Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Presentation
2.4. Risk of Bias
3. Results
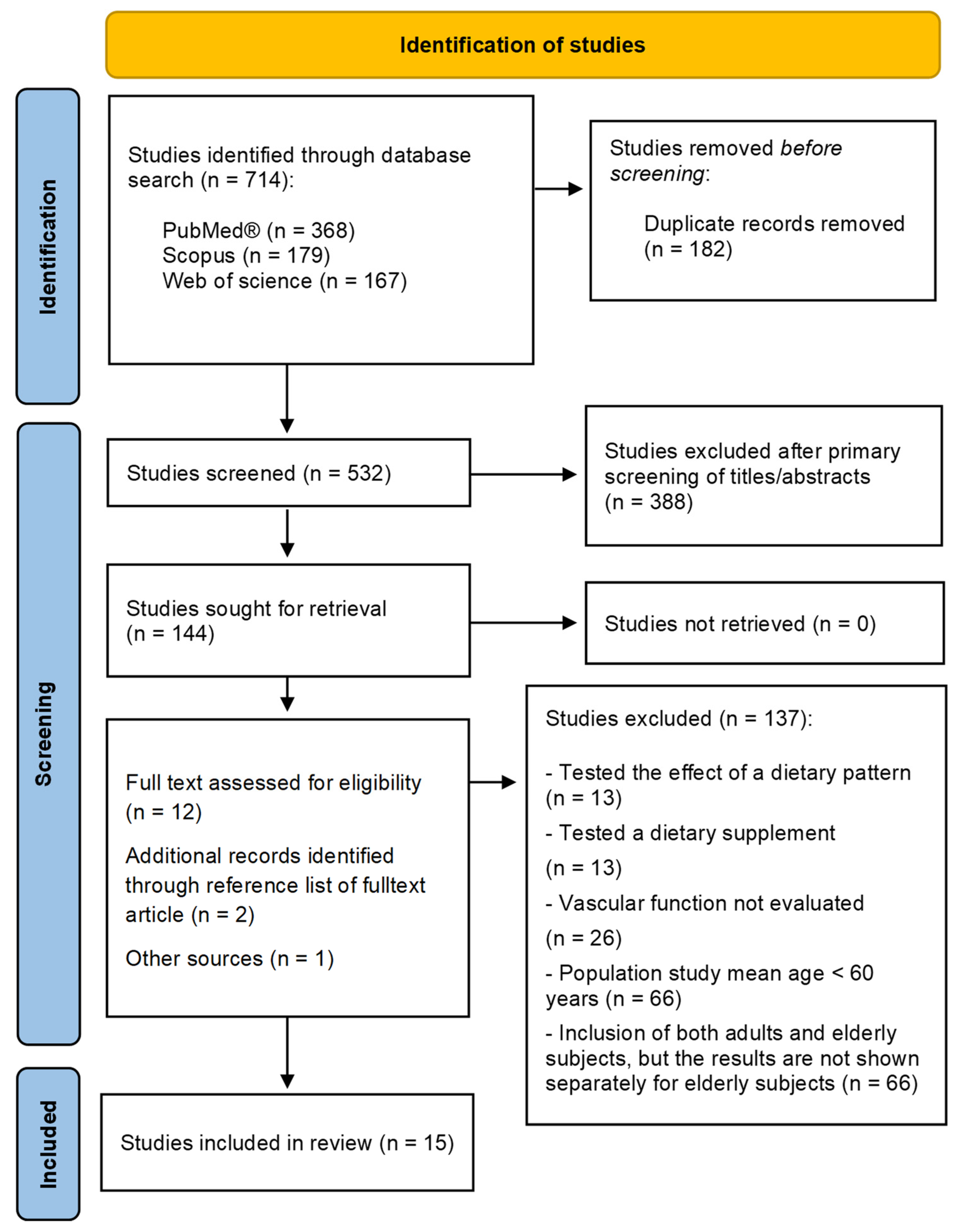
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Results from Acute Intervention Studies
3.5. Results from Chronic Intervention Studies
3.6. Other Markers Analyzed and Main Findings Obtained from Acute and Chronic Intervention Studies
4. Discussion
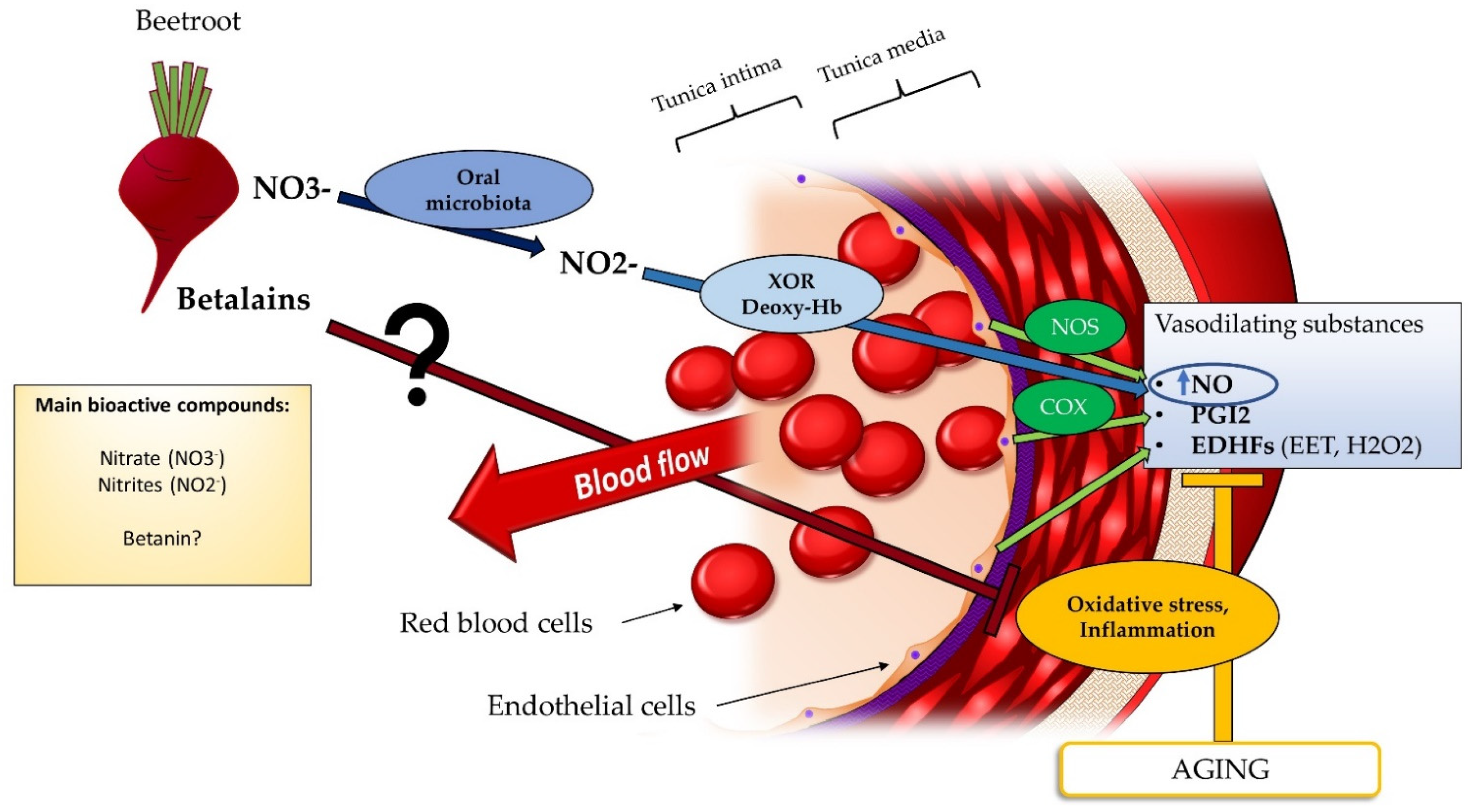
4.1. Beetroot
4.2. Effect of Fruit on Vascular Function
4.3. Effect of Vegetable Oils on Vascular Function
5. Strengths and Limitations
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 22 April 2022).
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Bansilal, S.; Castellano, J.M.; Fuster, V. Global burden of CVD: Focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 2015, 201, S1–S7. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savji, N.; Rockman, C.; Skolnick, A.H.; Guo, Y.; Adelman, M.; Riles, T.; Berger, J.S. Association Between Advanced Age and Vascular Disease in Different Arterial Territories. J. Am. Coll. Cardiol. 2013, 61, 1736–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Heidenreich, P.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [Green Version]
- North, B.J.; Sinclair, D.A. The Intersection Between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Merz, A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Feéleétou, M.; Vanhoutte, P.M. Endothelium-Derived Hyperpolarizing Factor. Arter. Thromb. Vasc. Biol. 2006, 26, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.L.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C7–C12. [Google Scholar] [CrossRef] [Green Version]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and Atherosclerosis: Human Trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneni, F.; Cañestro, C.D.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef]
- Busse, R.; Fleming, I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol. Sci. 2003, 24, 24–29. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Vallejo, C.P.; Peiró, C.; Sánchez-Ferrer, C.F.; Rodríguez-Mañas, L. Mechanisms Involved in the Aging-Induced Vascular Dysfunction. Front. Physiol. 2012, 3, 132. [Google Scholar] [CrossRef] [Green Version]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Pietri, P.; Stefanadis, C. Cardiovascular Aging and Longevity. J. Am. Coll. Cardiol. 2021, 77, 189–204. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [Green Version]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Zurbau, A.; Au-Yeung, F.; Mejia, S.B.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Different Fruit and Vegetable Sources with Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef]
- Knoops, K.T.B.; de Groot, L.; Kromhout, D.; Perrin, A.-E.; Moreiras-Varela, O.; Menotti, A.; Van Staveren, W.A. Mediterranean Diet, Lifestyle Factors, and 10-Year Mortality in Elderly European Men and Women. JAMA J. Am. Med. Assoc. 2004, 292, 1433–1439. [Google Scholar] [CrossRef]
- Gómez-Sánchez, L.; Rodríguez-Sánchez, E.; Ramos, R.; Marti-Lluch, R.; Gómez-Sánchez, M.; Lugones-Sánchez, C.; Tamayo-Morales, O.; Llamas-Ramos, I.; Rigo, F.; García-Ortiz, L.; et al. The Association of Dietary Intake with Arterial Stiffness and Vascular Ageing in a Population with Intermediate Cardiovascular Risk—A MARK Study. Nutrients 2022, 14, 244. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Dietary Fruits and Vegetables and Cardiovascular Diseases Risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [Green Version]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and Its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef]
- Del Bo’, C.; Marino, M.; Martini, D.; Tucci, M.; Ciappellano, S.; Riso, P.; Porrini, M. Overview of Human Intervention Studies Evaluating the Impact of the Mediterranean Diet on Markers of DNA Damage. Nutrients 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Marino, M.; Angelino, D.; Del Bo’, C.; Del Rio, D.; Riso, P.; Porrini, M. Role of berries in vascular function: A systematic review of human intervention studies. Nutr. Rev. 2019, 78, 189–206. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Marino, M.; Del Bo’, C.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M. Modulation of Adhesion Process, E-Selectin and VEGF Production by Anthocyanins and Their Metabolites in an In Vitro Model of Atherosclerosis. Nutrients 2020, 12, 655. [Google Scholar] [CrossRef] [Green Version]
- PubMed®. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 31 January 2022).
- Web of Science. Available online: http://apps.webofknowledge.com (accessed on 31 January 2022).
- Scopus Scopus Database. Available online: https://www.scopus.com/ (accessed on 1 December 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, G.V.; Morgado, M.; Pierucci, A.P.; Alvares, T.S. A single dose of a beetroot-based nutritional gel improves endothelial function in the elderly with cardiovascular risk factors. J. Funct. Foods 2016, 26, 301–308. [Google Scholar] [CrossRef]
- Hughes, W.E.; Ueda, K.; Treichler, D.P.; Casey, D.P. Effects of acute dietary nitrate supplementation on aortic blood pressure and aortic augmentation index in young and older adults. Nitric Oxide 2016, 59, 21–27. [Google Scholar] [CrossRef]
- Casey, D.; Treichler, D.P.; Ganger, C.T.; Schneider, A.C.; Ueda, K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J. Appl. Physiol. 2015, 118, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, W.E.; Kruse, N.T.; Ueda, K.; Feider, A.; Hanada, S.; Bock, J.M.; Casey, D.P. Dietary nitrate does not acutely enhance skeletal muscle blood flow and vasodilation in the lower limbs of older adults during single-limb exercise. Eur. J. Appl. Physiol. 2020, 120, 1357–1369. [Google Scholar] [CrossRef]
- Pekas, E.J.; Wooden, T.K.; Yadav, S.K.; Park, S.-Y. Body mass-normalized moderate dose of dietary nitrate intake improves endothelial function and walking capacity in patients with peripheral artery disease. Am. J. Physiol. Integr. Comp. Physiol. 2021, 321, R162–R173. [Google Scholar] [CrossRef]
- Dodd, G.F.; Williams, C.M.; Butler, L.T.; Spencer, J.P. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr. Health Aging 2019, 5, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Casey, D.P.; Bock, J.M. Inorganic nitrate supplementation attenuates conduit artery retrograde and oscillatory shear in older adults. Am. J. Physiol. Circ. Physiol. 2021, 320, H991–H998. [Google Scholar] [CrossRef]
- Rosario, V.A.D.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston-Green, K.; et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 950–960. [Google Scholar] [CrossRef]
- Rosario, V.A.D.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial. Clin. Nutr. 2020, 40, 879–889. [Google Scholar] [CrossRef]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oggioni, C.; Jakovljevic, D.G.; Klonizakis, M.; Ashor, A.W.; Ruddock, A.; Ranchordas, M.; Williams, E.; Siervo, M. Dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults: A randomised cross-over study. Int. J. Food Sci. Nutr. 2017, 69, 74–83. [Google Scholar] [CrossRef]
- Shaltout, H.; Eggebeen, J.; Marsh, A.P.; Brubaker, P.H.; Laurienti, P.J.; Burdette, J.H.; Basu, S.; Morgan, A.; Dos Santos, P.C.; Norris, J.L.; et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide 2017, 69, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.; VanBruggen, M.D.; Pieper, C.F.; Sloane, R.; Kraus, W.E.; Gow, A.J.; Allen, J.D. Beet the Best? Circ. Res. 2018, 123, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Dunn, E.L.; Macdonald, J.H.; Kubis, H.-P.; McMahon, N.; Sandoo, A. The Effects of Beetroot Juice on Blood Pressure, Microvascular Function and Large-Vessel Endothelial Function: A Randomized, Double-Blind, Placebo-Controlled Pilot Study in Healthy Older Adults. Nutrients 2019, 11, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, P.A.; Kovacs, C.; Moreira, P.; Magnoni, D.; Saleh, M.H.; Faintuch, J. Unsaturated Fatty Acids Improve Atherosclerosis Markers in Obese and Overweight Non-diabetic Elderly Patients. Obes. Surg. 2017, 27, 2663–2671. [Google Scholar] [CrossRef]
- Asgary, S.; Afshani, M.R.; Sahebkar, A.; Keshvari, M.; Taheri, M.; Jahanian, E.; Rafieian-Kopaei, M.; Malekian, F.; Sarrafzadegan, N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: A randomized crossover pilot study. J. Hum. Hypertens. 2016, 30, 627–632. [Google Scholar] [CrossRef]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, A.T.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2015, 103, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M. Titanium Alloys. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 213–224. ISBN 9780128012383. [Google Scholar]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles during Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [Green Version]
- Dinenno, F.A. Skeletal muscle vasodilation during systemic hypoxia in humans. J. Appl. Physiol. 2016, 120, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Casey, D.; Madery, B.D.; Curry, T.B.; Eisenach, J.H.; Wilkins, B.W.; Joyner, M.J. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J. Physiol. 2010, 588, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary Nitrate Supplementation and Exercise Performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Shi, J.; Xie, S.-Y.; Zhang, T.-Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Nitrite in nitric oxide biology: Cause or consequence? A systems-based review. Free Radic. Biol. Med. 2006, 41, 691–701. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO Generation from Nitrite and Its Role in Vascular Control. Arter. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.M.; Kapil, V.; Fuentes-Calvo, I.; Bubb, K.J.; Pearl, V.; Milsom, A.B.; Khambata, R.; Maleki-Toyserkani, S.; Yousuf, M.; Benjamin, N.; et al. Enhanced Vasodilator Activity of Nitrite in Hypertension. Hypertension 2013, 61, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Benjamin, N. Nitrates in the human diet-good or bad? Anim. Res. 2000, 49, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Del Bo’, C.; Porrini, M.; Fracassetti, D.; Campolo, J.; Klimis-Zacas, D.; Riso, P. A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: A randomized-controlled trial. Food Funct. 2014, 5, 3107–3116. [Google Scholar] [CrossRef] [Green Version]
- Del Bo’, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Deon, V.; Campolo, J.; Lanti, C.; Parolini, M.; Porrini, M.; Klimis-Zacas, D.; Riso, P. A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: Two randomized, controlled, crossover pilot studies. Food Funct. 2017, 8, 4108–4117. [Google Scholar] [CrossRef] [PubMed]
- Mateos, A.M.R.; Del Pino-García, R.; George, T.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P.E. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2012, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily Blueberry Consumption Improves Blood Pressure and Arterial Stiffness in Postmenopausal Women with Pre- and Stage 1-Hypertension: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; De Souza Gomes, R.; Do Carmo, M.M.; Da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 316. [Google Scholar] [CrossRef] [Green Version]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. Int. Rev. J. 2019, 11, 224–236. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D.; Morand, C.; Monfoulet, L.-E. An update on the role of nutrigenomic modulations in mediating the cardiovascular protective effect of fruit polyphenols. Food Funct. 2016, 7, 3656–3676. [Google Scholar] [CrossRef]
- Speciale, A.; Virgili, F.; Saija, A.; Cimino, F. Chapter 72: Anthocyanins in Vascular Diseases. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 923–941. ISBN 9780123984562. [Google Scholar]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Hall, W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr. Res. Rev. 2009, 22, 18–38. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2021, 477, 15–38. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [Green Version]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef]
- López, D.; Orta, X.; Casós, K.; Sáiz, M.P.; Puig-Parellada, P.; Farriol, M.; Mitjavila, M.T. Upregulation of endothelial nitric oxide synthase in rat aorta after ingestion of fish oil-rich diet. Am. J. Physiol. Circ. Physiol. 2004, 287, H567–H572. [Google Scholar] [CrossRef]
- Donnell, V.B.; Freeman, B.A. Interactions between nitric oxide and lipid oxidation pathways: Implications for vascular disease. Circ. Res. 2001, 88, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-H.; Hung, T.-M.; Wei, J.; Chiang, A.-N. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc. Res. 2004, 61, 169–176. [Google Scholar] [CrossRef]
- Weseler, A.R.; Bast, A. Oxidative Stress and Vascular Function: Implications for Pharmacologic Treatments. Curr. Hypertens. Rep. 2010, 12, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisaki, K.; Okuda, Y.; Suzuki, S.; Miyauchi, T.; Soma, M.; Ohkoshi, N.; Sone, H.; Yamada, N.; Nakajima, T. Eicosapentaenoic Acid Suppresses Basal and Insulin-Stimulated Endothelin-1 Production in Human Endothelial Cells. Hypertens. Res. 2003, 26, 655–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monahan, K.D.; Feehan, R.P.; Blaha, C.; McLaughlin, D.J. Effect of omega-3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol. Rep. 2015, 3, e12438. [Google Scholar] [CrossRef]
- Horrobin, D.F. Omega-6 and Omega-3 Essential Fatty Acids in Atherosclerosis. Semin. Thromb. Hemost. 1993, 19, 129–137. [Google Scholar] [CrossRef]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.; Toborek, M.; McClain, C.J. High-Energy Diets, Fatty Acids and Endothelial Cell Function: Implications for Atherosclerosis. J. Am. Coll. Nutr. 2001, 20, 97–105. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Effects of dietary fats on blood lipids: A review of direct comparison trials. Open Heart 2018, 5, e000871. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.H.; Fritsche, K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041.E15. [Google Scholar] [CrossRef]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef]
- Carpenter, K.; Taylor, S.E.; Ballantine, J.A.; Fussell, B.; Halliwell, B.; Mitchinson, M.J. Lipids and oxidised lipids in human atheroma and normal aorta. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1993, 1167, 121–130. [Google Scholar] [CrossRef]
- Al-Shudiefat, A.A.R.; Sharma, A.K.; Bagchi, A.K.; Dhingra, S.; Singal, P.K. Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Mol. Cell. Biochem. 2012, 372, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2009, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Hirata, K.; Kawashima, S.; Yokoyama, M. Unsaturated free fatty acids inhibit Ca2+ mobilization and NO release in endothelial cells. Kobe J. Med. Sci. 2001, 47, 211–219. [Google Scholar]
- Fattore, E.; Bosetti, C.; Brighenti, F.; Agostoni, C.; Fattore, G. Palm oil and blood lipid–related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014, 99, 1331–1350. [Google Scholar] [CrossRef] [Green Version]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef]
- Keogh, J.B.; Grieger, J.A.; Noakes, M.; Clifton, P.M. Flow-Mediated Dilatation Is Impaired by a High–Saturated Fat Diet but Not by a High-Carbohydrate Diet. Arter. Thromb. Vasc. Biol. 2005, 25, 1274–1279. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Elherik, K.; Bolton-Smith, C.; Barr, R.; Hill, A.; Murrie, I.; Belch, J.J. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc. Res. 2003, 59, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Newens, K.J.; Thompson, A.K.; Jackson, K.G.; Wright, J.; Williams, C.M. Acute effects of elevated NEFA on vascular function: A comparison of SFA and MUFA. Br. J. Nutr. 2010, 105, 1343–1351. [Google Scholar] [CrossRef] [Green Version]

| Reference, Country | Study Design | Study Population | Test Product | Control Product | Outcome Variables | Main Findings |
|---|---|---|---|---|---|---|
| Casey et al. 2015, USA [58] | Randomized, parallel, placebo-controlled | n = 12 healthy older subjects (9 M + 3 F), of which 7 completed the placebo trial Mean age = 64 ± 2 years Mean BMI = 25.5 ± 0.7 kg/m2 | 500 mL of beetroot juice providing 9.4 mmol of nitrate | 140 mL of nitrate-deprived beetroot juice + 360 mL of water | FBF, FVC, and CV tested in both condition of normoxia and hypoxia, at rest and after forearm exercise Tested prior and after 3 h from consumption of test product or placebo | ↑ FBF, and FVC and in hypoxia compared to control in older subjects, but not in young subjects ⟷ FBF and FVC observed in the placebo group under both normoxic and hypoxic condition |
| de Oliveira et al. 2016, Brazil [56] | Randomized, crossover, placebo-controlled, double-blind Washout period of at least 1 week | n = 20 older subjects with cardiovascular risk factors (7 M + 13 F). Mean age = 70.5 ± 5.6 years Mean BMI = 30.2 ± 5.3 kg/m2 | 100 g of beetroot gel providing 12.2 mmol of nitrate and 367.9 mg of phenolic acids (expressed as GAE) | 100 g of nitrate-deprived beetroot gel (placebo) | FMD, RH, BFV, PWV, AIx, Ep, and AC. Tested 2 h after consumption of the test or control product | ↑ FMD, RH, and BFV in test products vs. placebo ⟷ arterial stiffness parameters |
| Dodd et al. 2019, UK [61] | Randomized, crossover, controlled, double blind Washout period not indicated | n = 18 older subjects not taking anti-hypertensive medications (8 M + 13 F)Mean age = 68.7 ± 3.3 years Mean BMI = 25.9 ± 4.5 kg/m2 | 30 g of blueberry powder providing about 500 mg of anthocyanidins and 70 mg of procyanidins, homogenized with 300 mL of semi-skimmed milk and consumed after a standardized breakfast | 30 g of a powder providing the same amount of sugar and vit. C of blueberry powder. | DVP Tested 1 h after consumption of the test or control product | ⟷ arterial stiffness parameters |
| Hughes et al. 2016, USA [57] | Comparative parallel study evaluating the differences in response to acute ingestion of dietary nitrate between young and older subjects. Uncontrolled for older subject group. | n = 12 healthy non-obese, non-hypertensive older subjects (9 M + 3 F). Mean age = 64 ± 5 years Mean BMI = 25.5 ± 2 kg/m2 | 500 mL of beetroot juice providing 9.4 mmol of nitrate | - | AIx, AIx@75, and other hemodynamic measures Tested at six different timepoints (baseline, 1 h, 1,5 h, 2 h, 2,5 h, and 3 h). | ⟷ arterial stiffness and other parameters |
| Hughes et al. 2020, USA [59] | Randomized, crossover, placebo-controlled, double-blind | n = 10 healthy non-obese non-smokers older subjects (7 M + 3 F). Mean age = 68 ± 1 years Mean BMI = 25.8 ± 1 kg/m2 | Beetroot powder in 240 mL of water, providing 4 mmol of nitrates and 0.3 mmol of nitrites. | Nitrate-deprived beetroot powder in 240 mL of water. | Leg BF and VC, tested during exercise Tested prior and 2 h after consumption of test product or placebo | ⟷ vascular reactivity parameters |
| Pekas et al. 2021 USA [60] | Randomized, crossover, placebo-controlled, double-blind Washout period of 14 days | n = 11 older subjects with PAD (5 M + 6 F). Mean age = 70 ± 7 years Mean BMI = 29 ± 6 kg/m2 | Body mass-normalized beetroot juice, providing 0.11 mmol of nitrates/kg | Nitrate deprived beetroot beverage | Brachial and popliteal FMD, AIx, AI@75, AP, PP, PWV Tested prior and 1 h after consumption of test product or placebo | ↑ FMD in test products vs. placebo |
| Reference, Country | Study Design | Study Population | Test Product | Control Product | Duration | Outcome Variables | Main Findings |
|---|---|---|---|---|---|---|---|
| Casey and Bock. 2021, USA [62] | Randomized, cross-over, placebo-controlled, double-blind Washout period of at least 4 weeks | n = 10 healthy, non-obese, non-smokers older subjects (6 M + 4 F) Mean age = 67 ± 3 years Mean BMI >25.8 ± 3.3 kg/m2. | Beetroot powder mixed with approximately 200 mL of water, providing about 4 mmol of nitrate and 0.3 mmol of nitrate | Nitrate- and nitrite-depleted Beetroot powder | 4 weeks | Measures of shear profile at rest, and measure of exercise hyperemia during handgrip exercise (FBF and FVC). | Improvement in shear profile in test products group but not in placebo group |
| de Oliveira et al. 2017, Brazil [70] | Randomized, parallel, double-blind | n = 76 obese or overweight non-diabetic older subjects (23 M + 53 F) Mean age = 67.4 ± 5.16 years Mean BMI >28 kg/m2 | 30 mL/day of three different types of vegetable oil: olive, flaxseed or sunflower oil. This quantity of oil should not have been additional to the normal diet of the subjects | Absent | 90 days | FMD and CIMT | ↑ FMD in the group that consumed sunflower oil ↓ CIMT in all the intervention groups |
| do Rosario et al. 2020, Australia [64] | Randomized, crossover, placebo-controlled, double-blind Washout period of 14 days | n = 16 overweight or obese, but otherwise clinically healthy elderly subjects (non-hypertensive, non-diabetic, non-drug-treated, non-smoker subjects). Mean age = 65.9 ± 6 years Mean BMI = 30.6 ± 3.9 kg/m2 | 250 mL/day of high ACN queen garnet plum juice (about 200 mg of ACN)/day | 250 mL colored apricot juice (placebo). | 5 days | FMD and microvascular reactivity (peak shear rate, PV, PORH max, and IONT max) to evaluate postprandial response 2 h after challenging with a high energy/high fat test meal | ↑ FMD, and PORH max in test products group versus control |
| do Rosario et al. 2020, Australia [63] | Randomized, parallel, placebo-controlled, double-blind | n = 31 older subjects with mild cognitive impairment (12 M + 19 F). Mean age = 75.3 ± 6.9 years Mean BMI = 26.1 ± 3.3 kg/m2 | 250 mL/day of two different types of fruit juice: low ACN queen garnet plum (about 45 mg of ACN) or high ACN queen garnet plum (about 200 mg of ACN). | Colored apricot juice (placebo). Blinding strategies included advertising and consenting participants to a “fruit juice study”, without providing information on which fruit was being investigated. | 8 weeks | Microvascular reactivity evaluated POHR | ⟷ microvascular reactivity |
| Gilchrist et al. 2013, UK [65] | Randomized, crossover, placebo-controlled, double-blind Washout period of 4 weeks | n = 27 non-smokers older subjects with T2DM (of at least 5 years duration) and BP 4125/85 mm Hg or on one or more antihypertensive agents (18 M + 9 F). Mean age = 67.2 ± 4.9 years. Mean BMI = 30.8 ± 3.2 kg/m2 | 250 mL/day of beetroot juice, providing 7.5 mmol of nitrate | 250 mL of nitrate-depleted beetroot juice (placebo). | 2 weeks | FMD and microvascular endothelial function (perfusion response after skin iontophoresis of ACh and SNP) | ⟷ macro- and microvascular reactivity |
| Jones et al. 2019, UK [69] | Randomized, parallel, placebo-controlled, double-blind | n = 20 non-obese older subjects. Mean age = 63 ± 6 years. Mean BMI = 26,5 kg/m2 | 70 mL of beetroot juice providing 4.7 mmol of nitrate every day | 70 mL of prune juice | 4 weeks | FMD and markers of microvascular endothelial function (perfusion response after skin iontophoresis of ACh and SNP) | ⟷ compared to placebo |
| Oggioni et al. 2017, UK [66] | Randomized, crossover, placebo-controlled, double-blind Washout period of at least 7 days | n = 20 non-smokers healthy older subjects (10 M + 10 F). Mean age = 64.7 ± 3 years. Mean BMI = 25.6 ± 3.4 kg/m2. | 70 mL of beetroot juice twice a day (for a total of 12 mmol additional nitrate/day) | 70 mL of nitrate-depleted beetroot juice twice a day (placebo) | 1 week | AIx | ⟷ arterial stiffness parameters |
| Shaltout et al. 2017, USA [67] | Randomized, parallel, placebo-controlled, double-blind | 26 older hypertensive patients (13 M + 13 F). Mean age = 69 ± 7 years. Mean BMI = 33.7 kg/m2. | 70 mL of beetroot juice providing 6.1 mmol of nitrate every day + aerobic exercise training 3 times/week | 70 mL of nitrate-depleted beetroot juice twice a day (placebo) + aerobic exercise training | 6 weeks | Hemodynamic measures (SVR, TAC, VI, AI, and LCWI) | ⟷ hemodynamic measures |
| Woessner et al. 2018, USA [68] | Randomized, parallel, placebo-controlled, double-blind | n = 24 older subjects with PAD + IC (10 M + 10 F). Mean age = 64.7 ± 3 years. Mean BMI = 25.6 ± 3.4 kg/m2. | 70 mL of beetroot juice providing 4.2 mmol 3 h prior training session (30 min walking sessions 3 times/week) | 70 mL of nitrate-depleted beet-root juice twice a day (placebo) prior training session | 12 weeks | ABI and RHBF | ↑ ABI, and RHBF in the test group vs. placebo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, M.; Marino, M.; Martini, D.; Porrini, M.; Riso, P.; Del Bo’, C. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients 2022, 14, 2615. https://doi.org/10.3390/nu14132615
Tucci M, Marino M, Martini D, Porrini M, Riso P, Del Bo’ C. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients. 2022; 14(13):2615. https://doi.org/10.3390/nu14132615
Chicago/Turabian StyleTucci, Massimiliano, Mirko Marino, Daniela Martini, Marisa Porrini, Patrizia Riso, and Cristian Del Bo’. 2022. "Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action" Nutrients 14, no. 13: 2615. https://doi.org/10.3390/nu14132615
APA StyleTucci, M., Marino, M., Martini, D., Porrini, M., Riso, P., & Del Bo’, C. (2022). Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients, 14(13), 2615. https://doi.org/10.3390/nu14132615











