPN: NR | Type: mixed Frequency: NR Duration: NR Status: ongoing | Strategy: Strength, endurance, and sensorimotor training (n = 30) Regimen: 1 h session twice per wk Duration: 36 wks | Standard care (n = 31) | Tuning fork test; pre and post (12, 24 and 36 wks) | + | o | + | o | + | + | + | + | + | o | ||||
| Zimmer 2018 [34]; Germany | N: 30 Age (yrs): 50–81 Males: 70% | Type: colorectal Stage: 4 Existing CIPN: NR | Type: mixed Frequency: NR Duration: 2–3 cycles Status: ongoing and ceased | Strategy: Strength, endurance, and sensorimotor training (n = 17) Regimen: 1 hr session twice per wk Duration: 8 wks | Written exercise guidelines (n = 13) | FACT/GOG-NTX; pre and post (8 and 12 wks) | + | o | + | o | o | o | ||||||||
| Nutrition supplements | ||||||||||||||||||||
| Bradfield, 2015 [35]; USA | N: 200 Age (yrs): 9 ± 5 Males: 62% | Type: lymphoma Stage: NR Existing CIPN: no | Type: Vincristine Frequency: weekly Duration: ≥4 wks Status: ongoing | Strategy: L-glutamic acid in capsule form, taken orally (n = 101) Regimen: 3 times daily, total 0.75–1.5 g per day Duration: 5 wks | Placebo (n = 99) | mBPSPN; pre and post (5 wks) | o | |||||||||||||
| Howells, 2019 [36]; UK | N: 27 Age (yrs): 68 (53–78) Males: NR | Type: colorectal Stage: metastatic Existing CIPN: no | Type: 5FU and oxaliplatin Frequency: fortnightly Duration: ≤12 cycles Status: ongoing | Strategy: Curcumin powder in capsule form, taken orally (n = 18) Regimen: 4 times daily, total 2 g per day Duration: duration of CTX (from 7 days before C1D1) | Standard care (n = 9) | EORTC-QLQ-C30 and NCI-CTAE; pre and post | - | o | o | o | o | o | o | - | o | + | o | |||
| Sanchez-Lara, 2014 [37]; Mexico | N: 112 Age (yrs): 18–80 Males: 47% | Type: NSCL Stage: ≥3 b Existing CIPN: NR | Type: paclitaxel and cisplatin/carboplatin Frequency: every wks Duration: 2–6 cycles Status: ongoing | Strategy: omega 3 (EPA)-enriched oral nutrition supplement + isocaloric diet (n = 54) Regimen: 2 237 mL drinks per day (provides 2.2 g EPA) Duration: 2 CTX cycles, from C1D1 | Isocaloric diet (n = 58) | EORTC-QLQ-C30 and -LC13; pre and post (C1 and C2) | + | o | o | + | + | + | o | o | ||||||
| Wang, 2007 [38]; Taiwan | N: 86 Age (yrs): 60% ≥50 Males: 65% | Type: colorectal Stage: metastatic Existing CIPN: no | Type: 5FU and oxaliplatin Frequency: every 4 wks Duration: NR Status: ongoing | Strategy: Levo-Glutamine, taken orally (n = 42) Regimen: twice daily, total 30 g per day for 7 days every 2 wks Duration: 6 cycles, from C1D1 | Standard care (n = 44) | NCI-CTCAE and Electro-physiological exam; pre and post (C2, C4 and C6) | + | + | + | o | ||||||||||
| Japanese herbal medicine | ||||||||||||||||||||
| Motoo 2020 [39]; Japan | N: 52 Age (yrs): 35–79 Males: 60% | Type: colorectal Stage: 3 Existing CIPN: no | Type: capecitabine and oxaliplatin Frequency: every 3 wks Duration: 8 cycles Status: ongoing | Strategy: ninjin’yoeito powder 1, taken orally (n = 26) Regimen: 2–3 times daily, total 9 g per day Duration: 8 cycles, from C1D1 | Standard care (n = 26) | NCI-CTCAE; pre and post (C1–C8) | + | o | o | o | + | o | ||||||||
| Niskioka, 2011 [40]; Japan | N: 45 Age (yrs): 48–80 Males: 49% | Type: colorectal Stage: metastatic Existing CIPN: no | Type: 5FU and oxaliplatin Frequency: every 2 wks Duration: 4–32 cycles Status: ongoing | Strategy: Goshajinkigan 2, taken orally (n = 22) Regimen: 2–3 times daily, total 7.5 g per day Duration: entire CTX course (4–32 cycles), from C1D1 | Standard care (n = 23) | DEB-NTC; pre and post (at each CTX cycle) | + | o | o | o | ||||||||||
| Oki, 2015 [41]; Japan | N: 186 Age (yrs): 61 ± 11 Males: 55% | Type: colorectal Stage: 3 Existing CIPN: no | Type: 5FU and oxaliplatin Frequency: every 2 wks Duration: 12 cycles Status: ongoing | Strategy: Goshajinkigan 2, taken orally (n = 93) Regimen: daily with meals, total 7.5 g per day Duration: entire CTX course (12 cycles), from C1D1 | Placebo (n = 93) | NCI-CTCAE and DEB-NTC; pre and post (at each CTX cycle) | - | o | o | + | o | |||||||||
| Technology-facilitated education for symptom self-management | ||||||||||||||||||||
| Given, 2008 [42]; USA | N: 47 Age (yrs): ≥21 Males: 0% | Type: breast Stage: metastatic Existing CIPN: NR | Type: mixed Frequency: NR Duration: NR Status: ongoing | Strategy: Education for symptom self-management via automated telephone voice technology incorporating symptom monitoring (n = 24) Regimen: weekly phone calls for 4 wks, then at wk 6 and wk 8 Duration: 8 wks | Cognitive behavioral nurse-administered telephone symptom management (n = 23) | 11-point Likert scale; pre and post (10 and 16 wks) | ? | ? | ? | ? | ? | ? | ? | |||||||
| Kim, 2018 [43]; Korea | N: 76 Age (yrs): 51 ± 7 Males: 0% | Type: breast Stage: 4 Existing CIPN: NR | Type: mixed Frequency: NR Duration: NR Status: ongoing | Strategy: Education for symptom self-management via a mobile phone game (n = 36) Regimen: >30 min per day, 3 times per wk Duration: 3 wks | Symptom management booklet (n = 40) | NCI-CTCAE; pre and post (3 wks) | + | + | - | - | - | o | + | o | ||||||
 . Statistically significant positive effect favoring intervention.
. Statistically significant positive effect favoring intervention.  . Statistically significant negative effect favoring control.
. Statistically significant negative effect favoring control. . No statistically significant effect.
. No statistically significant effect.  . Statistical significance not tested. 5FU: Fluorouracil; C: chemotherapy cycle; CIPN: chemotherapy-induced peripheral neuropathy; CTX: chemotherapy; D: day; DEB-NTC: Neurotoxicity Criteria of Debiopharm; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-LC13: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Lung Cancer 13; EPA: eicosapentaenoic acid; FACT/GOG-NTX: Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity; GI: Gastrointestinal; hr: hour; min: minutes; mBPSPN: Modified Balis Pediatric Scale of Peripheral Neuropathies; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NSCL: non-small cell lung cancer; NR: not reported; UK: United Kingdom; USA: United States of America; wk: week; yrs: years.
. Statistical significance not tested. 5FU: Fluorouracil; C: chemotherapy cycle; CIPN: chemotherapy-induced peripheral neuropathy; CTX: chemotherapy; D: day; DEB-NTC: Neurotoxicity Criteria of Debiopharm; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-LC13: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Lung Cancer 13; EPA: eicosapentaenoic acid; FACT/GOG-NTX: Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity; GI: Gastrointestinal; hr: hour; min: minutes; mBPSPN: Modified Balis Pediatric Scale of Peripheral Neuropathies; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NSCL: non-small cell lung cancer; NR: not reported; UK: United Kingdom; USA: United States of America; wk: week; yrs: years.| Outcome | Pooled Estimate | Significance of Pooled Estimate | Heterogeneity | Number of Studies (Citations) | Sample Size | GRADE Level of Evidence |
|---|---|---|---|---|---|---|
| Physical exercise | ||||||
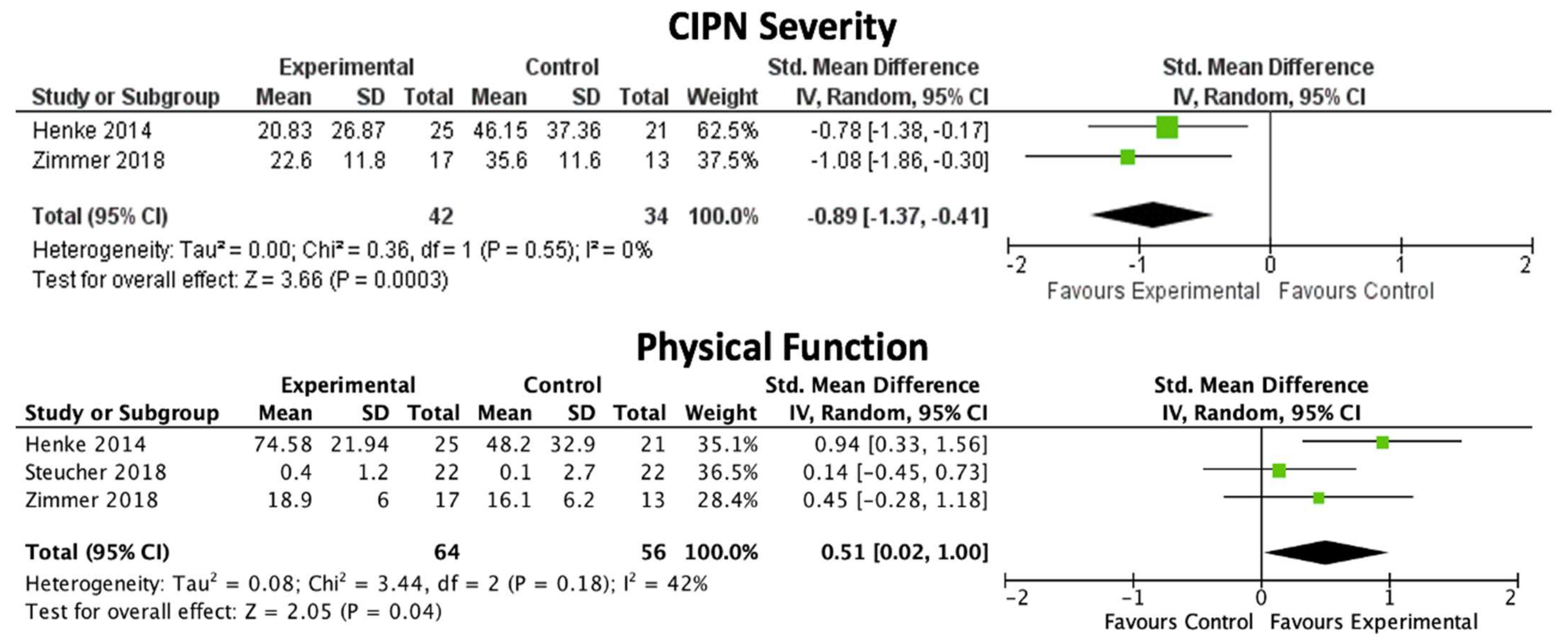
| CIPN severity | SMD: −0.89, 95% CI: −1.37, −0.41 | p = 0.0003 | 0% | 2 [31,34] | 76 | Moderate |
| Quality of life | SMD: 0.47, 95% CI: 0.01, 0.93 | p = 0.05 | 0% | 2 [31,34] | 76 | Very low |
| Physical function | SMD: 0.51, 95% CI: 0.02, 1.00 | p = 0.04 | 42% | 3 [31,32,34] | 120 | Moderate |
| Endurance | SMD: 1.11, 95% CI: −0.65, 2.87 | p = 0.22 | 93% | 2 [31,34] | 76 | Very low |
| Emotional wellbeing | SMD: 0.21, 95% CI: −0.42, 0.83 | p = 0.52 | 45% | 2 [31,34] | 76 | Very low |
| Social wellbeing | SMD: −0.02, 95% CI: −0.53, 0.50 | p = 0.95 | 21% | 2 [31,34] | 76 | Very low |
| Japanese herbal medicine | ||||||
| CIPN incidence - Grade 1 | OR: 1.98, 95% CI: 0.08, 48.14 | p = 0.68 | 92% | 2 [39,41] | 226 | Very low |
| CIPN incidence - Grade 2 | OR: 0.64, 95% CI: 0.06, 6.71 | p = 0.71 | 85% | 2 [39,41] | 226 | Very low |
| CIPN incidence - Grade 3 | OR: 0.37, 95% CI: 0.05, 2.52 | p = 0.31 | 80% | 3 [39,40,41] | 271 | Very low |
| CIPN incidence - Grade 2 and 3 | OR: 0.23, 95% CI: 0.01, 3.89 | p = 0.31 | 89% | 3 [39,40,41] | 271 | Very low |
| Fatigue | OR: 0.40, 95% CI: 0.06, 2.93 | p = 0.37 | 70% | 2 [39,41] | 238 | Very low |
| Nausea | OR: 0.80, 95% CI: 0.28, 2.23 | p = 0.66 | 30% | 3 [39,40,41] | 283 | Very low |
| Vomiting | OR: 0.63, 95% CI: 0.34, 1.16 | p = 0.13 | 0% | 3 [39,40,41] | 283 | Very low |
| Diarrhoea | OR: 1.20, 95% CI: 0.67, 2.17 | p = 0.54 | 0% | 2 [40,41] | 231 | Very low |
| Anorexia | OR: 0.71, 95% CI: 0.39, 1.27 | p = 0.25 | 0% | 3 [39,40,41] | 283 | Very low |
| Relative dose intensity of oxaliplatin | SMD: 1.77, 95% CI: −1.13, 4.68 | p = 0.23 | 98% | 2 [39,41] | 238 | Very low |
| Side effect: Neutropenia | OR: 0.74, 95% CI: 0.41, 1.31 | p = 0.30 | 0% | 3 [39,40,41] | 283 | Very low |
| Side effect: Thrombocytopenia | OR: 1.52, 95% CI: 0.87, 2.67 | p = 0.14 | 0% | 2 [39,41] | 238 | Very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crichton, M.; Yates, P.M.; Agbejule, O.A.; Spooner, A.; Chan, R.J.; Hart, N.H. Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2403. https://doi.org/10.3390/nu14122403
Crichton M, Yates PM, Agbejule OA, Spooner A, Chan RJ, Hart NH. Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(12):2403. https://doi.org/10.3390/nu14122403
Chicago/Turabian StyleCrichton, Megan, Patsy M. Yates, Oluwaseyifunmi Andi Agbejule, Amy Spooner, Raymond J. Chan, and Nicolas H. Hart. 2022. "Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis" Nutrients 14, no. 12: 2403. https://doi.org/10.3390/nu14122403
APA StyleCrichton, M., Yates, P. M., Agbejule, O. A., Spooner, A., Chan, R. J., & Hart, N. H. (2022). Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutrients, 14(12), 2403. https://doi.org/10.3390/nu14122403







