Abstract
Type 1 diabetes mellitus (T1DM) is a disease marked by oxidative stress, chronic inflammation, and the presence of autoantibodies. The gut microbiota has been shown to be involved in the alleviation of oxidative stress and inflammation as well as strengthening immunity, thus its’ possible involvement in the pathogenesis of T1DM has been highlighted. The goal of the present study is to analyze information on the relationship between the structure of the intestinal microbiome and the occurrence of T1DM. The modification of the intestinal microbiota can increase the proportion of SCFA-producing bacteria, which could in turn be effective in the prevention and/or treatment of T1DM. The increased daily intake of soluble and non-soluble fibers, as well as the inclusion of pro-biotics, prebiotics, herbs, spices, and teas that are sources of phytobiotics, in the diet, could be important in improving the composition and activity of the microbiota and thus in the prevention of metabolic disorders. Understanding how the microbiota interacts with immune cells to create immune tolerance could enable the development of new therapeutic strategies for T1DM and improve the quality of life of people with T1DM.
1. Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by insufficient insulin production due to the destruction of the insulin-secreting β cells in the islets of Langerhans of the pancreas [1]. In addition to antigen specificity, an important factor influencing the destruction of pancreatic β cells is the quality of the immune response to islet cell antigens [2]. The genetic factor is crucial in the development of T1DM (most often the presence of human leucocyte antigens HLA, DR3-DQ2, and DR4-DQ8), but environmental factors, including nutrition, stimulate the initiation and progression of the disease [3,4]. T1DM is characterized by disturbances in the efficiency of the antioxidant system, progressive inflammation, and the presence of autoantibodies [3]. In recent years, the number of T1DM cases has been increasing and some cases cannot always be explained by genetic factors [5]. It has been suggested that lifestyle changes, including the frequent use of antibiotics, may be responsible for the prevalence of T1DM, through the modification of the gut microbiome composition [5,6]. Therefore, in recent years, a relationship has been suggested between the structure and composition of the gut microbiota and the risk of T1DM development [7]. Studies in laboratory animals and clinical observations in humans with T1DM have shown changes in the number of bacteria, including Firmicutes, Bacteroides, Clostridium cluster, Lactobacillus, Bifidobacterium, and Prevotella, compared to that of healthy subjects [8], hence the suggestion that dysbiosis in various sections of the intestine may promote the development of T1DM by increasing intestinal permeability in predisposed individuals.
T1DM is a disease marked by oxidative stress, chronic inflammation, and the presence of autoantibodies [3]. The gut microbiota has been shown to be involved in the alleviation of oxidative stress [9] and inflammation [10], as well as strengthening immunity [11]; thus, its possible involvement in the pathogenesis of T1DM was highlighted. The intestinal commensal microbiota is a unique ecosystem associated with various functions of the living organism, especially those related to immunity. Dysbiosis of the intestinal microbiota plays a key role in the pathogenesis of autoimmune diseases [12]. Microbiome modulation is one of the most promising new strategies in medicine to improve the health of people with autoimmune diseases [12,13]. The goal of the present study is to analyze the information on the relationship between the structure of the intestinal microbiome and the occurrence of T1DM. The possibilities of supporting the intestinal microbiota by introducing certain food products into the diet, such as tea, herbs, and spices, as well as probiotics and prebiotics as methods of diet therapy supporting the pharmacological treatment of T1DM are also analyzed.
2. Materials and Methods
The analysis of information available in the global scientific literature was conducted in August 2021 using the following databases: PubMed, Scopus, Google Scholar, and Web of Science. The databases were searched for both joint and separate instances of the keywords, e.g., “autoimmune diabetes”, “diabetes”, “T1DM”, “gut microbiota”, “tea”, and “herbs” in both Polish and English languages (Figure 1). Based on the titles and synopses, articles unrelated to the substantive criteria were excluded, and the remaining original and review papers were intensely analyzed to identify the most pertinent publications. Bibliographies were also reviewed in all the selected articles to identify other potentially viable texts. The search was narrowed to papers published between 2008 and 2022. Ultimately, a total of 2646 publications were reviewed, of which 219 were used: 149 research reports and 70 reviews.
Figure 1.
Research strategy employed in the review of available literature. T1DM—Type 1 diabetes; SCFA—Short-chain fatty acids.
3. The Importance of Microbiota for Human Health
Microbiota are essential for the proper functioning of the digestive tract since they regulate digestive processes by affecting the length and thickness of the intestinal villi [14]. The microbiota also influence the motor activity of the intestines playing a regulatory role in the intestinal metabolism of bile acids and serotonin [6]. Intestinal bacteria also have a positive effect on the growth, maturation, and proliferation of enterocytes, all of which influence the renewal of the epithelium. The intestinal bacteria adhere to receptors on the surface of the epithelium, sealing the intestinal wall and maintaining the integrity of the intestinal barrier [15]. By competing for nutrients, acidifying the intestinal environment, and synthesizing bacteriocins, the gut microbiota inhibit the development of pathogenic microorganisms and neutralize toxins and xenobiotics. They are also involved in the synthesis of some B vitamins and vitamin K [12,14,16,17,18]. Bolan et al. [19] confirmed a reduction in intestinal permeability to toxic metals (As, Cd, Pb, Hg) under the influence of intestinal microorganisms and chelating agents using an in vitro intestinal epithelial model from Caco-2 cells. A previous study on mice showed that some bacteria and their metabolites modulate the transcription of the intestinal epithelium in different ways [20]. Plasmocytes located in the intestinal wall, under the influence of certain microflora, secrete immunoglobulin A (IgA), which is key in the humoral immune response [21]. The IgA responses of the mucosa are devoid of the classic features of memory, which in turn enables the immune system of the mucosa to react to the constantly changing microbiota composition [22]. IgA reactions eliminate pathogens and promote host–microbial symbiosis [23].
The gut microflora performs many important functions and plays a key role in maintaining the proper homeostasis of the organism. Intestinal flora is very diverse, and the exact number of species is not fully known; it is estimated that there may be as many as 1500 different species of bacteria in the human digestive tract [24]. The gut microbiome encodes over 3 million genes, which is 100 times more than the number of human genes, so it is often referred to as the “second human genome” [12]. Characterizing the processes that govern the formation of the human microbiome is essential in understanding human development and physiology. Previous studies have shown that the profile of the placental microbiome is similar to that of the human oral cavity, which suggests that microbial colonization of the gastrointestinal tract begins in utero [11]. Both environments are dominated by Firmicutes, Proteobacteria, Tenericutes, Bacteroidetes, and Fusobacteria [25]. It has also been shown that meconium from full-term newborns is not sterile and that the bacteria present in it are also present in the amniotic fluid, vagina, and oral cavity [11]. Colonization also occurs at the time of childbirth: in the case of natural birth, the gastrointestinal tract of the newborn contains bacteria that live in the gastrointestinal tract and vagina of the mother, while in newborns born by caesarean section, the bacterial flora contains bacteria inhabiting the mother’s skin, so they have a smaller number of bacteria of the type Bifidobacterium and Bacteroides and more pathogenic Enterococcus, Enterobacter, and Klebsiella [26,27]. Following delivery, the diet is one of the main factors that modulates the gut microbiota of infants. In the first week of life, the intestinal microbiome of full-term infants is dominated by Actinobacteria, Proteobacteria, Bacteroides, and Firmicutes (to a lesser extent), while in premature babies these are mainly bacteria of the Firmicutes and Tenericutes genera, as well as Actinobacteria (in smaller numbers) [28]. The composition of the microbiome changes dynamically until it reaches a relatively mature equilibrium at about 3 years of age [25,29]. Until then, the structure of the microbiota is very unstable and reacts to changes in the diet, such as the introduction of solid foods or disease. The maturation of the gut microbiota early in human life induces enterocyte proliferation via microbial metabolites [15].
In adults, the groups of microorganisms show a clear differentiation in individual sections of the gastrointestinal tract, which is mainly related to the pH of the environment and the presence of oxygen. Streptococcus, Peptococcus, Bifidobacterium, Staphylococcus, Lactobacillus, and Fusobacterium predominate in the oral cavity, while Helicobacter pylori, Lactobacillus, Streptococcus, and Candida albicans are the most abundant in the stomach and duodenum. Bacteroides, Lactobacillus, and Streptococcus dominate in the jejunum, Bacteroides, Clostridium, Enterococcus, Lactobacillus, and Veillonella in the ileum, while Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria predominate in the large intestine [14]. Changing the structure, abundance, and eubiotic state of the microbiota may contribute to intestinal and parenteral autoimmune and inflammatory disorders [12,30], which can lead to the occurrence of diseases associated with these conditions. It has been suggested that this condition could be the result of a lack of early stimulation of the immune system by biotic factors, which may interfere with the functioning of the immune system later in life and lead to hypersensitivity, autoimmune diseases, and/or inflammatory diseases [31]. This could explain the fact that there are more and more cases of autoimmune diseases, the occurrence of which cannot always be explained by genetic factors, but rather environmental factors, e.g., T1DM [5].
4. The Influence of Microbiota on the Immune System
The microbiome plays a key role in shaping and developing the main components of the host innate and adaptive immune system, while the immune system regulates the maintenance of the host–microbiota symbiosis [12,32]. Dysbiosis in a living organism, characterized by an overgrowth of facultative anaerobic bacteria and a decrease in diversity and balance, can induce an abnormal immune response that leads to a reduction in tolerance to the commensal microbiota. It can also interfere with the immune response, causing inflammation, oxidative stress, and insulin resistance [33].
An important function of the intestinal microbiota is the immuno-stimulation of cells of the lymphoid tissue associated with the intestine GALT (Gut-Associated Lymphoid Tissue), which is part of the immune system of the mucous membranes, MALT (Mucosa-Associated Lymphoid Tissue) [34]. About 70% of human lymphocytes, which are linked with intestinal GALT, which is made up of Peyer’s patches (sites of immune induction), mesenteric lymph nodes, and other lymphoid tissue (diffuse lymphoid regions not encapsulated) interact with autochthonous bacteria [34]. The modulation of the immune system by the microbiota begins even before birth, thanks to the microorganisms present in the amniotic fluid, which means that the fetus is exposed to bacterial antigens to which it must develop tolerance [29]. Following birth, diet and microbiota are factors that determine the proper maturation of the immune system, while the consumption of food, over and above supplying nutrients, also delivers antigens to the body [35,36]. Th infant’s immune system thus learns to control the balance between inflammatory and anti-inflammatory processes. The diversity of the gut microbiome during early colonization is critical to the formation of an immunoregulatory network that protects the body against mucosal IgE induction, which is associated with susceptibility to allergies [37]. The reactions initiated by commensal bacteria influence the antigen-specific adaptive immunity. Th17 lymphocytes (T-helper-17), a type of CD4 + T-helper cells, play a key role in coordinating the inflammatory immune response that provides protection against pathogens [38]. Th17 lymphocytes support actions against fungal and bacterial pathogens in the mucous membranes of the respiratory and digestive systems [38,39]. They limit side effects and ensure specific tolerance to food and bacterial antigens. The main pathological activity of Th17 lymphocytes is the maintenance of chronic inflammation, which contributes to the degradation of the body’s own tissues. The presence of Th17 has been demonstrated in allergic, autoinflammatory, and autoimmune diseases, including in people with T1DM [40,41,42]. Studies in mice have confirmed the role of intestinal bacterial metabolism in Th17 activation and in the development of Th17-dependent autoimmunity [43].
Undigested amino acids have the potential to become additional precursors for the production of SCFA by the gut microbiota; therefore, amino acids may have an indirect effect on the immune system [44]. Glycine, threonine, glutamine, and ornithine can be metabolized by anaerobic bacteria to produce acetate, while threonine, lysine, and glutamine can be used to synthesize butyrate [45]. One study has shown that the most abundant amino acid fermenting bacteria in the human small intestine are Clostridia, Bacillus-Lactobacillus-Streptococcus, Proteobacteria, and Peptostreptococcus [44].
Certain bacteria, such as Faecalibacterium prausnitzii, Roseburia intestinalis, and Anaerostipes butyraticus, produce short-chain fatty acids (SCFAs) during the fermentation of complex carbohydrates [46]. SCFAs, consisting mainly of butyrate, propionate, and acetate, by activating macrophages and dendritic cells, regulate the action of host immune cells by modulating the expression of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-12 (IL-12), and interleukin-6 (IL-6), as well as eicosanoids and chemokines [47]. SCFAs activate intestinal epithelial cells via G-protein coupled receptors (GPRs), GPR41 and GPR43, as shown in the studies in mice [47]. Kobayashi et al. [48] showed that GPR41 and GPR43 exert anti-inflammatory effects in human renal cortical epithelial cells. In addition, SCFAs inhibit the production of Th17 cells and promote the production of Treg lymphocytes (Regulatory T) from naive CD4 + T cells, which reduces inflammation [49,50]. Many autoimmune diseases result from an imbalance between pathogenic T cells and effector and regulatory T cells [51].
The intestinal microbiota stimulate the production of interleukin IL-10 by intestinal B cells through the activation of the TLR2/Myd88 (Toll-like receptor/myeloid differentiation factor 88 protein) signaling pathway, via PI3K (phosphoinositide 3-kinase) [52,53]. In addition to the initiation of B-cell differentiation, the microbiota stimulates the plasma cells that produce IL-10 and IgA+ [53]. In mice with a sterile digestive tract and with low-diversity microbiota, elevated serum IgE levels are found early in life [37]. This suggests that microbiota-derived immunoregulatory signals are essential in the maintenance of basal IgE levels, and that the proper induction of immune regulation requires adequate exposure to microbes early in life. Animals with a sterile digestive tract also have low levels of IgA in the gut and poorly developed lymphoid tissues associated with the gut, including small and sparse Peyer’s patches and mesenteric lymph nodes [54]. An efficient immune system is necessary to maintain a delicate balance between the elimination of pathogens and the maintenance of tolerance to one’s own tissues in order to avoid autoimmunity.
5. Pathogenesis of T1DM
T1DM is a chronic, genetically determined, autoimmune disease caused by the patient’s inability to secrete insulin as a result of autoimmune destruction of pancreatic β cells [3]. Pancreatic β-cell death is a result of the autoantibody-induced excessive production of proinflammatory cytokines, which in turn promotes phagocytosis, autophagy, and interferon activity. Pathological mechanisms, such as DNA methylation and modifications of histones and microRNAs, the action of which is related to the regulation of gene expression, are responsible for the development of T1DM [55]. About 95% of people with T1MD have the DR and DQ genes of the class-II human leukocyte HLA antigen (chromosome 6p21.3) or the UBASH3A mutation (chromosome 21q22.3), while 15% of people have an insulin-linked variable number of tandem repeats (INS-VNTR, chromosome 1p5,5), receptor cytotoxic T-Lymphocyte Antigen-4 (CTLA-4, chromosome 2q33), immune regulator (PTPN22, chromosome 1p13), and other genes [3,11].
In recent years, a relationship between the structure of the gut microbiota and the risk of T1DM has been suggested [5,8], which means that dysbiosis in various parts of the intestine may promote the development of T1DM by increasing intestinal permeability in predisposed individuals and lead to the occurrence of inflammation and oxidative stress. Moreover, T1DM is an autoimmune disease and the gut microbiota induces autoimmunity through TCR (T-cell receptors), thanks to which T cells recognize their antigens and are activated [51]. TCR have the ability to recognize many antigens (TCR cross-reactivity). One potential mechanism by which pathogenic autoreactive T cells may be activated is molecular mimicry, in which a foreign antigen exhibits significant structural or sequence similarity to its own antigen [51,56]. The activated autoreactive T cell can then enter the tissue containing its own antigen and trigger an autoimmune pathology. It is presumed that the development of autoimmunity is also influenced by metabolites and epigenetic modifications caused by the microbiota through the control of the immune system [57]. Bacterial metabolites can inhibit the production of inflammatory cytokines by β cells [58], and T cells that recognize β-cell antigens can be activated by bacterial products in the gut and then travel to the pancreatic lymph nodes to cause β-cell damage [59].
6. The Importance of Microbiota in T1DM
Increased intestinal permeability or ‘leaky gut’ is observed in many T1DM patients, which precedes clinical disease [51,60], as well as reduced microbiota diversity and an increased abundance of inflammatory species compared to healthy subjects [61]. A previous study on mice showed that the development of streptozotocin-induced T1DM (autoimmune diabetes) is dependent on the translocation of the intestinal microbiota into the pancreatic lymph node [62]. Moreover, the mice with T1DM were shown to have an altered intestinal microbiota composition, compared to that of control mice with an increase in the numbers of Bacteroides, Oscillospira (which disrupts the intestinal epithelial barrier), Sutterella, and Bifidobacterium [62]. Studies on the microbiome of children at risk of developing T1DM have shown a greater abundance of Bacterioides, Globicatella sanguinis, Dialister invisus, and Bifidobacterium longum and a reduction in the amount of Bifidobacterium pseudocatenulatum and Bifidobacterium adolescentis, compared to children without the burden of the disease [63,64]. In rats with T1DM, there was an increase in the number of pathogenic bacteria associated with infections and inflammation (Ruminococcaceae, Shigella, Enterococcus, Streptococcus, Rothia, Alistipes), while the number of beneficial SCFA-producing bacteria (Lactobacillus, Faecalitalea, Butyricicoccus) was reduced [65]. The microbiota of genetically predisposed infants, between the ages of 3 months and 3 years, are characterized by a greater number of Rikenellaceae, Ruminococcus, Streptococcus, and Blautia [61]. In addition, it was shown that the low abundance of lactate- and butyrate-producing strains was associated with the autoimmunity of β-cells in children, which may adversely affect the intestinal epithelial barrier function and stimulate inflammation [64].
The imbalance within the dominant genus, Bacteroides, is associated with the autoimmunity of the pancreatic islets, and the correlation analysis suggested a possible relationship between the signals of CrAssphage bacteriophages and the amount of Bacteroides dorei [66]. In this study, involving children who later developed islet autoimmunity, a lower number of Bacteroides vulgatus and Bifidobacterium bifidum was observed, compared to that observed in the control group. The study by Alkanani et al. [67], among people with islet autoimmunity, showed that changes in the gut microbiota correlate with susceptibility to T1DM. An increase in the relative abundance of Bacteroides and a decrease in the abundance of the anti-inflammatory, Prevotella, was observed in seropositive individuals with multiple antibodies, compared to those were only one autoantibody was found [67]. Similar results were obtained by Mejía-León et al. [29] when examining children with T1DM. In the microbiome of children with multiple islet auto-antibodies or confirmed T1DM, the anti-inflammatory species Prevotella and Butyricimonas were less frequent, and these differences were not related to diet. Moreover, these children had fewer SCFA-producing bacteria [60]. Giongo et al. [68] indicated that the high ratio of Firmicutes to Bacteroidetes and the instability of the microbiota may be one of the early diagnostic markers of the development of autoimmune disorders, including T1DM. Bedi et al. [69] found that changes in the gut microbiome in people with T1DM trigger the release of bacterial GAD (glutamic acid decarboxylase), thus injuring the host’s immune system due to the similarities between human and bacterial GAD. Talukdar et al. [70] showed that the composition of the gut microbiota differs significantly between people suffering from different types of diabetes: types 1, 2, and 3 (secondary pancreatogenic diabetes). The composition of the microbiome directly influences SCFA synthesis. Bacteroidetes mainly produce acetate and propionate, Firmicutes produce butyrate, and Bifidobacterium produce a large amount of acetate, as well as lactate, which is metabolized by other bacteria to butyrate [71]. This is why the large amount of Bacterioides contributes to an increase in intestinal permeability and thus to the development of autoantibodies against islet cells, which in turn leads to the translocation of microbes into the circulatory system, causing both direct damage and inflammation by immune damage of the pancreatic β cells [72].
7. Diet Components Positively Influencing the Development of Microbiota
Diet is a source of nutrients, but it is also the main route for the entry of antigens into the body. The intestinal microbiota metabolize proteins and complex carbohydrates, synthesize vitamins and produce a large number of metabolic products that can mediate contacts between the intestinal epithelium and cells of the immune system [33]. Questionable lifestyle and nutritional choices (albeit not always intentional), primarily in developed countries characterized by the West-type diet, may lead to the development of microbiota that lack the immunity and diversity necessary for a balanced immune response. It is believed that this may lead to an increase in autoimmune and inflammatory diseases [31,73]. Increased consumption of junk food has been shown to be associated with less gut microbiome diversity in children with T1DM [74]. The relationship between diet and immune response is largely regulated by the microbiota, as was shown in a study involving 482 healthy adult subjects [75].
7.1. Tea
Tea has positive effects on the health of people with T1DM due to the content of phenolic compounds that have antioxidant, anti-inflammatory, and immunomodulating properties [3]. At the same time, the presence of these compounds means that the consumption of tea can affect the composition of the intestinal microbiota by stimulating the growth of certain species or inhibiting the development of harmful species [76]. Green tea has been shown to have the strongest effect. Polyphenols are characterized by low bioavailability in the small intestine [77], therefore the participation of microbiota in their absorption is of great importance for human health. Most polyphenols are absorbed in the gut, but theaflavin and its galloyl derivatives, as well as teasinensin A, are more resistant to degradation by gut bacteria than most polyphenols [77,78]. Nevertheless, in vitro studies have shown that the microbiota plays an important role in the metabolism of theaflavins in both mice and humans. Lactobacillus plantarum 299v and Bacillus subtilis are of particular importance, as they metabolize the theaflavin-3,3′-digallate present in black tea [79].
Phenolic compounds show selective bactericidal activity. They damage bacterial cell membranes and thus inhibit the growth of Bacillus cereus, Campylobacter jejuni, Clostridium perfringens, Escherichia coli, Helicobacter pylori, Legionella pneumophila, and Mycobacterium spp. [80]. For example, epigallocatechin gallate (EGCG) can bind to peptidoglycans from the cell membranes of Gram-positive bacteria and cause them to break, while Gram-negative bacteria are protected from such effects by the outer membrane and negatively charged lipopolysaccharides that repel catechins [80].
Interactions between tea polyphenols and the gut microbiota lead to changes in the composition of the microbiota and the production of metabolites, including SCFAs, bile acids, and amino acids, which exert their biological effects both locally and systemically (Table 1). Glycans are formed as a result of the splitting out of glycosidic bonds in polyphenols. They are an important nutrient for the gut microbiota, especially for Bacteroidetes, as they have a greater ability to degrade glycans than Firmicutes [81]. By changing the composition of the microbiome and related metabolites, tea polyphenols may have a beneficial effect on the host organism, which contributes to the reduction in symptoms of some chronic metabolic disorders [82,83]. In vitro studies have shown that tea polyphenols promote the growth of Bacteroides, Faecalibacterium, Parabacteroides, and Bifidobacterium and inhibit Prevotella and Fusobacterium, and are therefore considered to be probiotic substances [76,77,84,85]. Flavonols can modulate the intestinal microbiota by affecting the adhesion of bacteria to enterocytes; this mainly applies to Lactobacillus acidophilus LA-5 and Lactobacillus plantarum IFPL379 [86]. Catechins inhibit the growth of certain pathogenic bacteria, such as Clostridium difficile and Staphylococcus spp., and may also stimulate the growth of beneficial Bifidobacterium bacteria [81]. Green tea extract, which is rich in catechins, especially EGCG, has been shown to improve the integrity of the intestinal barrier by reducing the translocation of gut-derived endotoxin and thus the resulting pro-inflammatory response in both animals and humans [87,88,89]. Campferol, which is present in tea leaves, improves the integrity of the intestinal barrier and inhibits inflammation in the intestines by reducing the activation of the TLR4/NF-κB pathway, and prevents the obesity-related gut dysbiosis [90].
Van Dorsten et al. [91] compared the microbial degradation of polyphenol-rich black tea extract and red wine extract in a five-step process, using an in vitro gastrointestinal model, which served as a simulator of the human intestinal microbial ecosystem. It was found that the production of phenolic acids and SCFAs by the gut microbiome was dependent on the region of the colon and on the source of the polyphenols. Tea extract inhibits the growth of Escherichia and Shigella, which are associated with gastrointestinal disorders, and increases the growth of Faecalibacterium, which synthesize butyrate [92]. A reduction in acetate synthesis was also found. A previous study by Unno and Osakabe [93] found that the administration of black or green tea extract to rats, at a dose of 10 g/kg, significantly modified the concentration of acetic and butyric acid observed in the cecum. It was found that black tea extract stimulates SCFA production more effectively than green tea extract, most likely by making it easier for the gut bacteria to use starch from the food consumed. In turn, the EGCG present in green tea was recognized as a potential modulator of the intestinal microbiota by stimulating an increase in the abundance of Akkermansia and the production of SCFAs in rats with colitis [94]. Green tea polyphenols have also been shown to lower the ratio of Bacteroidetes to Firmicutes and significantly increase the concentration of acetic and butyric acid in HFA mice [95]. Studies conducted on C57BL/6J mice showed a decrease in the number of Firmicutes and an increase in the number of Bacteroidetes in the caecum of the mice, due to polyphenols extracted from green and black tea, as well as an increase in SCFA synthesis correlated with an increase in the number of Pseudobutyrivibrio, which was especially pronounced in the groups receiving black tea [96]. Green, black, and oolong tea polyphenols inhibited the growth of Bacteroides, Prevotella and Clostridium histolyticum, and stimulated the growth of beneficial bacteria (Bifidobacterium, Lactobacillus, Enterococcus) in human Caco-2 cells [97]. The increased synthesis of SCFAs observed in this study was attributed to the microbial conversion of polyphenols in the pathways and processes occurring in the large intestine, which in turn stimulated the production of SCFA.
The administration of oolong tea polyphenols to obese mice for a period of four weeks caused an increase in the biodiversity of bacteria, with an increase in bacteria which produce butyrate and acetate, especially Bacteroidetes, and a simultaneous, favorable decrease in the proportion of Firmicutes and Bacteroidetes [84]. Guo et al. [85] highlighted the ability of oolong tea polyphenols to regulate the circadian rhythms of mice, by enhancing beneficial gut microbiota and influencing metabolic pathways by regulating gene expression. It is also possible that tea polyphenols influence the microbiome through the maintenance of an optimal redox status [98]. Lachnospiraceae, Bacteroides, Alistipes, and Faecalibaculum were identified as biomarkers of gut redox status in this study.

Table 1.
Influence of tea on the microbiota composition.
Table 1.
Influence of tea on the microbiota composition.
| Design | Effects on Gut Microbiota | Effect on SCFA Level | References | |
|---|---|---|---|---|
| Green tea | Post-menopausal females; four green tea pills/day for 12 months | =Firmicutes, =Bacteroidetes, =Actinobacteria | =acetic acid, = propionic acid, =butyric acid, =lithocholic acid, =deoxycholic acid | [99] |
| Green tea | C57BL/6J mice; 0.05, 0.2, or 0.8 g of green tea extract per 100 mL of water for 8 weeks | ↓Firmicutes, ↑Bacteroidetes, ↑Bacteroides, ↑Turicibacter, ↑Lachnospira, ↓Clostridium | Not measured | [95] |
| Green tea | C57BL/6J obese mice, 0.5 g of decaffeinated green tea extract or black tea extract per 100 g of diet for 4 weeks | ↑Bacteroidetes, ↓Firmicutes, ↓Actinobacteria, ↑Parabacteroides, ↑Clostridium, ↑Coprococcus, ↑Pseudobutyrivibrio | Green tea: = acetic acid, = propionic acid, = butyric acid, =valeric acid Black tea: = acetic acid, ↑propionic acid, ↑butyric acid, ↑valeric acid | [96] |
| Green tea | UVB-exposed Skh:HR-1 mice; diet containing 1% green tea extract for 10 weeks | ↓Lactobacillales, ↑Clostridia, ↑Erysipelotrichia | Not measured | [100] |
| Green tea, black tea | Male Wistar rats; green tea extract 10 g/kg diet or black tea extract 10 g/kg diet for 3 weeks | Green tea: ↓Prevotella, ↓Clostridium, ↑Lactobacillales, =Bifidobacterium, =Bacterioides; Black tea: ↓Clostridium, =Bifidobacterium, =Bacterioides | Green tea: ↓acetic acid, ↓butyric acid; Black tea: ↑acetic acid, ↑propionic acid | [93] |
| Green tea, oolong tea, black tea | Caco-2 cells; 100 mL/well, green tea polyphenols, oolong tea polyphenols or black tea polyphenols at a final concentration of 25, 50, 100, and 200 lg/mL incubated for 24, 48, and 72 h, respectively | ↑Bifidobacterium, ↑Lactobacillus/Enterococcus, ↓Bacteroides–Prevotella, ↓Clostridium histolyticum | ↑acetic acid, ↑formic acid, ↑propionic acid, ↑butyric acid | [97] |
| Green tea, black tea, oolong tea | C57BL/6J obese mice, 100 mL tea infusion of green tea, oolong tea, or black tea in diet for 13 weeks | ↑Alistipes, ↑Rikenella, ↑Lachnospiraceae, ↑Akkermansia, ↓Bacteroides, ↓Parabacteroides | Not measured | [101] |
| Pu-erh tea | Obese male Wistar rats; 750 mg/kg of ripe Pu-erh tea extract or 250 mg/kg of Pu-erh tea polyphenol or oxidized tea polyphenol for 12 weeks | ↑Firmicutes, ↓Bacteroidetes, ↑Actinobacteria | Not measured | [102] |
| Pu-erh tea | Normal and overweight males; 50 mg/kg/day of instant Pu-erh tea infusion for 4 weeks | ↓Bacilli, ↓Clostridia, ↓Lactobacillus, ↓Bacillus, ↓Streptococcus, ↓Lactococcus | Not measured | [103] |
| C57BL/6J male mice; Pu-erh tea infusion (450 mg/kg/day) or 1.5 mg/mL theabrownin infusion (225 mg/kg/day) for 26 weeks | ↓Bacilli, ↓Lactobacillus, ↓Bacillus, ↓Enterococcus, ↓Lactococcus, ↓Streptococcus | Not measured | ||
| Ganpu tea | C57BL/6J female mice; Ganpu tea-water extract in concentration: 0.1 g/mL, 0.2 g/mL, or 0.4 g/mL every day for 3 weeks | ↓Firmicutes; ↑Bacteroidetes; ↑microbial richness | ↑acetic acid, ↑propionic acid, ↓butyric acid, ↓isobutyric acid, ↓valeric acid, ↓isovaleric acid | [104] |
| Oolong tea | C57BL/6J male mice; 0.1 g of oolong tea polyphenols per 100 g of diet for 4 weeks | ↓Firmicutes, ↑Bacteroidetes, ↑Proteobacteria | Not measured | [84] |
| Liupao dark tea | Male Wistar rats with streptozotocin-induced diabetes; 150 mg/kg/day tea extract, or 30 mg/kg/day tea extract in vehicle for six weeks | ↑Clostridiales, ↑Ruminococcaceae, ↑Prevotella, ↑Bacteroides, ↑Lactobacillus | 150 mg/kg/day tea group: ↑butyric acid, ↑propionic acid, =acetic acid, ↑total SCFA30 mg/kg/day tea group: ↑butyric acid, =propionic acid, =acetic acid, =total SCFA | [105] |
↑ increased compared to control group, ↓ decreased compared to control group, = no effect compared to control group, SCFA—Short-chain fatty acids.
Previous studies in laboratory animals have shown that Pu-erh tea changes the composition and functioning of the intestinal microbiota, with an increase in the relative abundance of Firmicutes and a decrease in the relative abundance of Bacteroidetes, observed in the cecum [102,106,107]. Obese mice also showed an increase in mucin-degrading Akkermansia muciniphila, probably caused by the stimulation of type-II and -III secretion system proteins, elongation factor Tu, and glyceraldehyde-3-phosphate dehydrogenase by polyphenols and their metabolites [102]. In studies conducted on obese rats, a significant increase in the number of bacteria negatively correlated with obesity (Bacteroidaceae, Ruminococcaceae, Lachnospiraceae, Muribaculaceae, Rikenellaceae), and a reduction in the number of bacteria positively correlated with obesity (Erysipelotrichaceae, Lactobacillaceae) were found [107]. In animals receiving Pu-erh tea, an increase in SCFA synthesis was also found as a result of the improvement of the composition of the intestinal microbiota [108].
Wang et al. [104] investigated the effects of Ganpu tea (Pu-erh tea + mandarin peel) on the microbiome of C57 mice. After the administration of Ganpu tea, the number of Bacteroidetes increased and the number of Firmicutes decreased, which significantly influenced the content of various types of SCFAs in the stool: an increase in the concentration of acetic and propionic acid and a decrease in butyric acid. In mice consuming Ganpu tea, a reduction in the content of branched SCFAs, mainly isobutyric and isovaleric acids, was also found. Zheng et al. [32] found that Ganpu tea increased the number of Bifidobacterium, Lactobacillus and Lactococcus bacteria in rats. Liupao dark tea, which is popular in China and used in traditional medicine as an antidiabetic agent, when administered to rats with streptozotocin-induced diabetes and in the diet, was found to increase the Bacteroidetes/Firmicutes ratio and the number of SCFA-producing bacteria, especially Prevotella and Bacteroides [105].
7.2. Herbs and Spices
Herbs and spices are widely used as traditional medications to treat diabetes and its complications. The biochemical mechanisms of the antidiabetic action of herbs and spices include the stimulation of insulin secretion from pancreatic β cells, increased glucose uptake by adipose and muscle tissue, inhibition of glucose production by hepatocytes, inhibition of intestinal glucose digestion and absorption, and regulation of the activity of certain enzymes, such as lipoprotein lipase, glucose 6-phosphatase, lactate dehydrogenase, and aldose reductase [109]. The interactions between the gut microbiota and herbs and spices mainly occur in two ways: (1) the gut microbiota breaks down active substances into more easily digestible forms, thus inducing physiological changes in the body, and (2) the active substances within the herbs and spices regulate the composition of the gut microbiota, and in turn its metabolites, cause physiological changes in the host organism [110,111]. Moreover, some of the active ingredients show bactericidal properties, e.g., ferulic acid and quercetin, while some polyphenols, e.g., ellagitannins, tannins, and chlorogenic acids, can be metabolized by intestinal microorganisms [92]. Herbs and spices provide substrates for SCFA production by the host, thus modulating the composition of the gut microbiota and regulating SCFA production [112]. The active ingredients of herbs and spices influencing the composition and activity of the microbiota are mainly oligosaccharides and other prebiotic substances, polyunsaturated fatty acids (PUFAs), phenolic compounds, anthocyanins, essential oils, and organic acids [113,114,115]. The influence of herbs and spices on the gut microbiota composition are reported in Table 2.

Table 2.
Influence of herbs and spices on the microbiota composition.
There are many publications in the available literature on the anti-diabetic properties of herbs used in traditional Chinese medicine. They significantly affect glucose and lipid metabolism by modulating the intestinal microbiota, especially mucin-decomposing bacteria, bacteria with anti-inflammatory properties, and lipopolysaccharide- and SCFA-producing bacteria, as well as bacteria exhibiting bile salt hydrolase activity [128]. These effects are thanks to the content of flavonoids, alkaloids, terpenoids, saponins, polysaccharides, phenylpropanoids, berberine, resveratrol, and organic acids in the herbs [111]. In rats with streptozotocin-induced diabetes, Chinese medicinal herbs were shown to increase the number of bacteria producing SCFA and anti-inflammatory compounds, and reduce the number of pathogenic bacteria associated with the diabetes phenotype, which in turn proves that the hypoglycemic mechanism of these herbs is associated with the modulation of the intestinal microbiota [120]. Berberine, present in many Chinese herbs, caused an increase in the number of butyrate-producing bacteria, mainly Faecalibacterium and Roseburia, thus reducing intestinal inflammation and lowering glucose levels in diabetic rats. An increase in the level of SCFAs in the stool was also noted [129]. The prebiotic effect of berberine has been demonstrated in numerous studies [130,131,132,133]. A previous study performed on obese mice have shown that berberine is more effective in modifying the structure of the microbiome than curcumin [131]. This study also showed that the increase in the number of Bifidobacterium spp. and Akkermansia spp. in the cecum was correlated with an improvement in intestinal barrier function and a reduction in inflammation and oxidative stress indicators in the liver [131]. Berberine indirectly supports the growth of Akkermansia in mice by stimulating the secretion of intestinal mucin [132]. The use of berberine in diabetic rats resulted in an increase in the number of Bacteroidetes and Lactobacillaceae, and a decrease in the number of Proteobacteria and Verrucomicrobia [134]. In contrast, in rats subjected to chronic stress, berberine increased the abundance of Verrucomicrobia, Bacteroides, Lachnoclostridium, Akkermansia, and Anaerostipes, and decreased the ratio of Firmicutes to Bacteroidetes by reducing the relative amount of Firmicutes [135]. By stimulating the growth of SCFA-producing bacteria, berberine prevents obesity and insulin resistance through the TLR4 signaling pathway [136,137].
The influence of the aqueous sacha inchi leaf extract (P. volubilis L.), used in China as an adjunct in the treatment of diabetes, on the composition of the intestinal microbiota in mice with streptozotocin-induced diabetes was investigated [121]. This study showed an increase in species diversity of the intestinal microbiome, especially an increase in the abundance of Akkermansia, Parabacteroides, and Muribaculum, and a decrease in the abundance of Ruminiclostridium and Oscillibacter. Cyclocarya pallurus leaves are used in traditional Chinese medicine in anti-diabetes therapy because they regulate blood glucose levels, increase insulin synthesis, and inhibit apoptosis of pancreatic β cells [138]. Flavonoids isolated from the leaves of Cyclocarya pallurus lower the ratio of Firmicutes/Bacteroidetes in the large intestine, which proves their probiotic effect [122,123]. Some studies have shown a beneficial effect of phenolic-rich olive leaf extract (Olea europaea) in experimental models of the metabolic syndrome through the modification of the gut microbiota. In a study by Vezza et al. [139], an increase in the number of Firmicutes and a decrease in the Firmicutes/Bacteroidetes ratio, as well as a decrease in the level of inflammatory markers, were found in diet-induced obesity mice. The use of Anemarrhena asphodeloides extract, at a dose of 60 mg/kg/day in rats with induced diabetes reduced the number of Proteobacteria, Brachyspira, Facklamia, Klebsiella, Oligella, and Escherichia-Shigella and increased the numbers of Bacteroidetes, Actinobactercolia, Blautia, and Roseburocacteria [125]. Oregano essential oil has antibacterial properties. It inhibits the expression of genes related to virulence in Escherichia coli, increases the number of Lactobacillus, inhibits the expression of inflammatory cytokines, and enhances the antioxidant system of the organism [140,141].
Cinnamon, ginger, oregano, black pepper, and cayenne pepper are active against Fusobaterium, Ruminococcus spp., as well as some strains of Clostridium difficile, while promoting the growth of Bifidobaterium and Latobacillus [114]. In this study, cinnamon, oregano, and rosemary were active against selected Fusobacterium strains, while cinnamon, rosemary, and turmeric were active against selected Clostridium spp. The effect of the daily consumption of 5 g of mixed spices (cinnamon, Mediterranean oregano, ginger, rosemary, black, and cayenne pepper) by 29 healthy adults on the gut microbiota and SCFA synthesis was investigated, while the control group received maltodextrin [126]. The consumption of the spices resulted in a significant reduction in the number of Firmicutes and an increase in the number of Bacteroidetes. A significant negative correlation was also found between the concentration of SCFAs in the stool and the number of Firmicutes in the group receiving the spices, although there was no significant effect of the use of spices on the production of SCFAs. On the other hand, the average number of Actinobateria, Verrucomicrobia, Proteobacteria, Fusobacteria, Euryarchaeota, Spirochaetes, Tenericutes, Cyanobacteria, and Lentisphaerae was not different between groups. Turmeric and curcumin used in healthy people caused an increase in the number of Clostridium, Bacteroides, Citrobacter, Cronobacter, Enterobacter, Enterococcus, Klebsiella, Parabacteroides, and Pseudomonas strains and a decrease in the number of Blautia and Ruminococcus [142]. Just one serving of spices has a positive effect on the composition of the intestinal microbiota, which has been proven in studies conducted among people consuming 6 or 12 g of curry a day [116]. An in vitro study showed that curry paste increases the number of beneficial bacteria, especially Bifidobacteria and Lactobacteria, and causes an increase in SCFA synthesis, while reducing the number of Clostridia [117]. The main ingredient in curry powder is turmeric, and the active substance in turmeric is curcumin, which has been shown to stabilize blood glucose levels [143]. The administration of curcumin has been shown to protect against the overgrowth of opportunistic pathogens in nephropathic rats, including Escherichia-Shigella and Bacteroides, and increase the relative abundance of SCFA-producing bacteria, such as Lactobacillus and Ruminococcaceae [144]. Other studies have showed the inhibitory effect of curcumin on the growth of pathogenic strains, such as Prevotellaceae, Coriobacterales, Enterobacteria, and Rikenellaceae [145,146]. The administration of garlic to C57BL/6N mice fed a high-fat diet increased the diversity of the gut microbiome by increasing the number of Lachnospiraceae and reducing the number of Prevotella [119]. Due to the content of fructans and polysaccharides, garlic has prebiotic properties, stimulating the selective growth of beneficial bacteria, such as Bifidobacteria, and inhibiting pathogenic bacteria, such as Clostridia [147,148]. In addition to fructans, a component of garlic that favorably modulates the microbiome is allicin, which primarily increases the number of Bifidobacterium and Lactobacillus probiotic bacteria, as well as SCFA-synthesizing strains, e.g., Blautia [118]. In C57BL/6J mice with obesity caused by a high-fat diet, the use of black garlic melanoidins resulted in the modulation of the intestinal microbiota, with an increase in the number of SCFA-producing bacteria (Bacteroidaceae) and probiotic strains (Lactobacillaceae, Akkermansiaceae) and a reduction in the number of opportunistic pathogens (Enterobacterovibrionaceae) [149]. Cinnamon also positively modifies the microbiota of the gastrointestinal tract, mainly due to the presence of cinnamaldehyde in the essential oil, which has bactericidal and anti-inflammatory properties [133,150,151]. Cinnamon stimulates the production of SCFAs by having a positive impact on the growth of Alloprevotella and Lachnospiraceae bacteria [133]. It also stimulates the growth of beneficial bacteria Akkermansia, Bacteroides, Clostridium III, Psychrobacter, and Intestinimonas, and inhibits the growth of Ruminococcus and Escherichia-Shigella [151]. Cinnamon has also been found to lower blood glucose levels and prevents insulin resistance [152].
7.3. Probiotics, Prebiotics, Postbiotics, and Synbiotics
The main goal of the supplementation of probiotics and prebiotics in the diet is to increase the population of beneficial bacteria, eliminate pathogens, and introduce permanent changes in the composition of the intestinal flora (Table 3).
The mechanism of action of probiotics also includes: (1) communication between microorganisms and epithelial cells, regulating the adhesion of microorganisms to epithelial cells; (2) intercellular communication between tissues, regulating intercellular adhesion; (3) as well as the regulation of signaling and transport processes of epithelial cells [153,154]. Probiotic bacteria ferment, primarily by converting sugar to lactate; however, when sugar is in short supply, the bacteria are able to alter the fermentation process and produce acetate and formate instead [155]. The acidic environment created as a result of the fermentation inhibits the growth of pathogenic microorganisms. In turn, lactic acid bacteria inhibit the growth and multiplication of pathogenic strains by secreting antibacterial metabolites: bacteriocins, lysozymes, short-chain organic acids, and β-hydroxybutyrate [156]. In the large intestine, bacteria stimulate the production of immunoglobulins and they compete for nutrients and thus prevent the adherence of pathogenic microorganisms to epithelial cells [157]. Eliminating intestinal dysbiosis with probiotics and prebiotics is associated with a reduction in the autoimmune response, a reduction in inflammation, and an improvement in intestinal integrity, through the increased expression of proteins in the intestinal epithelium [158].
Lactic acid bacteria are classified as probiotic microorganisms and belong to the phylum Firmicutes, class Bacilli and order Lactobacillales, and include over 50 genera from 6 families and over 300 species [157]. Two groups of bacteria dominate the human intestines: the Gram-positive Firmicutes (Lactobacillus spp., Bacillus spp., and Clostridium spp.) and Gram-negative Bacteroidetes [159,160]. Probiotic Lactobacillus show anti-inflammatory effects and the ability to modulate the composition of the intestinal microbiota. Studies on obese mice have shown that Lactobacillus fermentum CECT5716 restores the number of Akkermansia sp., reduces the number of Erysipelotrichi and Clostridium spp., and increases the number of Bacteroides in the microbiome [161]. The administration of the probiotic Lactobacillus plantarum, Bifidobacterium breve, and Lactobacillus fermentum to obese mice exposed to Escherichia coli increased the content of SCFAs in the stool by modifying the structure of the microbiota and reducing inflammation [162,163]. The administration of Lactobacillus acidophilus (probiotic bacteria) has been shown to alleviate the effects of obesity in mice by modulating intestinal microbiota dysbiosis and tightening the intestinal mucosa, as well as increasing the activity of Akkermansia muciniphila [164,165]. Consuming foods containing probiotic bacteria has a positive effect on the gastrointestinal microbiome [157,159]. The consumption of fermented probiotic drinks containing Lactobacillus casei Shirota (LcS) increased the number of Lactobacillus spp. and Bifidobacterium spp. in children, which was positively correlated with changes in the content of SCFAs in the stool [159]. In a previous in vitro study, a probiotic containing Lactobacillus acidophilus LA-5, Bifidobacterium animalis, and inulin caused an increase in the number of beneficial microorganisms, such as Bifidobacterium spp., Bacteroides spp., and Faecalibacterium spp., as well as an increase in SCFA synthesis [166]. The probiotic strain Bifidobacterium longum BB-46, used in healthy people, increased the number of Firmicutes (especially Lachnospiraceae and Lactobacillaceae) and Bacteroidetes in the colon, but did not affect the production of SCFAs [167]. On the other hand, the simultaneous use of Bifidobacterium longum BB-46 and lemon citric pectin stimulated an increase in the number of Faecalibacterium, Eubacterium, Lactobacillus, and Ruminococcaceae in the colon, and reduced the number of proteolytic bacteria, including Bacteroides, Clostridium, Peptoniphilus, and Streptococcus [167].

Table 3.
Influence of prebiotics, probiotics, and synbiotics on the gut microbiota composition.
Table 3.
Influence of prebiotics, probiotics, and synbiotics on the gut microbiota composition.
| Design | Effects on Gut Microbiota | Effect on SCFA Level | References | |
|---|---|---|---|---|
| Bifidobacterium longum BB-46 with or without citric pectin | Probiotic strain Bifidobacterium longum BB-46 alone and in combination with a citric pectin from lemon on the gut microbiota using the Simulator of Human Intestinal Microbial Ecosystem | ↑Faecalibacterium, ↑Eubacterium, ↑Lactobacillus, ↑Ruminococcaceae, ↓Bacteroides, ↓Clostridium, ↓Peptoniphilus, ↓Streptococcus | ↑butyric acid | [167] |
| Lactobacillus fermentum CECT5716 | Male C57BL/6 J obese mice; probiotic Lactobacillus fermentum at 5 × 108 CFU in 100 μL/mouse/day for 11 weeks | ↑Bacterioidetes, ↓Firmicutes, ↑Verrumicrobia | Not measured | [161] |
| Lactobacillus acidophilus, Bifidobacterium longum, and Enterococcus faecalis | Female C57BL/6J mice fed high-fat or high-carbohydrate diets; intragastrically administered an encapsulated probiotics (Lactobacillus acidophilus, Bifidobacterium longum, Enterococcus faecalis), contained at a daily dose of 2.0 × 107 CFU for 30 days | ↑Bifidobacterium, ↑Lactococcus, ↑Akkermansia, ↓Alistipes, ↓Bacteroides, ↑Allobaculum, ↑Alloprevotella, ↑Lactobacillus, ↑Clostridium, ↓Escherichia/Shigella | Not measured | [168] |
| Lactobacillus casei Shirota | Normal-weight and overweight school children; Lactobacillus casei Shirota fermented probiotic drinks (80 mL bottle) for 15 weeks | ↑Lactobacillus, ↑Bifidobacterium | =acetic acid, =butyric acid, ↑propionic acid, ↑total SCFA | [159] |
| Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 | Commercial dietary supplement containing Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 plus inulin (each 1 g sachet contained 9 log CFU/g of LA-5, 10 log10 CFU/g of BB-12, and 0.22 g of inulin), under simulated gastrointestinal conditions for 14 days | ↑Bifidobacterium, ↑Bacteroides, ↑Faecalibacterium, ↑Lactobacillus | ↓acetic acid, ↓propionic acid, ↑butyric acid | [166] |
| Fructans | Female nonobese diabetic T1DM NOD/LtJ mice; inulin-type fructans | ↑Ruminococcaceae, ↑Lactobacilli | ↑acetic acid, ↑butytic acid, ↑propionic acid | [169] |
| Fructans | Male diet-induced obese C57BL/6J mice; 5% cellulose (control), 10% cellulose, 10% short-chain fructo-oligosaccharides (scFOSs), or 10% inulin in diet for 4 weeks | ↑Verrucomicrobia, ↓Firmicutes, ↑Actinobacteria (scFOS and inulin), ↑Bacteroidetes (inulin) | Not measured | [170] |
| Fructans | Patients with mild/moderately active ulcerative colitis; 7.5 g or 15 g daily oral oligofructose-enriched inulin for 9 weeks | 7.5 g inulin: no effect 15 g inulin: ↑Bacteroidaceae, ↑Porphyromonadaceae, ↑Bacteroides, ↑Parabacteroides | 7.5 g inulin: ↑acetic acid, =butyric acid, = propionic acid, =isobutyric acid, =isovaleric acid 15 g inulin: acetic acid, ↑butyric acid, ↑propionic acid, ↑isobutyric acid, ↑isovaleric acid | [171] |
| Fructans | T1DM patients aged 41–71 years consumed 16 g of inulin-type fructans (a mixture of oligofructose and inulin) for 6 weeks | ↑Bifidobacterium adolescentis, ↑Bacteroides ovatus, ↑Lachnospiraceae, ↑Faecalibacterium prausnitzii, ↓Ruminococcaceae, ↓Lachnospiraceae, ↓Erysipelotrichaceae | ↑total SCFA, ↑acetic acid, ↑propionic acid, =butyris acid, =isobutyric acid, =isovaleric acid, =valeric acid, =isocaproic acid, =caproic acid | [172] |
| Polysaccharides | Obese mice; 0.2% Lycium barbarum polysaccharides in water for 10 weeks | ↓Firmicutes, ↑Bacteroidetes | =acetic acid, =propionic acid, ↑butyric acid | [173] |
↑ increased compared to control group, ↓ decreased compared to control group, = no effect compared to control group, SCFA—Short-chain fatty acids, scFOSs—short-chain fructo-oligosaccharides, T1DM—Type 1 diabetes, NOD—non-obese diabetic.
Probiotics stabilize the gut microbiome and stimulate the production of SCFAs in animals exposed to xenobiotics and showing behavioral and neurodegenerative disorders [174,175,176]. In addition, probiotics can inhibit inflammation and oxidative stress by inhibiting lipid peroxidation, modulating the secretion of pro-inflammatory and anti-inflammatory cytokines, and maintaining the activity of antioxidant enzymes [177].
Postbiotics, which are products of the metabolism of living bacteria or are released from bacteria after their lysis, have beneficial health effects on the human body [178]. When used in appropriate amounts, postbiotics have been shown to promote host health and well-being, either directly or indirectly [179]. Postbiotics are identified as metabolites of probiotic bacterial strains, such as Bifidobacterium breve, B. lactis, B. infantis, Bacteroides fragilis, Lactobacillus, as well as Escherichia coli and Faecalibacterium prausnitzii [180]. They contain dead microorganisms and their metabolites, including enzymes, bacteriocins, peptides, lipids, teichoic acids, cell-surface proteins, polysaccharides, as well as organic acids and SCFAs [181]. The effects of postbiotics on the human body are similar to those of probiotics, however, their mechanisms of action are still unclear [182]. Postbiotics do not contain live microorganisms, therefore the risk associated with their intake is minimized, yet they are still able to modulate the intestinal microbiome. They also show immunomodulatory properties and, unlike bacteria inhabiting the intestines, they have the ability to penetrate the intestinal barrier into the GALT system and directly activate immune cells [183,184]. SCFAs are monocarboxylates produced by the gut microbiota as a result of the fermentation of polysaccharides [185]. SCFAs are involved in many metabolic processes, such as providing an energy source for microorganisms and colonocytes, controlling microbial function, fighting pathogens, protecting and maintaining intestinal integrity, and regulating immune cells and glucose metabolism [186,187,188]. Amino acid derivatives transformed by the intestinal microbiota are compounds that are potential postbiotics. Tryptophan metabolites, including tryptamine and indole-3-propionic acid, rebuild the intestinal mucosa [189]. Polysaccharide metabolites counteract oxidative stress (one of the markers of T1DM) and prevent diabetic complications [190]. They can also inhibit pancreatic α-amylase as demonstrated in in vitro studies, regulate the expression of genes involved in glucose metabolism, and increase hepatic glycogen reserves [190]. Bacteriocins and organic acids are responsible for inhibiting the growth and multiplication of pathogenic microorganisms, as well as for the maintenance of the intestinal barrier [156,191]. Postbiotics produced by Bifidobacterium breve and Streptococcus thermophilus strongly inhibit the secretion of the pro-inflammatory cytokine TNF-α, compared to other bacteria, which is indicative of specific anti-inflammatory effects of postbiotics obtained from specific bacteria [192]. This study also demonstrated the beneficial effect of commensal bacteria on intestinal homeostasis. Postbiotics also include strains of probiotic bacteria inactivated e.g., by high temperature, i.e., paraprobiotics, the health-promoting properties of which result, among others, from the immunomodulatory properties of elements of their cell walls [190]. Postbiotics obtained from various Lactobacillus species inhibit both Gram-positive and Gram-negative pathogenic bacteria, e.g., Listeria monocytogenes, Salmonella enterica, and Escherichia coli [193,194].
Prebiotics are food ingredients that have a positive effect on health and well-being by selective stimulation of the growth and/or activity of the intestinal microflora, thus also positively affecting the structure of the intestines [195]. Resistant oligosaccharides, mainly fructo-oligosaccharides and galacto-oligosaccharides, with a degree of polymerization from 3 to 9, demonstrate prebiotic activity. They form part of dietary fiber, along with the non-starch polysaccharides, lignin and cellulose [196]. Indigestible oligosaccharides have been shown to reduce inflammation in experimental colitis [197]. In this study, the enrichment of the rats’ diets with oligosaccharides resulted in changes in the microbiota, namely, an increase in Bifidobacteria and Enterobacteriaceae, and a decrease in Clostridium cluster IV. A higher concentration of SCFAs was also observed in the cecum of the rats. A study conducted on HLA-B27 transgenic rats with colitis showed that the use of long-chain inulin and oligofructose as a diet additive reduced the concentration of interleukin IL-1β and increased the concentration of transforming growth-factor β (TGF-β) in the cecum, as well as increased Lactobacillus and Bifidobacterium populations [198]. Fructo-oligosaccharides administered to Wistar rats with induced intestinal inflammation reduced the symptoms of inflammation [199]. This study also shows that the oligosaccharides are fermented in the upper parts of the large intestine, but their prebiotic effect extends to the distal parts of the large intestine, thus having a positive effect on inflammation in the large intestine. The administration of caramel, enriched with the prebiotic fructodisaccharides, to rats with inflammatory bowel disease, decreased the levels of pro-inflammatory cytokines TNF-α and IL-1β in the colon [200]. In female mice, inulin-type fructan promoted modulatory T-cell responses, as evidenced by an increase in CD25 + Foxp3 + CD4 + regulatory T cells, a decrease in IL17A + CD4 + Th17 cells, and modulation of the cytokine production profile in the pancreas, spleen, and large intestine [169]. Fructan was also shown to increase the Firmicutes/Bacteroidetes ratio, thereby strengthening the antidiabetogenic composition of the microbiota, as well as increasing the number of Ruminococcaceae and Lactobacilli [169]. On the other hand, Lycium barbarum polysaccharides increased the diversity of bacteria, decreased the Firmicutes/Bacteroidetes ratio, and improved intestinal dysbiosis caused by a high-fat diet, increasing the number of SCFA-producing bacteria Lacticigenium, Lachnospiraceae, and Butyricicoccus, and thus also the content of SCFAs in the feces [173]. It should be noted that the development of T1DM is associated with an abnormally increased proportion of Bacteroidetes/Firmicutes, but conflicting results are found in the literature. These data suggest that a decreased or increased proportion of Bacteroidetes/Firmicutes may be related to the obesity (more Bacterioidetes) or thinness (more Firmicutes) phenotypes [201]. Therefore, the influence of the Bacteroidetes/Firmicutes ratio remains unclear with respect to the development of T1DM, since differences in the composition of the gut microbiota may be due to the differential level of glucose in the host body fluids, which in turn may result from, e.g., diet. [158]. A commercial prebiotic containing the polysaccharides from Acacia senegal and Acacia seyal, used in an in vitro study involving an artificial intestine settled by human fecal microflora, caused an increase in the number of Bifidobacteria and a decrease in the number of Clostridium histolyticum, as well as an increase in SCFA content [202]. Some prebiotics benefit gut health by selectively stimulating gut microorganisms, including Bifidobacteria [203]. Enrichment of the diet with oligosaccharides in rats caused changes in the composition of the microbiota, with an increase in the number of Bifidobacterium and Enterobacteriaceae, and a decrease in the number of Clostridium cluster, as well as a higher concentration of SCFAs in the cecum [197]. A study conducted on HLA-B27 transgenic rats with colitis showed that the use of inulin and oligofructose in their diets reduced the concentration of inflammatory markers, and an increase in the Lactobacillus and Bifidobacterium populations in the cecum [198]. The combined use of long-chain fructo-oligosaccharides and postbiotics has beneficial effects in modulating the composition of the microbiome and immunological characteristics in early life [181].
Dietary fibers are edible carbohydrate polymers, with three or more monomeric units that are resistant to endogenous digestive enzymes, and therefore are not hydrolyzed and absorbed in the human small intestine [204]. However, they are fermented by gut bacteria and are the main source of carbon and energy for gut microbiota, which helps to increase gut microbiota diversity [205]. Apart from oligosaccharides, dietary fiber includes β-glucans and arabinoxylans from wholegrain cereals, pectin from fruits, vegetables and legumes, and resistant starch. After as little as 14 days of eating fiber, an increase in SCFA synthesis and an improvement in the intestinal function in adults was observed [206]. Firmicutes bacteria, Bifidobacac terium spp. and Bacteroides spp., are the main bacterial groups which degrade long-chain fiber components, e.g., resistant starch, while SCFAs are the products of anaerobic fermentation [204].
Synbiotics are a combination of a probiotic and a prebiotic with a synergistic effect [207]. The consumption of a combination of probiotics, prebiotics, postbiotics, and amino acids has been shown to help alleviate intestinal dysbiosis and degeneration, and thus may help improve the health of people with T1MD, and might be more effective in glycemic control than probiotics alone [208]. The beneficial effect of synbiotics on the metabolic profile of people with hyperglycemia (glycemia, biomarkers of inflammation, and oxidative stress) may result from the stimulation of the microbial production of SCFAs, which in turn leads to an increase in the concentration of glucagon-like peptide-1 (GLP-1) and inhibits hyperglycemia [177,209]. Oligosaccharides from cow’s milk were found to increase the production of acetates and lactates in the gut of human infants, leading to a lower pH [207,210]. They were also found to stimulate the growth of Bifidobacterium (B. longum, B. breve, B. bifidum, B. pseudocatenulatum) and enhance the efficiency of environmental colonization by these bacteria [207,210]. The administration of a synbiotic in the form of yogurt significantly reduced the development of hyperglycemia in streptozotocin-induced diabetic mice compared to the group receiving milk [211]. Synbiotic yoghurt favorably modulated the composition of the intestinal microbiota and protected the morphology of the pancreatic islets, thus preventing hyperglycemia through the microbiome-gut–pancreas axis. In diabetic mice, the administration of a synbiotic (polysaccharides isolated from Lactobacillus plantarum JY039 and Lactobacillus paracasei JY062) modified the intestinal microbiome, primarily causing an increase in the number of Bifidobacterium and Faecalibaculum and a reduction in Firmicutes, Muribaculaceae, and Lachnospiraceae [212]. The symbiotic also improved the functioning of other bacteria, which take part in metabolism within the intestinal tract, and it reduced inflammation by balancing the pro-inflammatory factors IL-6 and TNF-α and the anti-inflammatory factor IL-10 [212].
8. Perspectives
Huang et al. [58] showed that patients with newly diagnosed T1DM exhibit a different intestinal microbiota profile associated with reduced SCFA production, as well as altered IgA indirect immunity compared to healthy subjects. These authors also showed that the intestinal microbiota of mice with T1DM induced a more intense immune response, compared to the intestinal microbiota of healthy control mice; while the administration of SCFAs to the T1MD mice decreased the secretion of IgA. This may suggest that modification of the intestinal microbiota can increase the proportion of SCFA-producing bacteria, which could in turn be effective in the prevention and/or treatment of T1DM. SCFAs are bacterial metabolites resulting from the fermentation of polysaccharides [185]. They serve as a form of communication between the microbiome and the immune system and are responsible for maintaining the balance between the anti-inflammatory and pro-inflammatory response, e.g., by signaling via free fatty acid receptors (GPRs). SCFAs also induce Treg cells by inhibiting the enzyme histone deacetylase [213]. The production of SCFAs by gut bacteria is dependent on the availability of necessary substrates. Increased daily intake of soluble and non-soluble fiber, as well as the inclusion of probiotics, prebiotics, herbs, spices, and teas that are sources of phytobiotics, in the diet, could be important in improving the composition and activity of the microbiota and thus in the prevention of metabolic disorders (Figure 2). SCFAs produced by the gut microbiota have been found to positively affect glucose metabolism in a manner which is dependent on the activation of protein kinase by adenosine monophosphate, and involves the γ receptor that is activated by peroxisome proliferators [186,187]. This mechanism seems to be crucial in diabetes development. Targeting the diet to increase the production of SCFAs may be a way to alter the immune profile, thus promoting immune tolerance, and improving glycemic control in the treatment of T1DM.
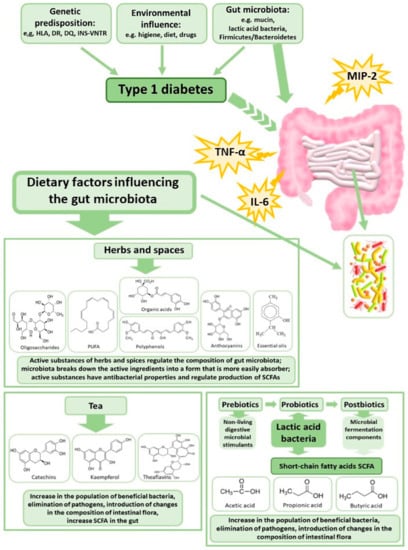
Figure 2.
Dietary factors modulating gut microbiota in the case of type 1 diabetes. SCFA—Short-chain fatty acids, TNF-α—tumor necrosis factor-α, MIP-2—Macrophage Inflammatory Protein 2, IL-6—interleukin-6, PUFA—Polyunsaturated fatty acids.
The results from previous studies suggest that there may be a diabetogenic microbiome [58,67,70,214], enabling a quick and accurate diagnosis of T1DM. Understanding how the microbiota interacts with immune cells to create immune tolerance could enable the development of new therapeutic strategies for T1DM and improve the quality of life of people with T1DM.
It is worth noting that some studies indicate the influence of dysbiosis on the occurrence of various types of diabetes: T1DM, T2DM, and T3DM [71,215,216]. Diabetes changes the composition of the microbiota, and the altered microbiota affects the pathophysiology of the disease. Changes in the gut microbiota, in combination with a specific diet, can lead to increased intestinal permeability, which, connected with low-grade inflammation, leads to the development of insulin resistance [216]. Differences in the microbiota composition in the different types of diabetes is directly associated with the physiological symptoms of the disease. In T1DM, pathogenic microbiota cause inflammation and an autoimmune reaction, which in turn causes damage to the pancreas due to the destruction of beta cells by lymphocytes [217]. Inflammatory factors produced by an altered gut microbiota also increase the risk of developing T2DM [217]. With regards to T3DM, which is associated with Alzheimer’s disease, it has been theorized that some cyanobacteria can produce neurotoxins, leading to neurodegeneration [218]. The gut microbiota, through the gut–brain axis, modulates normal behavioral reactions related to stress and anxiety, and the vagus nerve is an important means of communication between the gut microbiota and the central nervous system [216]. However, the results of previous studies are still inconclusive and future studies need to pay more attention with regard to distinguishing specific groups of gut microbiota that are indicative of the development or prevention of T1DM, T2DM, and T3DM.
9. Conclusions
The analysis of the microbiota could allow for the provision of targeted probiotic therapy and individual tailored treatment. It also allows us to extend the routine diagnostics and support the doctor in a holistic approach to the patient for affected by T1DM. The manipulation of the composition of the gut microbiome and, in turn, the level of SCFAs may be a promising tool in the treatment and prevention of autoimmune diseases. On the other hand, a full understanding of the etiology of T1DM would allow for the development of preventive strategies that will avoid or delay the onset of T1DM and help maintain control over the disease after its development.
Author Contributions
Conceptualization, A.W.-M.; Methodology, A.W.-M. and E.T.; Investigation, A.W.-M., E.T., J.D. and K.J.; Resources, A.W.-M. and E.T.; Data Curation, A.W.-M.; Writing—Original Draft Preparation, A.W.-M.; Writing—Review and Editing, K.J., J.D. and E.T.; Visualization, A.W.-M. and K.J.; Supervision, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Egan, A.M.; Dinneen, S.F. What is diabetes? Medicine 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Akerblom, H.K.; Vaarala, O.; Hyöty, H.; Ilonen, J.; Knip, M. Environmental factors in the etiology of type 1 diabetes. Am. J. Med. Genet. 2002, 115, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Jachimowicz, K. Antioxidant, anti-inflammatory, and immunomodulatory properties of tea—The positive impact of tea consumption on patients with autoimmune diabetes. Nutrients 2021, 13, 3972. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.M.; Simpson, M.D.; Seifert, J.; Scott, F.W.; Rewers, M.; Norris, J.M. The association between IgG4 antibodies to dietary factors, islet autoimmunity and type 1 diabetes: The Diabetes Autoimmunity Study in the Young. PLoS ONE 2013, 8, e57936. [Google Scholar] [CrossRef]
- Gülden, E.; Wong, F.S.; Wen, L. The gut microbiota and type 1 diabetes. Clin. Immunol. 2015, 159, 143–153. [Google Scholar] [CrossRef]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2016, 15, 13. [Google Scholar] [CrossRef]
- Hu, C.; Wong, F.S.; Wen, L. Type 1 diabetes and gut microbiota: Friend or foe? Pharmacol. Res. 2015, 98, 9–15. [Google Scholar] [CrossRef]
- Bibbò, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Ortega-Hernández, A.; Martínez-Martínez, E.; Gómez-Gordo, R.; López-Andrés, N.; Fernández-Celis, A.; Gutiérrrez-Miranda, B.; Nieto, M.L.; Alarcón, T.; Alba, C.; Gómez-Garre, D.; et al. The interaction between mitochondrial oxidative stress and gut microbiota in the cardiometabolic consequences in diet-induced obese rats. Antioxidants 2020, 9, 640. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Gałęcka, M.; Basińska, A.M.; Bartnicka, A. The importance of intestinal microbiota in shaping human health—Implications in the practice of the family physicia. Forum Med. Rodz. [Fam. Med. Forum] 2018, 12, 50–59. [Google Scholar]
- Dougherty, M.W.; Kudin, O.; Mühlbauer, M.; Neu, J.; Gharaibeh, R.Z.; Jobin, C. Gut microbiota maturation during early human life induces enterocyte proliferation via microbial metabolites. BMC Microbiol. 2020, 20, 205. [Google Scholar] [CrossRef]
- Breton, J.; Le Clère, K.; Daniel, C.; Sauty, M.; Nakab, L.; Chassat, T.; Dewulf, J.; Penet, S.; Carnoy, C.; Thomas, P.; et al. Chronic ingestion of cadmium and lead alters the bioavailability of essential and heavy metals, gene expression pathways and genotoxicity in mouse intestine. Arch. Toxicol. 2013, 87, 1787–1795. [Google Scholar] [CrossRef]
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; Chmiel, J.A.; Burton, J.P.; Reid, G. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef]
- Fernandez, B.; Le Lay, C.; Jean, J.; Fliss, I. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J. Appl. Microbiol. 2013, 114, 877–885. [Google Scholar] [CrossRef]
- Bolan, S.; Seshadri, B.; Keely, S.; Kunhikrishnan, A.; Bruce, J.; Grainge, I.; Talley, N.J.; Naidu, R. Bioavailability of arsenic, cadmium, lead and mercury as measured by intestinal permeability. Sci. Rep. 2021, 11, 14675. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef]
- Huus, K.E.; Petersen, C.; Finlay, B.B. Diversity and dynamism of IgA−microbiota interactions. Nat. Rev. Immunol. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef]
- Wołkowicz, T.; Januszkiewicz, A.; Szych, J. Gut microbiome and its dysbiosis as an important factor influencing the human health condition. Med. Dosw. Mikrobiol. 2014, 66, 223–235. [Google Scholar]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Ríos-Covian, D.; Langella, P.; Martín, R. From short-to long-term effects of c-section delivery on microbiome establishment and host health. Microorganisms 2021, 9, 2122. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef]
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; de la Barca, A.M.C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 2014, 4, 3814. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Dogra, S.K.; Chung, C.K.; Wang, D.; Sakwinska, O.; Colombo Mottaz, S.; Sprenger, N. Nurturing the early life gut microbiome and immune maturation for long term health. Microorganisms 2021, 9, 2110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W. Chemical profile, antioxidative, and gut microbiota modulatory properties of ganpu tea: A derivative of Pu-erh tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Alexander, M.; Turnbaugh, P.J. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity 2020, 53, 264–276. [Google Scholar] [CrossRef]
- Mennella, J.A.; Li, Y.; Bittinger, K.; Friedman, E.S.; Zhao, C.; Li, H.; Wu, G.D.; Trabulsi, J.C. The macronutrient composition of infant formula produces differences in gut microbiota maturation that associate with weight gain velocity and weight status. Nutrients 2022, 14, 1241. [Google Scholar] [CrossRef]
- Cahenzli, J.; Koller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef]
- Sano, T.; Kageyama, T.; Fang, V.; Kedmi, R.; Martinez, C.S.; Talbot, J.; Chen, A.; Cabrera, I.; Gorshko, O.; Kurakake, R.; et al. Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes. Cell Rep. 2021, 36, 109608. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Chen, X.; Zhao, Y.; Wu, Y.; Li, Y.; Wang, X.; Chen, H.; Xiang, C. Induction of intestinal Th17 cells by flagellins from segmented filamentous bacteria. Front. Immunol. 2019, 10, 2750. [Google Scholar] [CrossRef]
- Piekarski, R.; Szewczyk, L.; Wilczyńska, B. The dynamics of Th17 cells and clinical markers of type 1 diabetes. Pediatr. Endocrinol. 2015, 14, 37–42. [Google Scholar] [CrossRef]
- Honkanen, J.; Nieminen, J.K.; Gao, R.; Luopajarvi, K.; Salo, H.M.; Ilonen, J.; Knip, M.; Otonkoski, T.; Vaarala, O. IL-17 immunity in human type 1 diabetes. J. Immunol. 2010, 185, 1959–1967. [Google Scholar] [CrossRef]
- Ye, L.; Li, L.; Wan, B.; Hong, Y.; Gu, W.; Wang, S.W.; Ning, G. Immune response after autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus. Stem. Cell Res. Ther. 2017, 8, 90. [Google Scholar] [CrossRef]
- Alexander, M.; Ang, Q.Y.; Nayak, R.R.; Bustion, A.E.; Sandy, M.; Zhang, B.; Upadhyay, V.; Pollard, K.S.; Lynch, S.V.; Turnbaugh, P.J. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe 2022, 30, 17–30.e9. [Google Scholar] [CrossRef]
- Ciarlo, E.; Heinonen, T.; Herderschee, J.; Fenwick, C.; Mombelli, M.; Le Roy, D.; Roger, T. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci. Rep. 2016, 6, 37944. [Google Scholar] [CrossRef]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef]
- Venegas, D.P.; Marjorie, K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 43, 629–631. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Quigley, C.; Paw, C.; Butala, S.; Schneider, E.; Pandiyan, P. Role of short chain fatty acids in controlling tregs and immunopathology during mucosal infection. Front. Microbiol. 2018, 9, 1995. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Short, but smart: SCFAs train T cells in the gut to fight autoimmunity in the brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef]
- Sprouse, M.L.; Bates, N.A.; Felix, K.M.; Wu, H.J. Impact of gut microbiota on gut-distal autoimmunity: A focus on T cells. Immunology 2019, 156, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Oka, A.; Liu, B.; Herzog, J.W.; Eun, C.S.; Fan, T.J.; Bulik-Sullivan, E.; Carroll, I.M.; Hansen, J.J.; Chen, L.; et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J. Clin. Investig. 2019, 129, 3702–3716. [Google Scholar] [CrossRef] [PubMed]
- Botía-Sánchez, M.; Alarcón-Riquelme, M.E.; Galicia, G. B cells and microbiota in autoimmunity. Int. J. Mol. Sci. 2021, 22, 4846. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Xie, Z.; Chang, C.; Zhou, Z. Molecular mechanisms in autoimmune type 1 diabetes: A critical review. Clinic. Rev. Allerg. Immunol. 2014, 47, 174–192. [Google Scholar] [CrossRef]
- Munz, C.; Lunemann, J.D.; Getts, M.T.; Miller, S.D. Antiviral immune responses: Triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 2009, 9, 246–258. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Huang, J.; Pearson, J.A.; Peng, J.; Hu, Y.; Sha, S.; Xing, Y.; Huang, G.; Li, X.; Hu, F.; Xie, Z.; et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight 2020, 5, e135718. [Google Scholar] [CrossRef]
- Durazzo, M.; Ferro, A.; Gruden, G. Gastrointestinal microbiota and type 1 diabetes mellitus: The state of art. J. Clin. Med. 2019, 8, 1843. [Google Scholar] [CrossRef]
- Harbison, J.E.; Roth-Schulze, A.J.; Giles, L.C.; Tran, C.D.; Ngui, K.M.; Penno, M.A.; Thomson, R.L.; Wentworth, J.M.; Colman, P.G.; Craig, M.E.; et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr. Diabetes 2019, 20, 574–583. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Costa, F.R.; Françozo, M.C.; de Oliveira, G.G.; Ignacio, A.; Castoldi, A.; Zamboni, D.S.; Ramos, S.G.; Câmara, N.O.; de Zoete, M.R.; Palm, N.W.; et al. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J. Exp. Med. 2016, 213, 1223–1239. [Google Scholar] [CrossRef]
- Maffeis, C.; Martina, A.; Corradi, M.; Quarella, S.; Nori, N.; Torriani, S.; Plebani, M.; Contreas, G.; Felis, G.E. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 700–709. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Wang, J.; Li, P.; Duan, Y.; Dai, H.; An, Y.; Cheng, L.; Wang, T.; Wang, C.; et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed. Pharmacother. 2020, 124, 109873. [Google Scholar] [CrossRef]
- Cinek, O.; Kramna, L.; Lin, J.; Oikarinen, S.; Kolarova, K.; Ilonen, J.; Simell, O.; Veijola, R.; Autio, R.; Hyöty, H. Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: Parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr. Diabetes 2017, 18, 588–598. [Google Scholar] [CrossRef]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyoty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Bedi, S.; Richardson, T.M.; Jia, B.; Saab, H.; Brinkman, F.S.L.; Westley, M. Similarities between bacterial GAD and human GAD65: Implications in gut mediated autoimmune type 1 diabetes. PLoS ONE 2022, 17, e0261103. [Google Scholar] [CrossRef]
- Talukdar, R.; Sarkar, P.; Jakkampudi, A.; Sarkar, S.; Aslam, M.; Jandhyala, M.; Deepika, G.; Unnisa, M.; Reddy, N. The gut microbiome in pancreatogenic diabetes differs from that of Type 1 and Type 2 diabetes. Sci. Rep. 2021, 11, 10978. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. The role of the gut microbiota in the pathogenesis of diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut microbiota and type 1 diabetes mellitus: The effect of mediterranean diet. Front. Nutr. 2021, 7, 612773. [Google Scholar] [CrossRef]
- Yao, Q.; Li, H.; Fan, L.; Zhang, Y.; Zhao, S.; Zheng, N.; Wang, J. Dietary regulation of the crosstalk between gut microbiome and immune response in inflammatory bowel disease. Foods 2021, 10, 368. [Google Scholar] [CrossRef]
- Harbison, J.E.; Thomson, R.L.; Wentworth, J.M.; Louise, J.; Roth-Schulze, A.; Battersby, R.J.; Ngui, K.M.; Penno, M.A.S.; Colman, P.G.; Craig, M.E.; et al. Associations between diet, the gut microbiome and short chain fatty acids in youth with islet autoimmunity and type 1 diabetes. Pediatr. Diabetes 2021, 22, 425–433. [Google Scholar] [CrossRef]
- Shi, H.; Ter Horst, R.; Nielen, S.; Bloemendaal, M.; Jaeger, M.; Joosten, I.; Koenen, H.; Joosten, L.A.B.; Schweren, L.J.S.; Vasquez, A.A.; et al. The gut microbiome as mediator between diet and its impact on immune function. Sci. Rep. 2022, 12, 5149. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial metabolism of theaflavin-3,3’-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Chen, H.; Hayek, S.; Rivera Guzman, J.; Gillitt, N.D.; Ibrahim, S.A.; Jobin, C.; Sang, S. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS ONE 2012, 7, e51001. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.-P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Janssens, P.L.; Penders, J.; Hursel, R.; Budding, A.E.; Savelkoul, P.H.; Westerterp-Plantenga, M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS ONE 2016, 11, e0153134. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Reis, V.T.; Tangney, C.; Keshavarzian, A.; Racine, A.; Chai, W.; Krumbeck, J.; Shaikh, M.; Hamaker, B.; Hutkins, R.; Walter, J.; et al. Relation between total polyphenol intake and markers of intestinal health in obese humans. Curr. Dev. Nutr. 2020, 4, 1592. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Zhu, J.; Cheng, L.; Cao, J.; Wu, Z.; Weng, P.; Zheng, X. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. 2018, 9, 1079–1087. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Zhang, C.; Cao, J.; Wu, Z. Omics analyses of gut microbiota in a circadian rhythm disorder mouse model fed with oolong tea polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef]
- Bustos, I.; García-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2019, 17, 100495. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; de Vos, R.C.; Vaughan, E.E.; van Duynhoven, J.P.; Possemiers, S.; van de Wiele, T.; et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.A. Plant extracts as natural modulators of gut microbiota community structure and functionality. Heliyon 2020, 6, e05474. [Google Scholar] [CrossRef]
- Unno, T.; Osakabe, N. Green tea extract and black tea extract differentially influence cecal levels of short-chain fatty acids in rats. Food Sci. Nutr. 2018, 6, 728–735. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green tea polyphenols modulate colonic microbiota diversity and lipid metabolism in high-fat diet treated HFA mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.; Qin, R.; Lv, S.; Wang, S. Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, N.; Arikawa, A.Y.; Chen, C. Inhibitory effects of green tea polyphenols on microbial metabolism of aromatic amino acids in humans revealed by metabolomic analysis. Metabolites 2019, 9, 96. [Google Scholar] [CrossRef]
- Jung, E.S.; Park, H.M.; Hyun, S.M.; Shon, J.C.; Singh, D.; Liu, K.-H.; Whon, T.W.; Bae, J.-W.; Hwang, J.S.; Lee, C.H. The green tea modulates large intestinal microbiome and exo/endogenous metabolome altered through chronic UVB-exposure. PLoS ONE 2017, 12, e0187154. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Xia, Y.; Tan, D.; Akbary, R.; Kong, J.; Seviour, R.; Kong, Y. Aqueous raw and ripe Pu-erh tea extracts alleviate obesity and alter cecal microbiota composition and function in diet-induced obese rats. Appl. Microbiol. Biotechnol. 2019, 103, 1823–1835. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. The-abrownin from Pu-Erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Qian, Y.; Li, X.; Wang, J.; Ma, J.; Guo, J.; Fu, F. Effects of different concentrations of ganpu tea on fecal microbiota and short chain fatty acids in mice. Nutrients 2021, 13, 3715. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharmacother. 2019, 118, 109262. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened Pu-erh Tea extract protects mice from obesity by modulating gut microbiota composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.K. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Pu-erh tea extract promotes gut microbiota resilience against dextran sulfate sodium induced colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef]
- Pereira, A.S.P.; Banegas-Luna, A.J.; Peña-García, J.; Pérez-Sánchez, H.; Apostolides, Z. Evaluation of the anti-diabetic activity of some common herbs and spices: Providing new insights with inverse virtual screening. Molecules 2019, 24, 4030. [Google Scholar] [CrossRef]
- An, X.; Bao, Q.; Di, S.; Zhao, Y.; Zhao, S.; Zhang, H.; Lian, F.; Tong, X. The interaction between the gut microbiota and herbal medicines. Biomed. Pharmacother. 2019, 118, 109252. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Bo, A.; Shi, R.Y.; Li, Q.Y.; Lei, L.J.; Zhang, L.; Li, M.H. The interactions between gut microbiota and bioactive ingrdients of traditional Chinese medicines: A review. Pharmacol. Res. 2020, 157, 104824. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Thumann, T.A.; Pferschy-Wenzig, E.M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Summanen, P.H.; Lee, R.P.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic potential and chemical composition of seven culinary spice extracts. J. Food Sci. 2017, 82, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Khine, W.W.T.; Haldar, S.; De Loi, S.; Lee, Y.K. A single serving of mixed spices alters gut microflora composition: A dose–response randomised trial. Sci. Rep. 2021, 11, 11264. [Google Scholar] [CrossRef] [PubMed]
- Promjiam, P.; Siripongvutikorn, S.; Wichienchot, S. Functional properties of curry paste in relation to digestibility and fermentation by gut microbiota. Int. J. Food Prop. 2017, 20, 3204–3214. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Yang, C.; Xu, J.; Zheng, S.; Liu, J.; Xu, W.; Luo, Y.; Huang, K. Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. 2020, 34, 10682–10698. [Google Scholar] [CrossRef]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, A.; Hou, D.X. Preventive effects and mechanisms of garlic on dyslipidemia and gut microbiome dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef]
- Tian, J.; Bai, B.; Gao, Z.; Yang, Y.; Wu, H.; Wang, X.; Wang, J.; Li, M.; Tong, X. Alleviation effects of GQD, a traditional Chinese medicine formula, on diabetes rats linked to modulation of the gut microbiome. Front. Cell Infect. Microbiol. 2021, 11, 740236. [Google Scholar] [CrossRef]
- Lin, J.; Wen, J.; Xiao, N.; Cai, Y.T.; Xiao, J.; Dai, W.; Chen, J.P.; Zeng, K.W.; Liu, F.; Du, B.; et al. Anti-diabetic and gut microbiota modulation effects of sacha inchi (Plukenetia volubilis L.) leaf extract in streptozotocin-induced type 1 diabetic mice. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Y.; Zhang, X.; Zheng, X.; Cao, J.; Wu, Z.; Qin, W.; Cheng, K. A metagenomic analysis of the modulatory effect of Cyclocarya paliurus flavonoids on the intestinal microbiome in a high-fat diet-induced obesity mouse model. J. Sci. Food Agric. 2019, 99, 3967–3975. [Google Scholar] [CrossRef]
- Song, D.; Ho, C.T.; Zhang, X.; Wu, Z.; Cao, J. Modulatory effect of Cyclocarya paliurus flavonoids on the intestinal microbiota and liver clock genes of circadian rhythm disorder mice model. Food Res. Int. 2020, 138, 109769. [Google Scholar] [CrossRef]
- Li, A.L.; Ni, W.W.; Zhang, Q.M.; Li, Y.; Zhang, X.; Wu, H.Y.; Du, P.; Hou, J.C.; Zhang, Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef]
- Yan, D.; Fan, P.; Sun, W.; Ding, Q.; Zheng, W.; Xiao, W.; Zhang, B.; Zhang, T.; Zhang, T.; Shi, J.; et al. Anemarrhena asphodeloides modulates gut microbiota and restores pancreatic function in diabetic rats. Biomed. Pharmacother. 2021, 133, 110954. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Rasmussen, A.M.; Yang, J.; Lee, R.P.; Huang, J.; Shao, P.; Carpenter, C.L.; Gilbuena, I.; Thames, G.; Henning, S.M.; et al. Mixed spices at culinary doses have prebiotic effects in healthy adults: A pilot study. Nutrients 2019, 11, 1425. [Google Scholar] [CrossRef]
- Vita, A.A.; McClure, R.; Farris, Y.; Danczak, R.; Gundersen, A.; Zwickey, H.; Bradley, R. Associations between frequency of culinary herb use and gut microbiota. Nutrients 2022, 14, 1981. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tian, J.X.; Lian, F.M.; Li, M.; Liu, W.K.; Zhen, Z.; Liao, J.Q.; Tong, X.L. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 2021, 133, 110857. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Z.; Yang, F.; Yang, Y.; Chen, L.; Han, L.; Zhao, N.; Xu, J.; Wang, X.; Ma, Y.; et al. Antidiabetic effects of gegen qinlian decoction via the gut microbiota are attributable to its key ingredient berberine. Genom. Proteom. Bioinform. 2020, 18, 721–736. [Google Scholar] [CrossRef]
- Han, Z.; Ce, C.; Ou, Q.; Pan, Y.; Zhang, J.; Huo, D.; Chen, K. The potential prebiotic berberine combined with methimazole improved the therapeutic effect of Graves’ disease patients through regulating the intestinal microbiome. Front. Immunol. 2022, 12, 826067. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Sánchez, C.R.; Rodriguez, J.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Prebiotic effect of berberine and curcumin is associated with the improvement of obesity in mice. Nutrients 2021, 13, 1436. [Google Scholar] [CrossRef]
- Dong, C.; Yu, J.; Yang, Y.; Zhang, F.; Su, W.; Fan, Q.; Wu, C.; Wu, S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 2021, 139, 11595. [Google Scholar] [CrossRef]
- Li, C.N.; Wang, X.; Lei, L.; Liu, M.Z.; Li, R.C.; Sun, S.J.; Liu, S.N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020, 34, 1166–1174. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef]
- Zhang, J.D.; Liu, J.; Zhu, S.W.; Fang, Y.; Wang, B.; Jia, Q.; Hao, H.F.; Kao, J.Y.; He, Q.H.; Song, L.J.; et al. Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation. Acta Pharmacol. Sin. 2021, 42, 1821–1833. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef]
- Xiao, H.T.; Wen, B.; Ning, Z.W.; Zhai, L.X.; Liao, C.H.; Lin, C.Y.; Mu, H.X.; Bian, Z.X. Cyclocarya paliurus tea leaves enhances pancreatic β cell preservation through inhibition of apoptosis. Sci. Rep. 2017, 7, 9155. [Google Scholar] [CrossRef]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Mith, H.; Clinquart, A.; Zhiri, A.; Daube, G.; Delcenserie, V. The impact of oregano (Origanum heracleoticum) essential oil and carvacrol on virulence gene transcription by Escherichia coli O157:H7. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Wei, H.; Chen, G.; Wang, R.; Peng, J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods 2015, 18, 1191–1199. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: A double-blind, randomized, placebo-controlled pilot study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Association of curry consumption with blood lipids and glucose levels. Nutr. Res. Pract. 2016, 10, 212–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, X.; Zeng, Y.; Wu, X.; Peng, X. Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci. Hum. Wellness 2013, 2, 119–123. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Jin, Y.; Zhang, M. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. 2020, 11, 9585–9598. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, J.H.; Song, Y.; Kim, Y.T.; Lee, Y.H.; Kang, H. Oral consumption of cinnamon enhances the expression of immunity and lipid absorption genes in the small intestinal epithelium and alters the gut microbiota in normal mice. J. Funct. Foods 2018, 49, 96–104. [Google Scholar] [CrossRef]
- Qi, L.; Mao, H.; Lu, X.; Shi, T.; Wang, J. Cinnamaldehyde promotes the intestinal barrier functions and reshapes gut microbiome in early weaned rats. Front. Nutr. 2021, 8, 748503. [Google Scholar] [CrossRef]
- Shang, C.; Lin, H.; Fang, X.; Wang, Y.; Jiang, Z.; Qu, Y.; Xiang, M.; Shen, Z.; Xin, L.; Lu, Y.; et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021, 12, 12194–12220. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Pothuraju, R.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Roy, H.K.; Bouvet, M.; Batra, S.K. Mucins, gut microbiota, and postbiotics role in colorectal cancer. Gut Microbes 2021, 13, 1974795. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Russell, J.B.; Flythe, M.D.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef]
- Ren, H.; Saliu, E.M.; Zentek, J.; Goodarzi Boroojeni, F.; Vahjen, W. Screening of host specific lactic acid bacteria active against Escherichia coli from massive sample pools with a combination of in vitro and ex vivo methods. Front. Microbiol. 2019, 10, 2705. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Joseph, N.; Vasodavan, K.; Saipudin, N.A.; Yusof, B.N.M.; Kumar, S.; Nordin, S.A. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 2019, 57, 103–111. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, S.; Liu, X.; Huo, Y.; Su, B.; Li, X. Effects of a probiotic intervention on Escherichia coli and high-fat diet-induced intestinal microbiota imbalance. Appl. Microbiol. Biotechnol. 2020, 104, 1243–1257. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Zhang, Y.; Song, Y.; Ma, X.; Shi, Y.; Li, X. L. plantarum, L. fermentum, and B. breve Beads modified the intestinal microbiota and alleviated the inflammatory response in high-fat diet-fed mice. Probiotics Antimicrob. Proteins. 2020, 12, 535–544. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef]
- Da Cruz Rodrigues, V.C.; Duque, A.L.R.F.; Fino, L.C.; Simabuco, F.M.; Sartoratto, A.; Cabral, L.; Noronha, M.F.; Sivieri, K.; Antunes, A.E.C. Modulation of the intestinal microbiota and the metabolites produced by the administration of ice cream and a dietary supplement containing the same probiotics. Br. J. Nutr. 2020, 124, 57–68. [Google Scholar] [CrossRef]
- Bianchi, F.; Larsen, N.; Tieghi, T.M.; Adorno, M.A.T.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin. Food Res. Int. 2019, 120, 595–602. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006. [Google Scholar] [CrossRef]
- Liu, T.W.; Cephas, K.D.; Holscher, H.D.; Kerr, K.R.; Mangian, H.F.; Tappenden, K.A.; Swanson, K.S. Nondigestible fructans alter gastrointestinal barrier function, gene expression, histomorphology, and the microbiota profiles of diet-induced obese C57BL/6J mice. J. Nutr. 2016, 146, 949–956. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2019, 10, 334–357. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, Y.; Wang, F.; Zhang, H.; Ma, X.; Yin, Y.; Tan, B.; Chen, J. Supplementation with Lycium barbarum polysaccharides reduce obesity in high-fat diet-fed mice by modulation of gut microbiota. Front. Microbiol. 2021, 12, 719967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, S.; Yu, Y.; Li, S.; Wu, L.; Zhang, Y. The role of probiotic intervention in regulating gut microbiota, short-chain fatty acids and depression-like behavior in lead-exposed rats. Int. J. Occup. Med. Environ. Health 2022, 35, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Golovko, S.; Golovko, M.Y.; Singh, S.; Darland, D.C.; Combs, C.K. Effects of probiotic supplementation on short chain fatty acids in the AppNL-G-F mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2020, 76, 1083–1102. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 2017, 39, 322–336. [Google Scholar] [CrossRef]
- Soleimani, A.; Motamedzadeh, A.; Zarrati Mojarrad, M.; Bahmani, F.; Amirani, E.; Ostadmohammadi, V.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: A randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob. Proteins 2019, 11, 1248–1256. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Karbowiak, M.; Zielińska, D. Postbiotics—Properties, application and impact on human health. Food Sci. Technol. Qual. 2020, 2, 22–37. [Google Scholar] [CrossRef]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Tims, S.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; et al. Effects of a postbiotic and prebiotic mixture on suckling rats’ microbiota and immunity. Nutrients 2021, 13, 2975. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for preventing and treating common infectious diseases in children: A systematic review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef]
- Cukrowska, B.; Kwiecień, J. Postbiotics—A new meaning of infant immunity support. Opinions of the immunologist and pediatrician. Forum Med. Rodz. [Fam. Med. Forum] 2019, 13, 103–110. [Google Scholar]
- Hoarau, C.; Lagaraine, C.; Martin, L.; Velge-Roussel, F.; Lebranchu, Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006, 117, 696–702. [Google Scholar] [CrossRef]
- Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In vitro fermentation of polysaccharides from aloe vera and the evaluation of antioxidant activity and production of short chain fatty acids. Molecules 2019, 24, 3605. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol. Motil. 2017, 30, e13178. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Araña, M.; Urtasun, R.; Encio, I.J.; Barajas, M. Role of postbiotics in diabetes mellitus: Current knowledge and future perspectives. Foods 2021, 10, 1590. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Prost, Ł.; Dobrowolski, P.; Chand, D.K.P.; Donaldson, J.; Czech, A.; Klebaniuk, R.; Fabjanowska, J.; Muszyński, S. Prenatal programming of the small intestine in piglets: The effect of supplementation with 3-hydroxy-3-methylbutyric acid (HMB) in pregnant sows on the structure of jejunum of their offspring. Ann. Anim. Sci. 2022, 22, 612–623. [Google Scholar] [CrossRef]
- Ménard, S.; Candalh, C.; Bambou, J.C.; Terpend, K.; Cerf-Bensussan, N.; Heyman, M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004, 53, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Calix-Lara, T.F.; Rajendran, M.; Talcott, S.T.; Smith, S.B.; Miller, R.K.; Castillo, A.; Sturino, J.M.; Taylor, T.M. Inhibition of Escherichia coli O157:H7 and Salmonella enterica on spinach and identification of antimicrobial substances produced by a commercial lactic acid bacteria food safety intervention. Food Microbiol. 2014, 38, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, F.; Chai, J.; Wang, J. Effect of lactic acid bacteria on Listeria monocytogenes infection and innate immunity in rabbits. Czech. J. Anim. Sci. 2020, 65, 23–30. [Google Scholar] [CrossRef]
- Bogusławska-Tryk, M.; Ziółkowska, E.; Sławińska, A.; Siwek, M.; Bogucka, J. Modulation of intestinal histology by probiotics, prebiotics and synbiotics delivered in ovo in distinct chicken genotypes. Animals 2021, 11, 3293. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Koleva, P.; Ketabi, A.; Valcheva, R.; Gänzle, M.G.; Dieleman, L.A. Chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats. PLoS ONE 2014, 9, e111717. [Google Scholar] [CrossRef]
- Hoentjen, F.; Welling, G.W.; Harmsen, H.J.; Zhang, X.; Snart, J.; Tannock, G.W.; Lien, K.; Churchill, T.A.; Lupicki, M.; Dieleman, L.A. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm. Bowel Dis. 2005, 11, 977–985. [Google Scholar] [CrossRef]
- Lara-Villoslada, F.; de Haro, O.; Camuesco, D.; Comalada, M.; Velasco, J.; Zarzuelo, A.; Xaus, J.; Galvez, J. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur. J. Nutr. 2006, 45, 418–425. [Google Scholar] [CrossRef]
- Arribas, B.; Suárez-Pereira, E.; Ortiz Mellet, C.; García Fernández, J.M.; Buttersack, C.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, N.; Bailon, E.; Guerra-Hernández, E.; Zarzuelo, A.; et al. Di-D-fructose dianhydride-enriched caramels: Effect on colon microbiota, inflammation, and tissue damage in trinitrobenzenesulfonic acid-induced colitic rats. J. Agric. Food Chem. 2010, 58, 6476–6484. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Rawi, M.H.; Abdullah, A.; Ismail, A.; Sarbini, S.R. Manipulation of gut microbiota using Acacia gum polysaccharide. ACS Omega 2021, 6, 17782–17797. [Google Scholar] [CrossRef]
- Petersen, A.; Bergström, A.; Andersen, J.B.; Hansen, M.; Lahtinen, S.J.; Wilcks, A.; Licht, T.R. Analysis of the intestinal microbiota of oligosaccharide fed mice exhibiting reduced resistance to Salmonella infection. Benef. Microbes. 2010, 1, 271–281. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Stachowska, E.; Maciejewska, D.; Palma, J.; Jamioł-Milc, D.; Szczuko, M.; Marlicz, W.; Wunsch, E.; Skonieczna-Żydecka, K. Improvement of bowel movements among people with a sedentary lifestyle after prebiotic snack supply–preliminary study. Gastroenterol. Rev. 2022, 17, 73–80. [Google Scholar] [CrossRef]
- Marsaux, B.; Van den Abbeele, P.; Ghyselinck, J.; Prioult, G.; Marzorati, M.; Bogićević, B. Synbiotic effect of Bifidobacterium lactis CNCM I-3446 and bovine milk-derived oligosaccharides on infant gut microbiota. Nutrients 2020, 12, 2268. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, pre-biotics and synbiotics in the treatment of pre-diabetes: A systematic review of randomized controlled trials. Front. Public Health 2021, 9, 645035. [Google Scholar] [CrossRef]
- Zeinali, F.; Aghaei Zarch, S.M.; Vahidi Mehrjardi, M.Y.; Kalantar, S.M.; Jahan-Mihan, A.; Karimi-Nazari, E.; Fallahzadeh, H.; Hosseinzadeh-Shamsi-Anar, M.; Rahmanian, M.; Fazeli, M.R.; et al. Effects of synbiotic supplementation on gut microbiome, serum level of TNF-α, and expression of microRNA-126 and microRNA-146a in patients with type 2 diabetes mellitus: Study protocol for a double-blind controlled randomized clinical trial. Trials 2020, 21, 324. [Google Scholar] [CrossRef]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Szajewska, H.; et al. Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef]
- Miller, B.; Mainali, R.; Nagpal, R.; Yadav, H. A Newly. Developed synbiotic yogurt prevents diabetes by improving the microbiome–intestine–pancreas axis. Int. J. Mol. Sci. 2021, 22, 1647. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y.A. Potential synbiotic strategy for the prevention of type 2 diabetes: Lactobacillus paracasei JY062 and exopolysaccharide isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Mejía-León, M.E.; Barca, A.M. Diet, microbiota and immune system in type 1 diabetes development and evolution. Nutrients 2015, 7, 5461. [Google Scholar] [CrossRef] [PubMed]
- Craciun, C.-I.; Neag, M.-A.; Catinean, A.; Mitre, A.-O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.-D.; Muntean, D.-M.; Craciun, A.-E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, P.; Jafri, I.; van Overveld, F.J.; Rijkers, G.T. The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev. Clin. Immunol. 2013, 9, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Jayaswal, R.P.; Vijayasimha, M.; Prabhakar, P.K. Gut microbiota and diabetes mellitus—An interlinkage. Asian J. Pharm. Clin. Res. 2018, 11, 13–16. [Google Scholar] [CrossRef][Green Version]
- Brenner, S.R. Blue-green algae or cyanobacteria in the intestinal micro-flora may produce neurotoxins such as beta-N-methylamino-L-alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson-Dementia-Complex in humans and Equine Motor Neuron Disease in horses. Med. Hypotheses 2013, 80, 103. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).