Active Compounds in Fruits and Inflammation in the Body
Abstract
1. Introduction
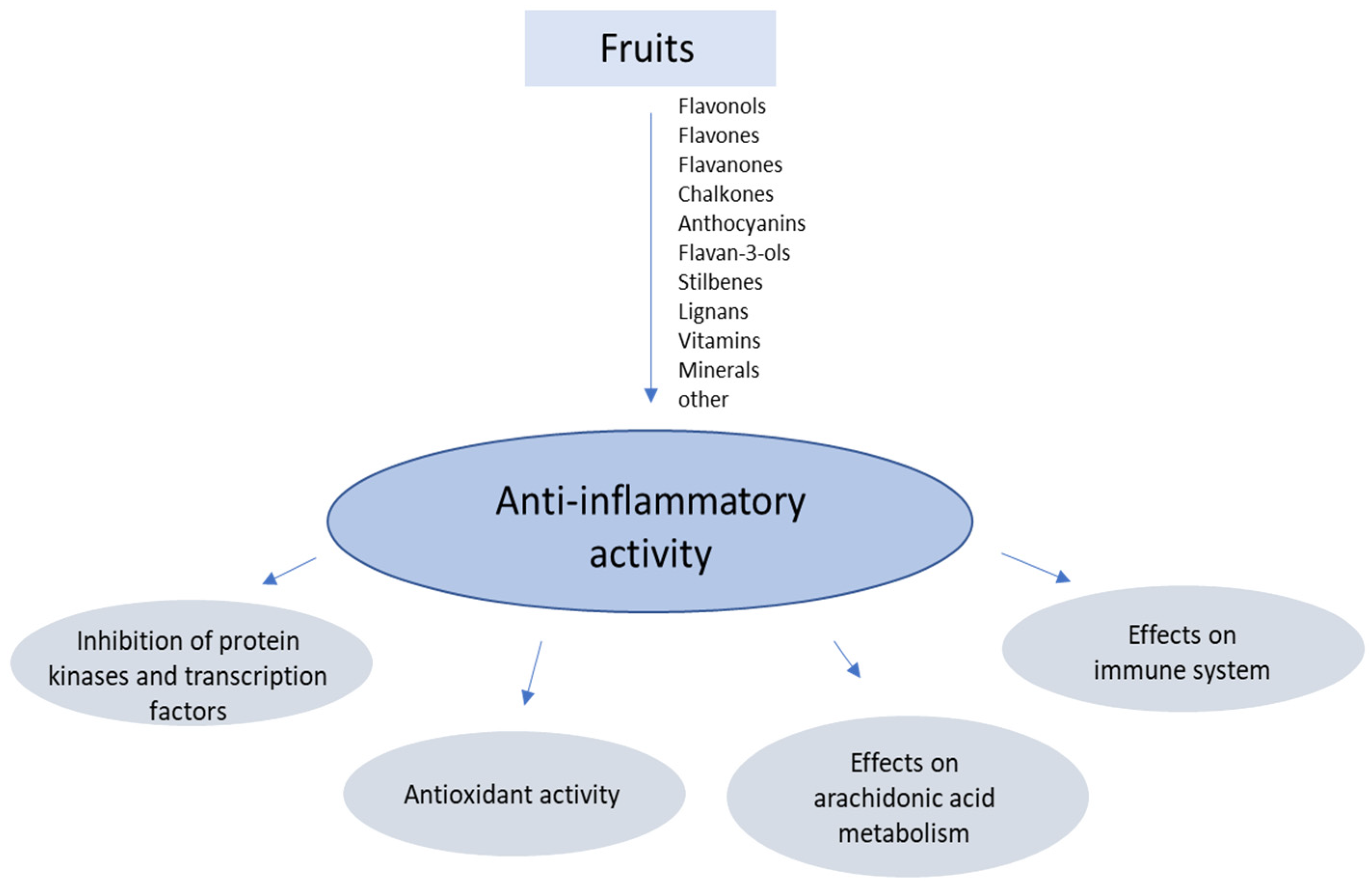
2. Targets of the Anti-Inflammatory Action
2.1. Inhibition of Protein Kinases and Transcription Factors
2.2. Antioxidant Activity
2.3. Effects on Arachidonic Acid Metabolism
2.4. Effects on the Immune System
3. Inflammation and Tumor Induction
4. Mechanism of the Anti-Inflammatory Action of Phenolic Compounds in Fruits
4.1. Flavonoids
4.1.1. Flavonols
4.1.2. Flavones
4.1.3. Flavanones
4.1.4. Chalcones
4.1.5. Anthocyanins
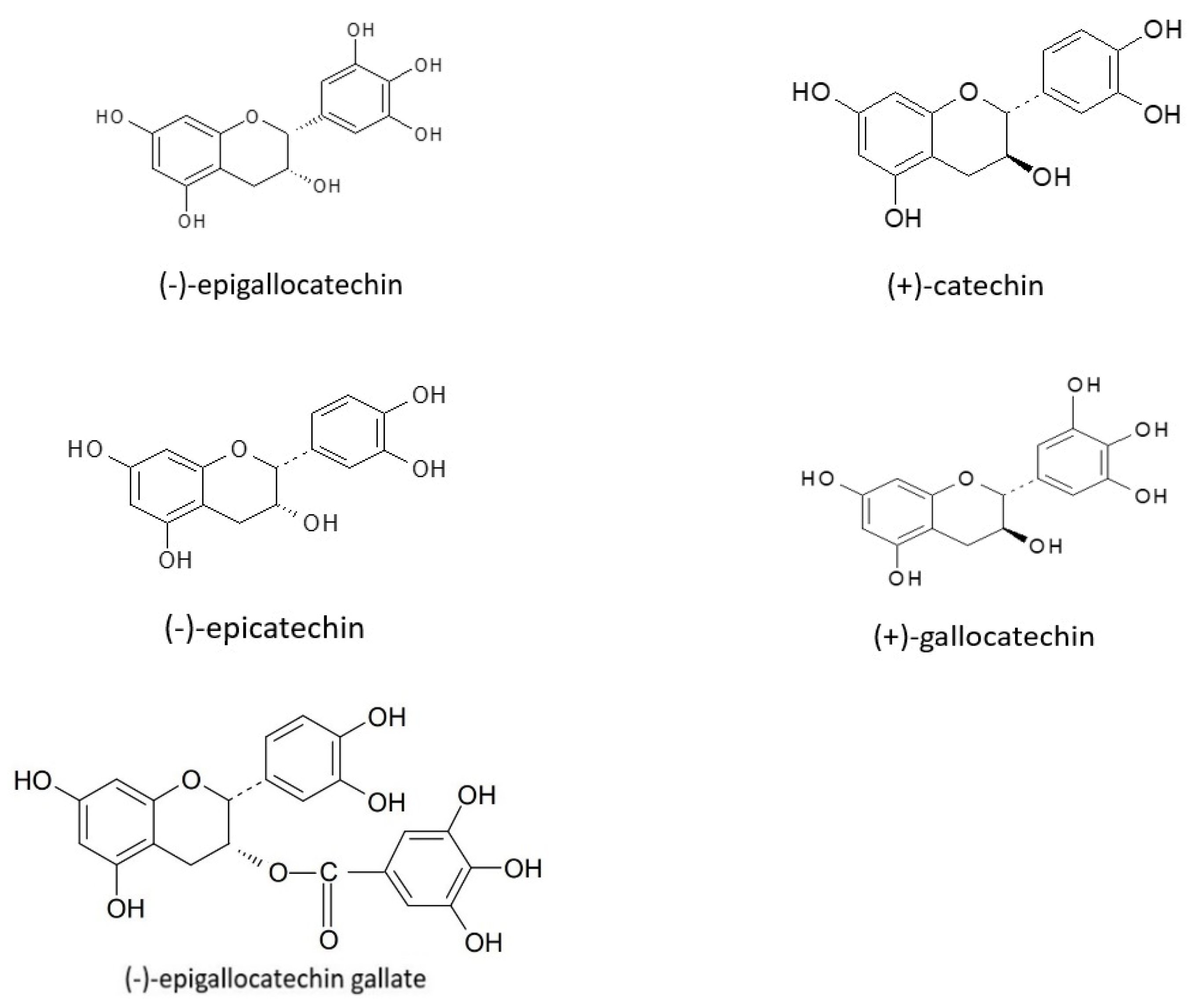
4.1.6. Flavan-3-ols
4.2. Elagitannins
4.3. Phenolic Acids
4.4. Stilbenes
4.5. Lignans
5. Mechanism of the Anti-Inflammatory Action of Essential Oil Components, Vitamins, Minerals, and Other Compounds in Fruits
5.1. Essential Oil Components
5.2. Vitamins
5.3. Minerals
5.4. Fiber
5.5. Other Compounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maximova, K.; Moez, E.K.; Dabravolskaj, J.; Ferdinands, A.R.; Dinu, I.; Siou, G.L.; Al Rajabi, A.; Veugelers, P.J. Co-consumption of Vegetables and Fruit, Whole Grains, and Fiber Reduces the Cancer Risk of Red and Processed Meat in a Large Prospective Cohort of Adults from Alberta’s Tomorrow Project. Nutrients 2020, 12, 2265. [Google Scholar] [CrossRef] [PubMed]
- Krusinska, B.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Drozdowski, M.; Chadzynski, T. Associations of Dietary Patterns and Metabolic-Hormone Profiles with Breast Cancer Risk: A Case-Control Study. Nutrients 2018, 10, 2013. [Google Scholar] [CrossRef] [PubMed]
- Van Breda, S.G.; De Kok, T.M. Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases. Mol. Nutr. Food Res. 2018, 62, 1700597. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-L.; Huang, W.-C.; Cheng, S.-C.; Liou, C.-J. Fisetin inhibits the generation of inflammatory mediators in interleukin-1β–induced human lung epithelial cells by suppressing the NF-κB and ERK1/2 pathways. Int. Immunopharmacol. 2018, 60, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2015, 56, 419–444. [Google Scholar] [CrossRef]
- Guo, L.; Liu, W.; Lu, T.; Guo, W.; Gao, J.; Luo, Q.; Wu, X.; Sun, Y.; Wu, X.; Shen, Y.; et al. Decrease of Functional Activated T and B Cells and Treatment of Glomerulonephitis in Lupus-Prone Mice Using a Natural Flavonoid Astilbin. PLoS ONE 2015, 10, e0124002. [Google Scholar] [CrossRef]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2006, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, M.; Wei, J.; Bhatt, S.; Paul, M.; Yakir, S.; Sampson, H.A. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J. Allergy Clin. Immunol. 2011, 128, 1242–1250.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Q.; Zhao, W.; Zhang, J.; Liu, W.; Huang, M.; Zeng, X. Oligomeric proanthocyanidins attenuate airway inflammation in asthma by inhibiting dendritic cells maturation. Mol. Immunol. 2017, 91, 209–217. [Google Scholar] [CrossRef]
- Del Bo’, C.; Cao, Y.; Roursgaard, M.; Riso, P.; Porrini, M.; Loft, S.; Møller, P. Anthocyanins and phenolic acids from a wild blueberry (Vaccinium angustifolium) powder counteract lipid accumulation in THP-1-derived macrophages. Eur. J. Nutr. 2016, 55, 171–182. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2014, 135, 1044–1052.e5. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005, 2, 518–525. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Song, X.-D.; Wang, Y.-N.; Zhang, A.; Liu, B. Advances in research on the interaction between inflammation and cancer. J. Int. Med. Res. 2020, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ritter, B.; Greten, F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019, 216, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of bioactive Compounds: A Review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef]
- Hopia, A.; Heinonen, M. Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J. Am. Oil Chem. Soc. 1999, 76, 139–144. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Deng, S.; Palu, K.; West, B.J.; Su, C.X.; Zhou, B.-N.; Jensen, J.C. Lipoxygenase Inhibitory Constituents of the Fruits of Noni (Morinda citrifolia) Collected in Tahiti. J. Nat. Prod. 2007, 70, 859–862. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Lange, M.L.B.; Sultana, R.; Butterfield, D.A. Nutritional Approaches to Modulate Oxidative Stress in Alzheimers Disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, T.P.; Määttä, K.R.; Hukkanen, A.T.; Kokko, H.I.; Törrönen, A.R.; Kärenlampi, S.O.; Karjalainen, R.O. Flavonol Content Varies among Black Currant Cultivars. J. Agric. Food Chem. 2001, 49, 3274–3277. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Marniemi, J.; Hakala, P.; Alfthan, G.; Meririnne, E.; Aro, A. Consumption of black currants, lingonberries and bilberries increases serum quercetin concentrations. Eur. J. Clin. Nutr. 2003, 57, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Park, S.-E.; Sapkota, K.; Choi, J.-H.; Kim, M.-K.; Kim, Y.H.; Kim, K.M.; Kim, K.J.; Oh, H.-N.; Kim, S.-J.; Kim, S. Rutin from Dendropanax morbifera Leveille Protects Human Dopaminergic Cells against Rotenone Induced Cell Injury Through Inhibiting JNK and p38 MAPK Signaling. Neurochem. Res. 2014, 39, 707–718. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farm. 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Aruna, R.; Geetha, A.; Suguna, P. Expression of caspase activation recruitment and pyrin domain levels of apoptosis-associated speck-like protein complex in the pancreas of rats subjected to experimental pancreatitis. Hum. Exp. Toxicol. 2013, 33, 940–948. [Google Scholar] [CrossRef]
- Mascaraque, C.; Aranda, C.; Ocón, B.; Monte, M.J.; Suárez, M.D.; Zarzuelo, A.; Marín, J.J.G.; Martínez-Augustin, O.; de Medina, F.S. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Selloum, L.; Bouriche, H.; Tigrine, C.; Boudoukha, C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003, 54, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Ku, S.-K.; Baek, Y.-D.; Bae, J.-S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Agents Actions 2013, 63, 197–206. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Nikfarjam, B.A.; Adineh, M.; Hajiali, F. Treatment with Rutin—A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases. J. Pharmacopunct. 2017, 20, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.T.; Takano-Ishikawa, Y.; Yamaki, K. Inhibitory Effect of Some Flavonoids on Tumor Necrosis Factor-αProduction in Lipopolysaccharide-Stimulated Mouse Macrophage Cell Line J774.1. J. Med. Food 2003, 6, 365–370. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Huang, Y.-T.; Tsai, S.-H.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 1999, 20, 1945–1952. [Google Scholar] [CrossRef]
- Kang, H.-K.; Ecklund, D.; Liu, M.; Datta, S.K. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res. Ther. 2009, 11, R59. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Felder, S.L.; Chhoker, S.; Edwards, J.Y.; Gros, R.; Huff, M.W. Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr mice. J. Lipid Res. 2013, 54, 711–724. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytother. Res. 2014, 29, 323–331. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, T.; Luo, Y.; Zhang, Y.; Xuan, H.; Ma, Y.; Pan, D.; Li, D.; Zhu, H. Luteolin Enhances Sarcoplasmic Reticulum Ca2+-ATPase Activity through p38 MAPK Signaling thus Improving Rat Cardiac Function after Ischemia/Reperfusion. Cell. Physiol. Biochem. 2017, 41, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, M. Antibacterial activity and mechanism of luteolin on Staphylococcus aureus. Acta Microbiol. Sin. 2010, 50, 1180–1184. [Google Scholar]
- Zhang, Q.; Yang, J.; Wang, J. Modulatory effect of luteolin on redox homeostasis and inflammatory cytokines in a mouse model of liver cancer. Oncol. Lett. 2016, 12, 4767–4772. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017, 39, 113–125. [Google Scholar] [CrossRef]
- Yao, Z.-H.; Yao, X.-L.; Zhang, Y.; Zhang, S.-F.; Hu, J.-C. Luteolin Could Improve Cognitive Dysfunction by Inhibiting Neuroinflammation. Neurochem. Res. 2018, 43, 806–820. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Shima, Y.; Ohshima, S.; Fujimoto, M.; Yamadori, T.; Kawase, I.; Tanaka, T. Flavonoids such as Luteolin, Fisetin and Apigenin Are Inhibitors of Interleukin-4 and Interleukin-13 Production by ActivatedHuman Basophils. Int. Arch. Allergy Immunol. 2004, 134, 135–140. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, Chalcones and Dihydrochalcones—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Hirai, S.; Kim, Y.-I.; Goto, T.; Kang, M.-S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.; Manchope, M.F.; Casagrande, R.; Verri, W.A. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Lätti, A.K.; Kainulainen, P.S.; Hayirlioglu-Ayaz, S.; Ayaz, F.A.; Riihinen, K.R. Characterization of Anthocyanins in Caucasian Blueberries (Vaccinium arctostaphylos L.) Native to Turkey. J. Agric. Food Chem. 2009, 57, 5244–5249. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Beekwilder, J.; Hall, R.D.; De Ric Vos, C.H. Identification and dietary relevance of antioxidants from raspberry. BioFactors 2005, 23, 197–205. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant Activity and Phenolic Content of Oregon Caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant Activity of Anthocyanins and Their Aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Ramesh, R. Mitogen-Activated Protein Kinases and Their Role in Radiation Response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-L.; Chen, P.-N.; Chu, S.-C.; Kuo, D.-Y.; Kuo, W.-H.; Chen, J.-Y.; Hsieh, Y.-S. Peonidin 3-Glucoside Inhibits Lung Cancer Metastasis by Downregulation of Proteinases Activities and MAPK Pathway. Nutr. Cancer 2010, 62, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate Fruit Extract Modulates UV-B-mediated Phosphorylation of Mitogen-activated Protein Kinases and Activation of Nuclear Factor Kappa B in Normal Human Epidermal Keratinocytes. Photochem. Photobiol. 2007, 81, 38–45. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Lyros, O.; Schmidt, J.L.; Jovanovic, N.; Nie, L.; Link, B.J.; Otterson, M.F.; Stoner, G.D.; Shaker, R.; Rafiee, P. Anti inflammatory and anti angiogenic effect of black raspberry extract on human esophageal and intestinal microvascular endothelial cells. Microvasc. Res. 2014, 97, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Beaux, D.; Fleurentin, J.; Mortier, F. Effect of Extracts of Orthosiphon Stamineus Benth, Hieracium Pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in Rats. Phytother. Res. PTR 1999, 13, 222–225. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2017, 40, 377–390. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A. Separation of Active and Toxic Portions in Sambucus ebulus. Pak. J. Biol. Sci. 2007, 10, 4171–4173. [Google Scholar] [CrossRef]
- Tall, J.; Seeram, N.P.; Zhao, C.; Nair, M.G.; Meyer, R.A.; Raja, S.N. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 2004, 153, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Yoshida, K.; Kase, S.; Suzuki, Y.; Ohno, S.; Jin, X.-H.; Kitaichi, N.; et al. Anti-inflammatory Effects of Aronia Extract on Rat Endotoxin-Induced Uveitis. Investig. Opthalmol. Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Paulrayer, A.; Adithan, A.; Lee, J.H.; Moon, K.H.; Kim, D.G.; Im, S.Y.; Kang, C.-W.; Kim, N.S.; Kim, J.-H. Aronia melanocarpa (Black Chokeberry) Reduces Ethanol-Induced Gastric Damage via Regulation of HSP-70, NF-κB, and MCP-1 Signaling. Int. J. Mol. Sci. 2017, 18, 1195. [Google Scholar] [CrossRef] [PubMed]
- Cebova, M.; Klimentova, J.; Janega, P.; Pechanova, O. Effect of Bioactive Compound of Aronia melanocarpaon Cardiovascular System in Experimental Hypertension. Oxidative Med. Cell. Longev. 2017, 2017, 8156594. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2011, 51, 563–572. [Google Scholar] [CrossRef]
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure–activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, A.S.; Gebhardt, S. Flavonoid Content of U.S. Fruits, Vegetables, and Nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Van De Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2003, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.B.; Zhang, Y.-T.; Du, Y.; Jiang, Y.-W. Preventive Effects of Catechins on Cardiovascular Disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, R.; Yoshigai, E.; Okuyama, T.; Mori, M.; Murase, H.; Machida, T.; Okumura, T.; Nishizawa, M. The Anti-Inflammatory Effects of Flavanol-Rich Lychee Fruit Extract in Rat Hepatocytes. PLoS ONE 2014, 9, e93818. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chiou, Y.-S.; Wang, Y.-J.; Ho, C.-T.; Lin, J.-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, Y.; Luo, X.; Ruan, Y.; Zhou, L.; Wang, Q.; Yan, Y.J.; Liu, Q.; Chen, J. Against NF-κB/thymic stromal lymphopoietin signaling pathway, catechin alleviates the inflammation in allergic rhinitis. Int. Immunopharmacol. 2018, 61, 241–248. [Google Scholar] [CrossRef]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical Composition of Caneberry (Rubus spp.) Seeds and Oils and Their Antioxidant Potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, Flavonoids, and Other Phenolics in Red Raspberries and Their Contribution to Antioxidant Capacity and Vasorelaxation Properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Jung, H.; Lee, S.-R.; Lee, H.-J.; Hwang, K.T.; Kim, T.-Y. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010, 123, 338–344. [Google Scholar] [CrossRef]
- Vuorela, S.; Kreander, K.; Karonen, M.; Nieminen, R.; Hämäläinen, M.; Galkin, A.; Laitinen, L.; Salminen, J.-P.; Moilanen, E.; Pihlaja, K.; et al. Preclinical Evaluation of Rapeseed, Raspberry, and Pine Bark Phenolics for Health Related Effects. J. Agric. Food Chem. 2005, 53, 5922–5931. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, I.C.O.; Seeram, N.P. Anti-inflammatory Effects of Polyphenolic-Enriched Red Raspberry Extract in an Antigen-Induced Arthritis Rat Model. J. Agric. Food Chem. 2011, 60, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and Anti-Inflammatory Activities of Tannin Fraction of the Extract from Black Raspberry Seeds Compared to Grape Seeds. J. Food Biochem. 2013, 38, 259–270. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Winand, J.; Hérent, M.-F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.-J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012, 3, 875–885. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Lim, J.W.; Hwang, H.J.; Shin, C.S. Polyphenol Compounds and Anti-inflammatory Activities of Korean Black Raspberry (Rubus coreanus Miquel) Wines Produced from Juice Supplemented with Pulp and Seed. J. Agric. Food Chem. 2012, 60, 5121–5127. [Google Scholar] [CrossRef] [PubMed]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, A.T.; Pölönen, S.S.; Kärenlampi, A.S.O.; Kokko, H.I. Antioxidant Capacity and Phenolic Content of Sweet Rowanberries. J. Agric. Food Chem. 2005, 54, 112–119. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic Acid Profiles in Some Small Berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; McPhee, D.J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Fu, L.; Li, C.; Xu, L.-P.; Zhang, L.-X.; Zhang, W.-M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Rozentsvit, A.; Vinokur, K.; Samuel, S.; Li, Y.; Gerdes, A.M.; Carrillo-Sepulveda, M.A. Ellagic Acid Reduces High Glucose-Induced Vascular Oxidative Stress Through ERK1/2/NOX4 Signaling Pathway. Cell. Physiol. Biochem. 2017, 44, 1174–1187. [Google Scholar] [CrossRef]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-inflammatory Effects of Ellagic Acid. J. PeriAnesthesia Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef]
- Rogerio, A.D.P.; Fontanari, C.; Melo, M.C.C.; Ambrosio, S.R.; de Souza, G.E.P.; Pereira, P.S.; Franca, S.; Da Costa, F.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory, analgesic and anti-oedematous effects of Lafoensia pacari extract and ellagic acid. J. Pharm. Pharmacol. 2006, 58, 1265–1273. [Google Scholar] [CrossRef]
- Kroes, B.H.; Berg, A.J.J.V.D.; van Ufford, H.C.Q.; van Dijk, H.; Labadie, R.P. Anti-Inflammatory Activity of Gallic Acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2017, 124, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-Y.; Mong, M.-C.; Chan, K.-C.; Yin, M.-C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-inflammatory Properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Fregoni, C.; Cantù, E.; Trevisan, M. Stilbene compounds: From the grapevine to wine. Drugs Under Exp. Clin. Res. 1999, 25, 57–63. [Google Scholar]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2018, 18, 37–67. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial activity of resveratrol structural analogues: A mechanistic evaluation of the structure-activity relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Wark, P.A.; Garg, M.L. Antioxidant and Anti-Inflammatory Effects of Resveratrol in Airway Disease. Antioxid. Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Buryanovskyy, L.; Fu, Y.; Boyd, M.; Ma, Y.; Hsieh, T.-C.; Wu, J.M.; Zhang, Z. Crystal Structure of Quinone Reductase 2 in Complex with Resveratrol, Biochemistry 2004, 43, 11417–11426. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Moreno, J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef]
- Zhong, L.-M.; Zong, Y.; Sun, L.; Guo, J.-Z.; Zhang, W.; He, Y.; Song, R.; Wang, W.-M.; Xiao, C.-J.; Lu, D. Resveratrol Inhibits Inflammatory Responses via the Mammalian Target of Rapamycin Signaling Pathway in Cultured LPS-Stimulated Microglial Cells. PLoS ONE 2012, 7, e32195. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.M.L.; de Oliveira, A.H.S.; Coutinho, L.G.; Fontes, F.L.; Oliveira, R.K.D.M.; Oliveira, T.T.; Faustino, A.L.F.; da Silva, V.L.; Campos, J.T.A.D.M.; Lajus, T.B.P.; et al. Resveratrol decreases the expression of genes involved in inflammation through transcriptional regulation. Free Radic. Biol. Med. 2018, 130, 8–22. [Google Scholar] [CrossRef]
- Lee, I.-T.; Lin, C.-F.; Huang, Y.-L.; Chong, K.-Y.; Hsieh, M.-F.; Huang, T.-H.; Cheng, C.-Y. Protective mechanisms of resveratrol derivatives against TNF-α-induced inflammatory responses in rat mesangial cells. Cytokine 2018, 113, 380–392. [Google Scholar] [CrossRef]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. A dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of oesophageal cancer. Br. J. Nutr. 2014, 112, 2002–2009. [Google Scholar] [CrossRef]
- Jin, M.; Moon, T.C.; Quan, Z.; Lee, E.; Kim, Y.K.; Yang, J.H.; Suh, S.-J.; Jeong, T.C.; Lee, S.H.; Kim, C.-H.; et al. The Naturally Occurring Flavolignan, Deoxypodophyllotoxin, Inhibits Lipopolysaccharide-Induced iNOS Expression through the NF-κB Activation in RAW264.7 Macrophage Cells. Biol. Pharm. Bull. 2008, 31, 1312–1315. [Google Scholar] [CrossRef]
- Chen, P.; Pang, S.; Yang, N.; Meng, H.; Liu, J.; Zhou, N.; Zhang, M.; Xu, Z.-H.; Gao, W.; Chen, B.; et al. Beneficial Effects of Schisandrin B on the Cardiac Function in Mice Model of Myocardial Infarction. PLoS ONE 2013, 8, e79418. [Google Scholar] [CrossRef]
- Huang, X.-X.; Bai, M.; Zhou, L.; Lou, L.-L.; Liu, Q.-B.; Zhang, Y.; Li, L.-Z.; Song, S.-J. Food Byproducts as a New and Cheap Source of Bioactive Compounds: Lignans with Antioxidant and Anti-inflammatory Properties from Crataegus pinnatifida Seeds. J. Agric. Food Chem. 2015, 63, 7252–7260. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Ko, Y.-J.; Yang, H.-M.; Ham, Y.-M.; Roh, S.W.; Jeon, Y.-J.; Ahn, G.; Kang, M.-C.; Yoon, W.-J.; Kim, D.; et al. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013, 57, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Elmadfa, I. Biological Relevance of Terpenoids. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.-Y.; Gao, W.-Y.; Wang, Y.; Wang, H.-Y.; Cao, J.-G.; Huang, L.-Q. Chemical Composition and Anti-inflammatory and Antioxidant Activities of Eight Pear Cultivars. J. Agric. Food Chem. 2012, 60, 8738–8744. [Google Scholar] [CrossRef]
- Mueller, D.; Triebel, S.; Rudakovski, O.; Richling, E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem. 2013, 139, 339–346. [Google Scholar] [CrossRef]
- Hakala, M.; Lapveteläinen, A.; Huopalahti, R.; Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compos. Anal. 2003, 16, 67–80. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J. Comparison of six cultivars of strawberries (Fragaria × ananassa Duch.) grown in northwest Poland. Eur. Food Res. Technol. 2004, 219, 66–70. [Google Scholar] [CrossRef]
- Rodriguez, M.A.R.; Oderiz, M.L.V.; Lopez-Hernandez, J.; Lozano, J.S. Determination of Vitamin C and Organic Acids in Various Fruits by HPLC. J. Chromatogr. Sci. 1992, 30, 433–437. [Google Scholar] [CrossRef]
- Najwa, F.R.; Azrina, A. Comparison of Vitamin C Content in Citrus Fruits by Titration and High Performance Liquid Chromatography (HPLC) Methods. Int. Food Res. J. 2017, 24, 726–733. [Google Scholar]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects their Phenolic and Vitamin C Composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ernawita; Thieme, C.; Westphal, A.; Malarski, A.; Böhm, V. Polyphenols, Vitamin C, in Vitro Antioxidant Capacity, α-Amylase and COX-2 Inhibitory Activities of Citrus Samples from Aceh, Indonesia. Int. J. Vitam. Nutr. Res. 2019, 89, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Nojavan, S.; Khalilian, F.; Kiaie, F.M.; Rahimi, A.; Arabanian, A.; Chalavi, S. Extraction and quantitative determination of ascorbic acid during different maturity stages of Rosa canina L. fruit. J. Food Compos. Anal. 2008, 21, 300–305. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; O Lowe, G.D.; Rumley, A.; Bruckdorfer, K.R.; Whincup, P. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006, 83, 567–574. [Google Scholar] [CrossRef]
- Van Straten, M.; Josling, P. Preventing the common cold with a vitamin C supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2002, 19, 151–159. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Wilson, J.X. Regulation of Vitamin C Transport. Annu. Rev. Nutr. 2005, 25, 105–125. [Google Scholar] [CrossRef]
- Ford, E.S.; Liu, S.; Mannino, D.; Giles, W.H.; Smith, S.J. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur. J. Clin. Nutr. 2003, 57, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.-S.; Ko, Y.-H.; Moon, Y.-S.; Sohn, S.-H. Effects of Vitamin C or E on the Pro-inflammatory Cytokines, Heat Shock Protein 70 and Antioxidant Status in Broiler Chicks under Summer Conditions. Asian-Australas. J. Anim. Sci. 2014, 27, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986. [Google Scholar] [CrossRef]
- Muraoka, K.-I.; Shimizu, K.; Sun, X.; Zhang, Y.K.; Tani, T.; Hashimoto, T.; Yagi, M.; Miyazaki, I.; Yamamoto, K.-I. Hypoxia, but not reoxygenation, induces interleukin 6 gene expression through NF-Kappa B activation. Transplantation 1997, 63, 466–470. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2018, 71, 487–494. [Google Scholar] [CrossRef]
- Nazrun, A.S.; Norazlina, M.; Norliza, M.; Nirwana, S.I. The Anti-Inflammatory Role of Vitamin E in Prevention of Osteoporosis. Adv. Pharmacol. Sci. 2011, 2012, 142702. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla, B.; Blanco, I.; Rojas-Hidalgo, E. Major Fruit and Vegetable Contributors to the Main Serum Ca-rotenoids in the Spanish Diet. Eur. J. Clin. Nutr. 1996, 50, 246–250. [Google Scholar]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla-Alonso, B.; Granado, F.; Blanco, I.; Berg, H.V.D.; Hininger, I.; Rousell, A.-M.; Chopra, M.; et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A. Dietary carotenoids and human immune function. Nutrition 2001, 17, 823–827. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.-Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Institute of Medicine. Military Strategies for Sustainment of Nutrition and Immune Function in the Field; The National Academies Press: Washington, DC, USA, 1999; p. 722. [Google Scholar]
- Hiemstra, I.H.; Beijer, M.R.; Veninga, H.; Vrijland, K.; Borg, E.G.F.; Olivier, B.J.; Mebius, R.E.; Kraal, G.; Haan, J.M.M.D. The identification and developmental requirements of colonic CD169+ macrophages. Immunology 2014, 142, 269–278. [Google Scholar] [CrossRef]
- Twining, S.S.; Schulte, D.P.; Wilson, P.M.; Zhou, X.; Fish, B.L.; Moulder, J.E. Neutrophil cathepsin G is specifically decreased under vitamin A deficiency. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1996, 1317, 112–118. [Google Scholar] [CrossRef][Green Version]
- Yuen, D.E.; Stratford, A.F. Vitamin A Activation of Transforming Growth Factor-β1 Enhances Porcine Ileum Wound Healing In Vitro. Pediatr. Res. 2004, 55, 935–939. [Google Scholar] [CrossRef]
- Semba, R.; West, K.P.; Sommer, A.; Griffin, D.; Ward, B.; Scott, A.; Muhilal; Natadisastra, G. Abnormal T-cell subset proportions in vitamin-A-deficient children. Lancet 1993, 341, 5–8. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; A Tanumihardjo, S. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. Int. Rev. J. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Albasini, A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007, 100, 419–427. [Google Scholar] [CrossRef]
- Hardisson, A.; Rubio, C.; Baez, A.; Martin, M.; Alvarez, R.; Diaz, E. Mineral composition of the banana (Musa acuminata) from the island of Tenerife. Food Chem. 2001, 73, 153–161. [Google Scholar] [CrossRef]
- Bo, S.; Durazzo, M.; Guidi, S.; Carello, M.; Sacerdote, C.; Silli, B.; Rosato, R.; Cassader, M.; Gentile, L.; Pagano, G. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am. J. Clin. Nutr. 2006, 84, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium Intake, C-Reactive Protein, and the Prevalence of Metabolic Syndrome in Middle-Aged and Older U.S. Women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.Y.; van Dam, R.; E Manson, J.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, W.H. Nutritional quality of 18 date fruit varieties. Int. J. Food Sci. Nutr. 2011, 62, 544–551. [Google Scholar] [CrossRef]
- Grembecka, M.; Szefer, P. Comparative assessment of essential and heavy metals in fruits from different geographical origins. Environ. Monit. Assess. 2013, 185, 9139–9160. [Google Scholar] [CrossRef]
- De Domenico, I.; Zhang, T.Y.; Koening, C.L.; Branch, R.W.; London, N.; Lo, E.; Daynes, R.A.; Kushner, J.P.; Li, D.; Ward, D.M.; et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J. Clin. Investig. 2010, 120, 2395–2405. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Corna, G.; Bosurgi, L.; Rovere-Querini, P.; Camaschella, C.; Silvestri, L. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood 2011, 118, 736–746. [Google Scholar] [CrossRef]
- Sprietsma, J. Modern diets and diseases: NO–zinc balance. Med. Hypotheses 1999, 53, 6–16. [Google Scholar] [CrossRef]
- Kocabaş, C.N.; Adalioglu, G.; Coşkun, T.; Tuncer, A.; Sekerel, B.E. The relationship between serum selenium levels and frequent wheeze in children. Turk. J. Pediatr. 2007, 48, 308–312. [Google Scholar]
- Guoa, C.-H.; Wangb, C.-L.; Chen, P.-C.; Yang, T.-C. Linkage of Some Trace Elements, Peripheral Blood Lymphocytes, Inflammation, and Oxidative Stress in Patients Undergoing Either Hemodialysis or Peritoneal Dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2011, 31, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2019, 78, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside Suppresses Lipopolysaccharide-Induced Inflammatory Mediators by Inhibiting Nuclear Factor-Kappa B Activation in RAW 264.7 Macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef]
- Fu, G.X.; Li, J.M.; Zhou, Y.; Zhao, S.P. Anti-inflammatory and immune suppressive effects of Conus officinalis glucosides in rats. Chin. J. Microbiol. Immunol. 2007, 27, 16–320. [Google Scholar]
- Sung, Y.-H.; Chang, H.-K.; Kim, S.-E.; Kim, Y.-M.; Seo, J.-H.; Shin, M.-C.; Shin, M.-S.; Yi, J.-W.; Shin, N.-H.; Kim, H.; et al. Anti-Inflammatory and Analgesic Effects of the Aqueous Extract of Corni Fructus in Murine RAW 264.7 Macrophage Cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef]
- Zhu, Q.; Liao, C.; Liu, Y.; Wang, P.; Guo, W.; He, M.; Huang, Z. Ethanolic extract and water-soluble polysaccharide from Chaenomeles speciosa fruit modulate lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. J. Ethnopharmacol. 2012, 144, 441–447. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, J.-A.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of muscle wasting by the plant-derived compound ursolic acid in a model of chronic kidney disease. J. Cachex Sarcopenia Muscle 2016, 8, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.; Kharazmi, A.; Christensen, L.P.; Christensen, S.B. An Antiinflammatory Galactolipid from Rose Hip (Rosa canina) that Inhibits Chemotaxis of Human Peripheral Blood Neutrophils in Vitro. J. Nat. Prod. 2003, 66, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Christensen, R.; Bartels, E.; Altman, R.; Astrup, A.; Bliddal, H. Does the hip powder of Rosa canina (rosehip) reduce pain in osteoarthritis patients? A meta-analysis of randomized controlled trials. Osteoarthr. Cartil. 2008, 16, 965–972. [Google Scholar] [CrossRef]
- Petcharat, A.; Wongsuphasawat, K. The efficacy of a standardized Rose-hip powder containing seeds and shells compared with glucosamine sulfate in patients with osteoarthritis of the knee—A blinded, parallel, randomized study. Osteoarthr. Cartil. 2013, 21, S216. [Google Scholar] [CrossRef]
- Willich, S.; Rossnagel, K.; Roll, S.; Wagner, A.; Mune, O.; Erlendson, J.; Kharazmi, A.; Sörensen, H.; Winther, K. Rose hip herbal remedy in patients with rheumatoid arthritis—A randomised controlled trial. Phytomedicine 2010, 17, 87–93. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 |
| Kaemferol | H | H | H | H | OH |
| Kaemferol 3-O-glucoside | H | H | H | H | O-glucose |
| Kaemferol 7-O-glucoside | glucose | H | H | H | OH |
| Kaemferol 7-O-rhamnoside | rhamnose | H | H | H | OH |
| Quercetin | H | OH | H | H | OH |
| Quercetin 3-O-glucoside | H | OH | H | H | O-glucose |
| Quercetin 3-O-galactoside | H | OH | H | H | O-galactose |
| Quercetin 3-O-rhamnoside | H | OH | H | H | O-rhamnose |
| Quercetin 7-O-glucoside | glucose | OH | H | H | OH |
| Rutin | H | OH | H | H | O-rutinose |
| Quercetin 3-O-glucuronide | H | OH | H | H | O-glucuronic acid |
| Apigenin | H | H | H | H | H |
| Luteolin | H | OH | H | H | H |
| Myricetin | H | OH | H | OH | OH |
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 |
| Cyanidin | H | OH | H | H |
| Cyjanidin 3-O-glucoside | glucose | OH | H | H |
| Cyjanidin 3-O-rutinoside | Glucose + rhamnose | OH | H | H |
| Cyjanidin 3-O-(2G-glukosylorutinoside) | glucose + rhamnoza + glucose | OH | H | H |
| Cyjanidin 3-O-soforoside | glucose + glucose | OH | H | H |
| Cyjanidin 3-O-sambubioside | OH | H | H | |
| Cyjanidin 3,5-O-diglucoside | glucose | OH | H | glucose |
| Pelargonidin 3-O-glucoside | glucose | H | H | H |
| Pelargonidin 3-O-rutinoside | glucose + rhamnose | H | H | H |
| Pelargonidin 3,5-O-diglucoside | glucose | H | H | glucose |
| Malvidin 3-O-glucoside | glucose | OCH3 | OCH3 | H |
| Delfinidin 3-O-glucoside | glucose | OH | OH | H |
 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 |
| benzoic acid | H | H | H | H | H |
| salicylic acid | OH | H | H | H | H |
| m-hydroxybenzoic acid | H | OH | H | H | H |
| p-hydroxybenzoic acid | H | H | OH | H | H |
| 2,3-dihydroxybenzoic acid | OH | OH | H | H | H |
| β-rezorcylic acid | OH | H | OH | H | H |
| gentysinic acid | OH | H | H | OH | H |
| γ-rezorcylic acid | OH | H | H | H | OH |
| protocatechic acid | H | OH | OH | H | H |
| α-rezorcylic acid | H | OH | H | OH | H |
| gallic acid | H | OH | OH | OH | H |
| 2,4-dimetoxybenzoic acid | OCH3 | H | OCH3 | H | H |
| veratric acid | H | OCH3 | OCH3 | H | H |
| vanillic acid | H | OCH3 | OH | H | H |
| syryngic acid | H | OCH3 | OH | OCH3 | H |
 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 |
| cinamonic acid | H | H | H | H |
| p-cumaric acid | H | H | OH | H |
| m-cumaric acid | OH | H | H | H |
| o-cumaric acid | H | OH | H | H |
| ferulic acid | H | OCH3 | OH | H |
| isoferulic acid | H | OH | OCH3 | H |
| caffeic acid | H | OH | OH | H |
| synapic acid | H | OCH3 | OH | OCH3 |
| 4-metoxycynamonic acid | H | H | OCH3 | H |
| 3,4-dimetoxycynamonic acid | H | OCH3 | OCH3 | H |
| 2,4-dimetoxycynamonic acid | OCH3 | H | OCH3 | H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majdan, M.; Bobrowska-Korczak, B. Active Compounds in Fruits and Inflammation in the Body. Nutrients 2022, 14, 2496. https://doi.org/10.3390/nu14122496
Majdan M, Bobrowska-Korczak B. Active Compounds in Fruits and Inflammation in the Body. Nutrients. 2022; 14(12):2496. https://doi.org/10.3390/nu14122496
Chicago/Turabian StyleMajdan, Magdalena, and Barbara Bobrowska-Korczak. 2022. "Active Compounds in Fruits and Inflammation in the Body" Nutrients 14, no. 12: 2496. https://doi.org/10.3390/nu14122496
APA StyleMajdan, M., & Bobrowska-Korczak, B. (2022). Active Compounds in Fruits and Inflammation in the Body. Nutrients, 14(12), 2496. https://doi.org/10.3390/nu14122496