The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect
Abstract
:1. Introduction
2. Depression Mechanism and Existing Treatment Options
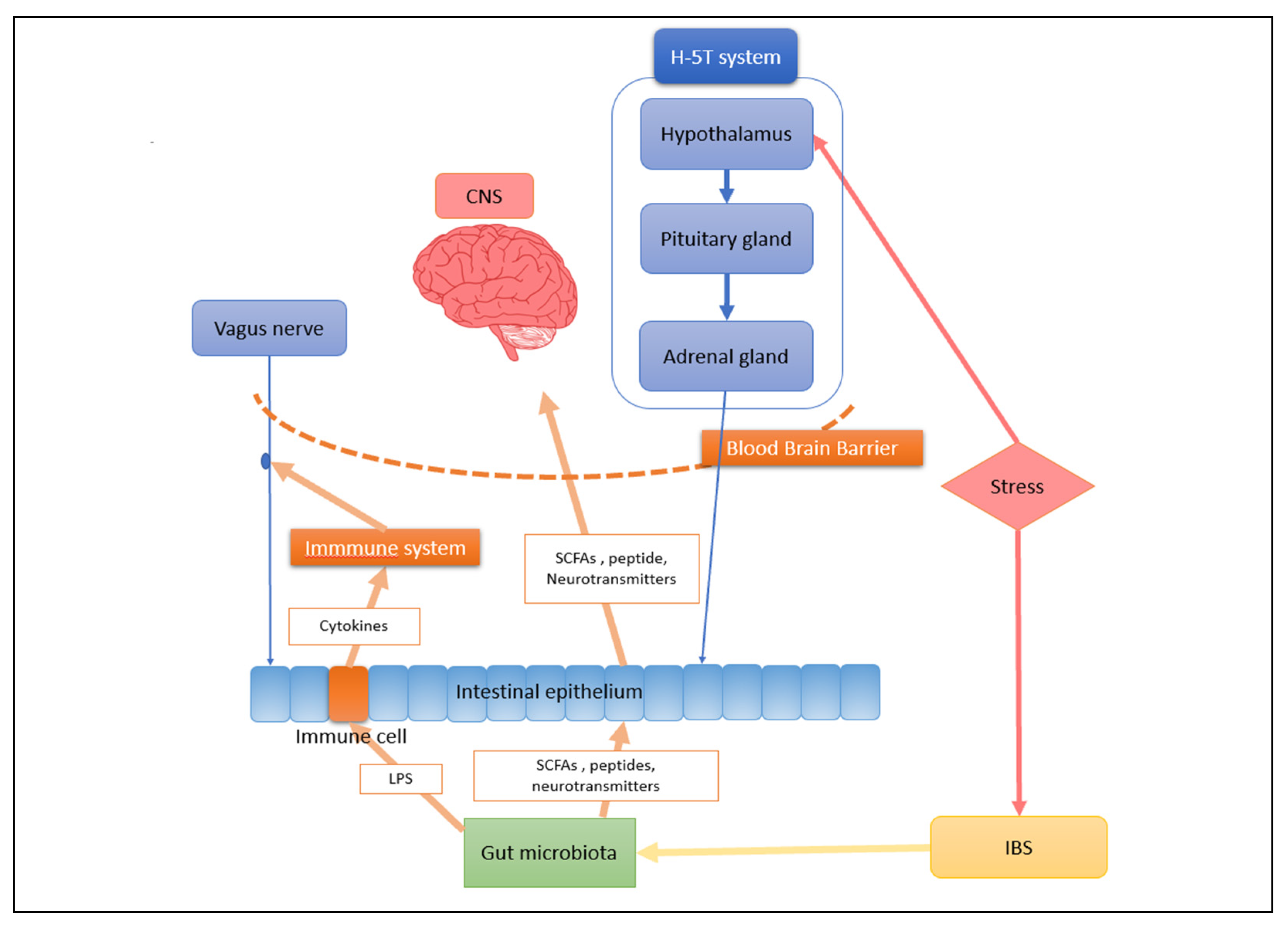
3. The Crucial Role the Gut Microbiota and Gut–Brain Axis Play in Central Nervous System Diseases
4. The Gut Microbiota and Microbes Interact Bidirectionally in People with Depression
4.1. Variation Occurs in the Intestinal Microbiota of Depression Patients
4.2. Intestinal Microbiota Can Regulate CNS in Depression Patients
5. Treatments of Depression
5.1. Traditional Treatment
5.2. Diet and Prebiotics
5.3. Psychobiotics
5.4. Engineered Bacteria
5.5. Fecal Microbiota Transplantation (FMT)
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowes, L.; Joinson, C.; Wolke, D.; Lewis, G. Peer victimisation during adolescence and its impact on depression in early adulthood: Prospective cohort study in the United Kingdom. BMJ 2015, 350, h2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klengel, T.; Binder, E.B. Gene-environment interactions in major depressive disorder. Can. J. Psychiatry 2013, 58, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Bennabi, D.; Aouizerate, B.; El-Hage, W.; Doumy, O.; Moliere, F.; Courtet, P.; Nieto, I.; Bellivier, F.; Bubrovsky, M.; Vaiva, G.; et al. Risk factors for treatment resistance in unipolar depression: A systematic review. J. Affect. Disord. 2015, 171, 137–141. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharm. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar]
- Aziz, M.N.M.; Kumar, J.; Nawawi, K.N.M.; Ali, R.A.R.; Mokhtar, N.M. Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain. Nutrients 2021, 13, 3061. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Konturek, S.; Konturek, P.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55, 137–154. [Google Scholar]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/depression#:~:text=Depression%20is%20a%20common%20mental%20disorder.%20Globally%2C%20more,More%20women%20are%20affected%20by%20depression%20than%20men (accessed on 13 September 2021).
- Li, B.J.; Friston, K.; Mody, M.; Wang, H.N.; Lu, H.B.; Hu, D.W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. 2018, 24, 1004–1019. [Google Scholar] [CrossRef]
- Herzog, D.P.; Wagner, S.; Engelmann, J.; Treccani, G.; Dreimüller, N.; Müller, M.B.; Tadic, A.; Murck, H.; Lieb, K. Early onset of depression and treatment outcome in patients with major depressive disorder. J. Psychiatr. Res. 2021, 139, 150–158. [Google Scholar] [CrossRef]
- Edwards, V.J.; Holden, G.W.; Felitti, V.J.; Anda, R.F. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. Am. J. Psychiatry 2003, 160, 1453–1460. [Google Scholar] [CrossRef]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Dube, S.R.; Anda, R.F.; Felitti, V.J.; Chapman, D.P.; Williamson, D.F.; Giles, W.H. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA 2001, 286, 3089–3096. [Google Scholar] [CrossRef] [Green Version]
- Alesci, S.; Martinez, P.E.; Kelkar, S.; Ilias, I.; Ronsaville, D.S.; Listwak, S.J.; Ayala, A.R.; Licinio, J.; Gold, H.K.; Kling, M.A.; et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: Clinical implications. J. Clin. Endocrinol. Metab. 2005, 90, 2522–2530. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut–brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Diamond, D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002, 3, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Brovedani, P. The Hippocampus, Neurotrophic Factors and Depression. CNS Drugs 2011, 25, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocr. 2019, 54, 100746. [Google Scholar] [CrossRef]
- Shamsuddin, K.; Fadzil, F.; Ismail, W.S.; Shah, S.A.; Omar, K.; Muhammad, N.A.; Jaffar, A.; Ismail, A.; Mahadevan, R. Correlates of depression, anxiety and stress among Malaysian university students. Asian J. Psychiatr. 2013, 6, 318–323. [Google Scholar] [CrossRef]
- Leucht, S.; Hierl, S.; Kissling, W.; Dold, M.; Davis, J.M. Putting the efficacy of psychiatric and general medicine medication into perspective: Review of meta-analyses. Br. J. Psychiatry 2012, 200, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Rush, A.J.; Kraemer, H.C.; Sackeim, H.A.; Fava, M.; Trivedi, M.H.; Frank, E.; Ninan, P.T.; Thase, M.E.; Gelenberg, A.J.; Kupfer, D.J.; et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006, 31, 1841–1853. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar]
- Moszak, M.; Szulinska, M.; Bogdanski, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Bocci, V. The neglected organ: Bacterial flora has a crucial immunostimulatory role. Perspect. Biol. Med. 1992, 35, 251–260. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.W.; Liu, Y.Y. Comparative study of classifiers for human microbiome data. Med. Microecol. 2020, 4, 100013. [Google Scholar] [CrossRef]
- Shen, G.; Wu, J.; Ye, B.C.; Qi, N. Gut Microbiota-Derived Metabolites in the Development of Diseases. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6658674. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Morais, L.H.; Schreiber, H.L.t.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Li, S.; Wei, J.; Chen, T. Editorial: Gut Microbiota in the Occurrence, Development and Treatment of Gut-Brain Disorders. Front. Cell Infect. Microbiol. 2021, 11, 808454. [Google Scholar] [CrossRef]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the Mammalian gut-brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.M.; Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Pedrogo, D.A.M.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.F.; Ma, C.L.; Wei, H.; Li, B.M.; Luo, J. Alleviation of Anxiety/Depressive-Like Behaviors and Improvement of Cognitive Functions by Lactobacillus plantarum WLPL04 in Chronically Stressed Mice. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6613903. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Y.; Li, C.; Li, N.; Zhu, S.; Tan, X.; Li, M.; Zhang, Y.; Xu, Z.; Ding, Z.; et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC epsilon levels in mouse. Biofactors 2020, 46, 38–54. [Google Scholar] [CrossRef]
- Pu, Y.; Tan, Y.; Qu, Y.; Chang, L.; Wang, S.; Wei, Y.; Wang, X.; Hashimoto, K. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav. Immun. 2021, 94, 318–326. [Google Scholar] [CrossRef]
- Dalile, B.; van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.J.; Zhu, L.; et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [Green Version]
- Sommansson, A.; Nylander, O.; Sjöblom, M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 2013, 54, 282–291. [Google Scholar] [CrossRef]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [Green Version]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Mayer, S.E.; Lopez-Duran, N.L.; Sen, S.; Abelson, J.L. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology 2018, 92, 57–65. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.A.M. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Balchin, R.; Linde, J.; Blackhurst, D.; Rauch, H.L.; Schönbächler, G. Sweating away depression? The impact of intensive exercise on depression. J. Affect. Disord. 2016, 200, 218–221. [Google Scholar] [CrossRef]
- Daley, A.J.; Foster, L.; Long, G.; Palmer, C.; Robinson, O.; Walmsley, H.; Ward, R. The effectiveness of exercise for the prevention and treatment of antenatal depression: Systematic review with meta-analysis. BJOG 2015, 122, 57–62. [Google Scholar] [CrossRef]
- Croarkin, P.E.; Nakonezny, P.A.; Deng, Z.D.; Romanowicz, M.; Voort, J.L.V.; Camsari, D.D.; Schak, K.M.; Port, J.D.; Lewis, C.P. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J. Affect. Disord. 2018, 239, 282–290. [Google Scholar] [CrossRef]
- Zhang, E.; Liao, P. Brain-derived neurotrophic factor and post-stroke depression. J. Neurosci. Res. 2020, 98, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostert, J.P.; Koch, M.W.; Heerings, M.; Heersema, D.J.; de Keyser, J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci. 2008, 14, 153–164. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; Whitworth, L.A.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Liu, S.; Zhang, W.; Zhu, H.; Tao, Q.; Wang, H.; Yan, H. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: Intestinal microbiota and gut microbiome function. Food Funct. 2019, 10, 5886–5897. [Google Scholar] [CrossRef]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef]
- Yan, T.; Nian, T.; Liao, Z.; Xiao, F.; Wu, B.; Bi, K.; He, B.; Jia, Y. Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int. J. Biol. Macromol. 2020, 144, 427–440. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Akbari, E.; Asemi, Z.; Kakhaki, R.D.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Bachner, H.A. Focus: Microbiome: Integrative Therapies in Anxiety Treatment with Special Emphasis on the Gut Microbiome. Yale J. Biol. Med. 2016, 89, 397. [Google Scholar]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef] [Green Version]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Buffington, S.A.; di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Q.; Liu, H.; Yang, Y.; Wang, Y.; Xia, C.; Tian, P.; Wei, J.; Li, S.; Chen, T. Probiotic Supplement Preparation Relieves Test Anxiety by Regulating Intestinal Microbiota in College Students. Dis. Markers 2021, 2021, 5597401. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented Milk Containing Lactobacillus casei Strain Shirota Preserves the Diversity of the Gut Microbiota and Relieves Abdominal Dysfunction in Healthy Medical Students Exposed to Academic Stress. Appl. Env. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Brown, L.C.; Cockburn, C.L.; Eyre, H.A. An Introduction to Antidepressant Pharmacomicrobiomics and Implications in Depression. In Convergence Mental Health: A Transdisciplinary Approach to Innovation; Oxford University Press: Oxford, UK, 2021; p. 329. [Google Scholar]
- Luhavaya, H.; Sigrist, R.; Chekan, J.R.; McKinnie, S.M.; Moore, B.S. Biosynthesis of l-4-chlorokynurenine, an antidepressant prodrug and a non-proteinogenic amino acid found in lipopeptide antibiotics. Angew. Chem. 2019, 131, 8482–8487. [Google Scholar]
- Rafferty, J.; Nagaraj, H.; McCloskey, A.P.; Huwaitat, R.; Porter, S.; Albadr, A.; Laverty, G. Peptide Therapeutics and the Pharmaceutical Industry: Barriers Encountered Translating from the Laboratory to Patients. Curr. Med. Chem. 2016, 23, 4231–4259. [Google Scholar] [CrossRef]
- Yin, L.; Yuvienco, C.; Montclare, J.K. Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials 2017, 134, 91–116. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; el Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Fond, G.B.; Lagier, J.C.; Honore, S.; Lancon, C.; Korchia, T.; de Verville, P.L.S.; Llorca, P.M.; Auquier, P.; Guedj, E.; Boyer, L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients 2020, 12, 1024. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.; Qiao, Y.; Xie, R.; Lin, L.; Jiang, J.; Wang, C.; Li, G. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J. Psychiatr. Res. 2021, 137, 147–157. [Google Scholar] [CrossRef]
- Yan, Z.X.; Gao, X.J.; Li, T.; Wei, B.; Wang, P.P.; Yang, Y.; Yan, R. Fecal Microbiota Transplantation in Experimental Ulcerative Colitis Reveals Associated Gut Microbial and Host Metabolic Reprogramming. Appl. Environ. Microbiol. 2018, 84, e00434-18. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Cheng, S.; Li, Y.; Wen, Z.; Ma, X.; Jiang, X.; Wang, Y.; Han, X. Faecal Microbiota Transplantation Reduces Susceptibility to Epithelial Injury and Modulates Tryptophan Metabolism of the Microbial Community in a Piglet Model. J. Crohns Colitis 2018, 12, 1359–1374. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Chen, V.L.; Steiner, C.A.; Berinstein, J.A.; Eswaran, S.; Waljee, A.K.; Higgins, P.D.R.; Owyang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1043–1050. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. Fecal Microbiota Transplantation and Its Usage in Neuropsychiatric Disorders. Clin. Psychopharmacol. Neurosci. 2016, 14, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, Z.; Shen, M.J.; Twardus, S.W.; Beuttler, M.M.; Chen, L.A.; Bateman-House, A. Fecal microbiota transplantation: Uses, questions, and ethics. Med. Microecol. 2020, 6, 100027. [Google Scholar] [CrossRef]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Kao, D.; Wong, K.; Franz, R.; Cochrane, K.; Sherriff, K.; Chui, L.; Lloyd, C.; Roach, B.; Bai, A.D.; Petrof, E.O.; et al. The effect of a microbial ecosystem therapeutic MET-2) on recurrent Clostridioides difficile infection: A phase 1, open-label, single-group trial. Lancet Gastroenterol. Hepatol. 2021, 6, 282–291. [Google Scholar] [CrossRef]
- Iqbal, U.; Anwar, H.; Karim, M.A. Safety and efficacy of encapsulated fecal microbiota transplantation for recurrent Clostridium difficile infection: A systematic review. Eur. J. Gastroenterol. Hepatol. 2018, 30, 730–734. [Google Scholar] [CrossRef]
- de Leon, L.M.; Watson, J.B.; Kelly, C.R. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2013, 11, 1036–1038. [Google Scholar] [CrossRef]
- Fischer, M.; Kao, D.; Kelly, C.; Kuchipudi, A.; Jafri, S.M.; Blumenkehl, M.; Rex, D.; Mellow, M.; Kaur, N.; Sokol, H.; et al. Fecal Microbiota Transplantation is Safe and Efficacious for Recurrent or Refractory Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 2402–2409. [Google Scholar] [CrossRef] [Green Version]
- Colman, R.J.; Rubin, D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohns Colitis 2014, 8, 1569–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author | Treatment | Experimental Subjects | Main Results |
|---|---|---|---|
| Yan et al. [88] | A polysaccharide (OP) which is isolated from okra (Abelmoschus esculentus (L) Moench) | CUMS-induced mice and fecal microbiological transplantation (FMT)-induced mice were used as models of depression | OP can treat depression through the microbial–gut–brain axis |
| Vulevic et al. [92] | Prebiotics-FOS1 and GOS | C57BL/6J male mice | Chronic prebiotics FOS1 GOS showed antidepressant and anti-anxiety effects |
| Tarr et al. [93] | Oligosaccharides 3′sialyllactose (3′SL) or 6′sialyllactose (6′SL) | 6~8-week-old male C57/BL6 mice used of the social disruption stressor | This prebiotic has a preventive effect on anxious behavior and inhibits nervous anxiety-related responses |
| Benton et al. [107] | Milk with probiotics | 124 healthy members of general population | There was an improvement in subjects, a positive effect on mood, and probiotic intake was also associated with demonstrated memory |
| Qin et al. [105] | Probiotic supplement preparation (PSP) | 120 college students with anxiety trend | Anxiety parameters decreased in the experimental group compared to the control group |
| Bravo et al. [100] | Probiotic Lactobacillus rhamnosus (JB-1) administration | Stress induced anxiety- and depression-like behaviors mice | Reduced stress-induced anxiety- and depression-like behaviors were found in mice |
| Messaoudi et al. [108] | Lactobacillus casei strain Shirota | Subjects’ mild depressive symptoms and low scores | Long-term combined use of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduced the symptoms of people with mild depression and effectively alleviated anxiety |
| Kang et al. [116] | Human fecal extract and FMT | Adult germ-free (GF), Swiss Webster female mice | Fecal bacteria transplantation can change the neural structure of the brain and affect the brain |
| Geng et al. [121] | FMT | Newborn piglets and subsequent lipopolysaccharide (LPS) challenge | FMT can regulate tryptophan metabolism and improve intestinal microbial disorders |
| Rao et al. [119] | FMT | Mice with chronic depression-like behavior induced by mild external stressors | FMT improves stress-induced depression-like behavior associated with inhibition of rat brain glial cells and NLRP3 inflammasome |
| Zhang et al. [122] | Transplantation of the NLRP3 KO microbiota from NLRP3 KO mice | NLRP3 KO mice | FMT from NLRP3 KO mice significantly alleviates the depressive-like behavior induced by chronic unpredictable stress (CUS)-induced depressive-like behaviors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. https://doi.org/10.3390/nu14102081
Zhu F, Tu H, Chen T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients. 2022; 14(10):2081. https://doi.org/10.3390/nu14102081
Chicago/Turabian StyleZhu, Fangyuan, Huaijun Tu, and Tingtao Chen. 2022. "The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect" Nutrients 14, no. 10: 2081. https://doi.org/10.3390/nu14102081
APA StyleZhu, F., Tu, H., & Chen, T. (2022). The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients, 14(10), 2081. https://doi.org/10.3390/nu14102081







