The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews
Abstract
:1. Introduction
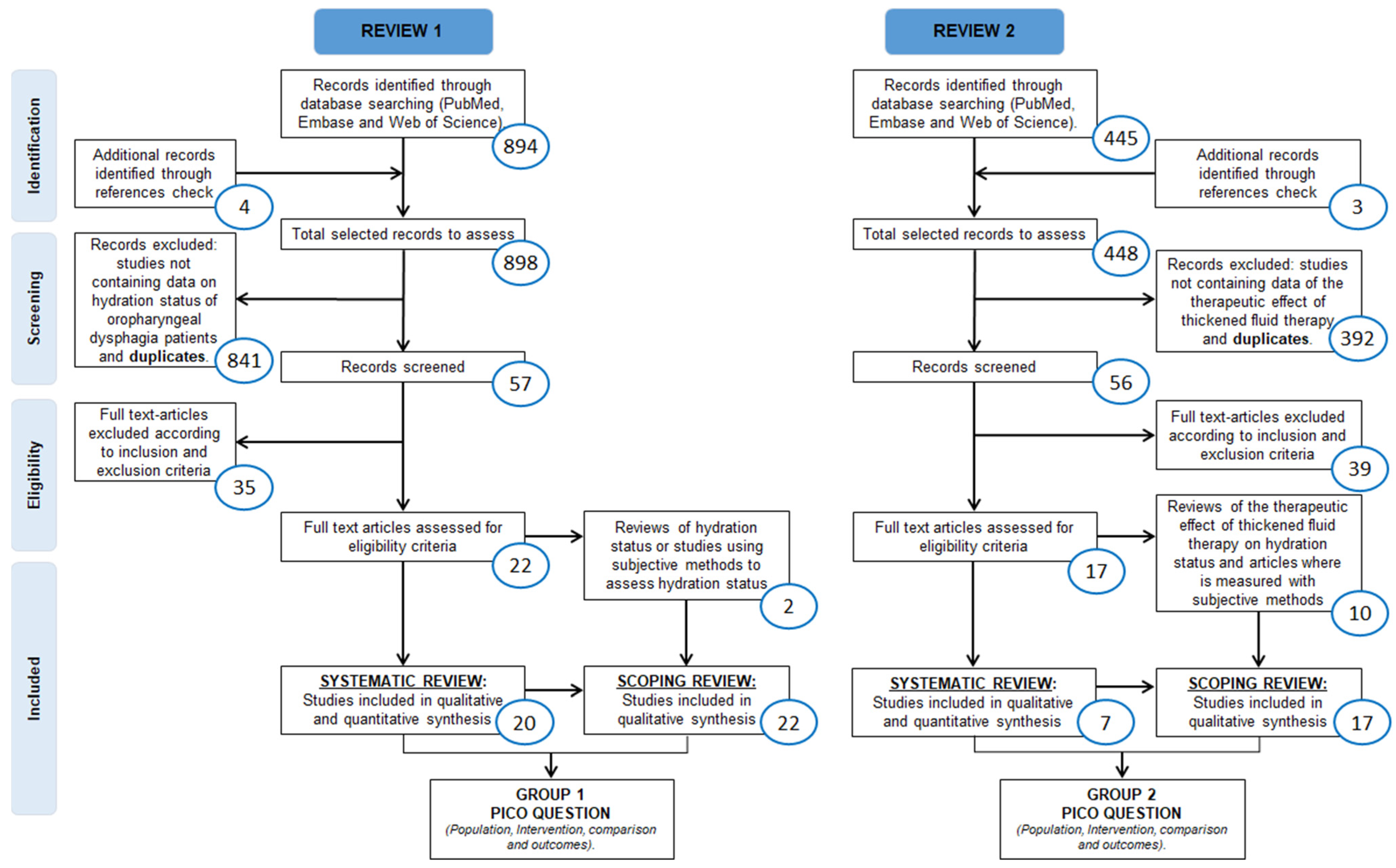
2. Materials and Methods
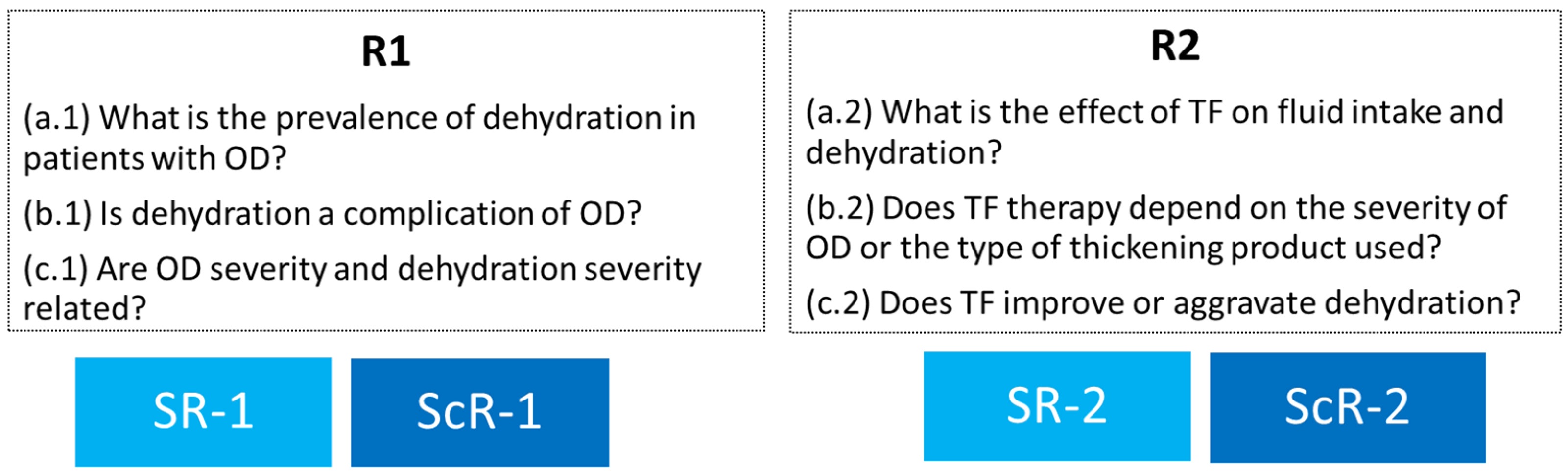
- Review 1 (SR + ScR)—Hydration status of patients with OD: a. (1) What is the prevalence of dehydration in adult patients with OD? b. (1) Is dehydration a complication of OD in adult patients with OD? c. (1) Is there a relationship between OD severity and dehydration severity?
- Review 2 (SR + ScR)—Effect of TF therapy on hydration status: a. (2) What is the effect of TF therapy on the fluid intake and hydration status of patients with OD? b. (2) Does the effect of TF therapy depend on the severity of OD or the type of thickening product used? c. (2) In patients with OD, does treatment with TF improve or aggravate dehydration?
2.1. Patient and Public Involvement Statement
2.2. Search Strategy
2.3. Eligibility Criteria and Selection Process
2.4. Data Presentation and Summary Measures
2.5. Quality Evaluation and Strength of the Evidence
3. Results
3.1. Review 1—Hydration Status of Patients with Oropharyngeal Dysphagia
3.1.1. Systematic Review (SR-1)
3.1.2. Scoping Review (ScR-1)
3.1.3. Synthesis of the Studies’ Findings on the Hydration Status of Patients with OD
3.1.4. Quality and Strength of Evidence across Studies
3.2. Review 2—Effect of Thickened Fluid Therapy on Hydration Status
3.2.1. Systematic Review (SR-2)
3.2.2. Scoping Review (ScR-2)
3.2.3. Synthesis of the Studies’ Findings
3.2.4. Quality and Strength of Evidence across Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lin, L.C.; Wu, S.C.; Chen, H.S.; Wang, T.G.; Chen, M.Y. Prevalence of impaired swallowing in institutionalized older people in taiwan. J. Am. Geriatr. Soc. 2002, 50, 1118–1123. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Hinojosa, G.; López, D.; Juan, M.; Fabré, E.; Voss, D.S.; Calvo, M.; Marta, V.; Ribó, L.; Palomera, E.; et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J. Am. Geriatr. Soc. 2011, 59, 186–187. [Google Scholar] [CrossRef]
- Baijens, L.W.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; del Nozal, J.M.; Ortega, O.; et al. European society for swallowing disorders-European union geriatric medicine society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-Prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol. Motil. 2010, 22, 851-e230. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Palomera, M.; Gomez, C.; Sar-Shalom, D.; Saiz, A.; Montoya, J.G.; Navajas, M.; Palomera, E.; Clavé, P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: A population-based prospective study. Age Ageing 2012, 41, 376–381. [Google Scholar] [CrossRef]
- Marin, S.; Serra-Prat, M.; Ortega, O.; Fericgla, M.A.; Valls, J.; Palomera, E.; Cunillera, R.; Palomeras, E.; Ibàñez, J.M.; Clavé, P. Healthcare costs of post-stroke oropharyngeal dysphagia and its complications: Malnutrition and respiratory infections. Eur. J. Neurol. 2021, 28, 3670–3681. [Google Scholar] [CrossRef]
- Carrión, S.; Roca, M.; Costa, A.; Arreola, V.; Ortega, O.; Palomera, E.; Serra-Prat, M.; Cabré, M.; Clavé, P. Nutritional status of older patients with oropharyngeal dysphagia in a chronic versus an acute clinical situation. Clin. Nutr. 2017, 36, 1110–1116. [Google Scholar] [CrossRef]
- Tomsen, N.; Ortega, O.; Nascimento, W.; Carrión, S.; Clavé, P. Oropharyngeal Dysphagia in Older People is Associated with Reduced Pharyngeal Sensitivity and Low Substance P and CGRP Concentration in Saliva. Dysphagia 2022, 37, 48–57. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, Assessment, and Performance Effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar]
- Reber, E.; Gomes, F.; Dähn, I.A.; Vasiloglou, M.F.; Stanga, Z. Management of Dehydration in Patients Suffering Swallowing Difficulties. J. Clin. Med. 2019, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Crary, M.A.; Carnaby, G.D.; Shabbir, Y.; Miller, L.; Silliman, S. Clinical Variables Associated with Hydration Status in Acute Ischemic Stroke Patients with Dysphagia. Dysphagia 2016, 31, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, A.; Baumoehl, Y.; Lubart, E.; Yaina, A.; Platinovitz, N.; Segal, R. Dehydration among Long-Term Care Elderly Patients with Oropharyngeal Dysphagia. Gerontology 2007, 53, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; De Kraa, M.; Arreola, V.; Girvent, M.; Farré, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment. Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef]
- Bolivar-Prados, M.; Rofes, L.; Arreola, V.; Guida, S.; Nascimento, W.V.; Martin, A.; Vilardell, N.; Ortega Fernández, O.; Ripken, D.; Lansink, M.; et al. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2019, 31, e13695. [Google Scholar] [CrossRef]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W.V.; Tomsen, N.; Gallegos, C.; Brito-de La Fuente, E.; Clavé, P. Therapeutic Effect, Rheological Properties and α-Amylase Resistance of a New Mixed Starch and Xanthan Gum Thickener on Four Different Phenotypes of Patients with Oropharyngeal Dysphagia. Nutrients 2020, 12, 1873. [Google Scholar] [CrossRef]
- Gallegos, C.; Brito-de la Fuente, E.; Clavé, P.; Costa, A.; Assegehegn, G. Nutritional Aspects of Dysphagia Management. Adv. Food Nutr. Res. 2017, 81, 271–318. [Google Scholar]
- Martín, A.; Ortega, O.; Roca, M.; Arús, M.; Civit, P.C. Effect of A Minimal-Massive Intervention in Hospitalized Older Patients with Oropharyngeal Dysphagia: A Proof of Concept Study. J. Nutr. Health Aging 2018, 22, 739–747. [Google Scholar] [CrossRef]
- O’Keeffe, S.T. Use of modified diets to prevent aspiration in oropharyngeal dysphagia: Is current practice justified? BMC Geriatr. 2018, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Yver, C.M.; Kennedy, W.P.; Mirza, N. Taste acceptability of thickening agents. World, J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, SA, Australia, 2017; Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 25 September 2021).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Goldberg, L.R.; Heiss, C.J.; Parsons, S.D.; Foley, A.S.; Mefferd, A.S.; Hollinger, D.; Parham, D.F.; Patterson, J. Hydration in older adults: The contribution of bioelectrical impedance analysis. Int. J. Speech Lang. Pathol. 2014, 16, 273–281. [Google Scholar] [CrossRef]
- Sezgin, B.; Durusoy, D.; Demirci, M.S.; Ozturk, K.; Kaya, I.; Eyigor, S.; Gode, S. The effect of “xanthan gum-based fluid thickener” on hydration, swallowing functions and nutritional status in total maxillectomy patients. Eur. Arch. Otorhinolaryngol. 2018, 275, 2997–3005. [Google Scholar] [CrossRef]
- Ramos-Vázquez, A.G.; Reyes-Torres, C.A.; Castillo-Martínez, L.; Serralde Zúñiga, A.E. Body composition by bioelectrical impedance, muscle strength, and nutritional risk in oropharyngeal dysphagia patients. Nutr. Hosp. 2021, 38, 315–320. [Google Scholar]
- Crary, M.A.; Humphrey, J.L.; Carnaby-Mann, G.; Sambandam, R.; Miller, L.; Silliman, S. Dysphagia, Nutrition, and Hydration in Ischemic Stroke Patients at Admission and Discharge from Acute Care. Dysphagia 2013, 28, 69–76. [Google Scholar] [CrossRef]
- Botigué, T.; Masot, O.; Miranda, J.; Nuin, C.; Viladrosa, M.; Lavedán, A.; Zwakhalen, S. Prevalence and Risk Factors Associated With Low Fluid Intake in Institutionalized Older Residents. J. Am. Med Dir. Assoc. 2019, 20, 317–322. [Google Scholar] [CrossRef]
- Murray, J.; Scholten, I.; Doeltgen, S. Factors Contributing to Hydration, Fluid Intake and Health Status of Inpatients With and Without Dysphagia Post Stroke. Dysphagia 2018, 33, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Goroff, H.; Herzog, L.; Cardi, R.; Reding, M. Use of Oral Hydration Protocols for Dysphagic Patients Following Stroke. Rehabil. Nurs. 2018, 43, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Doeltgen, S.; Miller, M.; Scholten, I. Does a Water Protocol Improve the Hydration and Health Status of Individuals with Thin Liquid Aspiration Following Stroke? A Randomized Controlled Trial. Dysphagia 2016, 31, 424–433. [Google Scholar] [CrossRef]
- Churchill, M.; Grimm, S.; Reding, M. Risks of diuretic usage following stroke. Neurorehabil. Neural. Repair 2004, 18, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Holas, M.; Halvorson, K.; Reding, M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia 1994, 9, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.M.; Nissenson, P.M.; Meeks, L.; Rosario, E.R. Use of Textured Thin Liquids in Patients With Dysphagia. Am. J. Speech Lang. Pathol. 2018, 27, 827–835. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Gaio, M.; Furlanis, G.; Douglas, P.; Naccarato, M.; Manganotti, P. Fluid and energy intake in stroke patients during acute hospitalization in a stroke unit. J. Clin. Neurosci. 2019, 62, 27–32. [Google Scholar] [CrossRef]
- Sala, R.; Muntó, M.J.; de la Calle, J.; Preciado, I.; Miralles, T.; Cortés, A.; Molla, R.; Alcaide, M. Alteraciones de la deglución en el accidente cerebrovascular: Incidencia, historia natural y repercusiones sobre el estado nutricional, la morbilidad y la mortalidad. Rev. Neurol. 1998, 27, 759–766. [Google Scholar] [CrossRef]
- Smithard, D.G.; O’Neill, P.A.; Parks, C.; Morris, J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke 1996, 27, 1200–1204. [Google Scholar] [CrossRef]
- Lee, A.; Sitoh, Y.Y.; Lieu, P.K.; Phua, S.Y.; Chin, J.J. Swallowing impairment and feeding dependency in the hospitalised elderly. Ann. Acad. Med. Singap. 1999, 28, 371–376. [Google Scholar]
- Kim, K.L.; Park, G.Y.; Kwon, D.R.; Kwon, D.Y.; Kwak, S.G.; Cho, H.K. Airway invasion in non-neurologically ill patients with dysphagia: Contributing factors and associated problems during swallowing process. A retrospective observational study. Medicine (Baltimore) 2020, 99, e22977. [Google Scholar] [CrossRef] [PubMed]
- Chumlea, W.C.; Guo, S.S.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Heymsfield, S.B.; Lukaski, H.C.; Friedl, K.; Hubbard, V.S. Body composition estimates from NHANES III bioelectrical impedance data. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Via, M.A.; Mechanick, J.I. Malnutrition, dehydration, and ancillary feeding options in dysphagia patients. Otolaryngol. Clin. North Am. 2013, 46, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Schettino, M.S.T.B.; Silva, D.C.C.; Pereira-Carvalho, N.A.V.; Vicente, L.C.C.; Friche, A.A.D.L. Dehydration, stroke and dysphagia: Systematic review. Audiol.-Commun. Res. 2020, 24, e2236. [Google Scholar] [CrossRef]
- Whelan, K. Inadequate fluid intakes in dysphagic acute stroke. Clin. Nutr. 2001, 20, 423–428. [Google Scholar] [CrossRef]
- DePippo, K.L.; Holas, M.A.; Reding, M.J.; Mandel, F.S.; Lesser, M.L. Dysphagia therapy following stroke: A controlled trial. Neurology 1994, 44, 1655–1660. [Google Scholar] [CrossRef]
- Garon, B.R.; Engle, M.; Ormiston, C. A Randomized Control Study to determine the effects of unlimited oral Intake of water in patients with identified aspiration. J. Neurol. Rehabil. 1997, 11, 139–148. [Google Scholar] [CrossRef]
- Karagiannis, M.J.; Chivers, L.; Karagiannis, T.C. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011, 11, 9. [Google Scholar] [CrossRef]
- McGrail, A.; Kelchner, L.N. Adequate oral fluid intake in hospitalized stroke patients: Does viscosity matter? Rehabil. Nurs. 2012, 37, 252–257. [Google Scholar] [CrossRef]
- McGrail, A.; Kelchner, L. Barriers to oral fluid intake: Beyond thickened liquids. J. Neurosci. Nurs. 2015, 47, 58–63. [Google Scholar] [CrossRef]
- Karagiannis, M.; Karagiannis, T.C. Oropharyngeal dysphagia, free water protocol and quality of life: An update from a prospective clinical trial. Hell. J. Nucl. Med. 2014, 17 (Suppl. 1), 26–29. [Google Scholar] [PubMed]
- McCormick, S.E.; Stafford, K.M.; Saqib, G.; Chroinin, D.N.; Power, D. The efficacy of pre-thickened fluids on total fluid and nutrient consumption among extended care residents requiring thickened fluids due to risk of aspiration. Age Ageing 2008, 37, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.P.; Campbell, K.L.; Suter, M.S.; Hannan-Jones, M.T.; Hulcombe, J.A. Contribution of thickened drinks, food and enteral and parenteral fluids to fluid intake in hospitalised patients with dysphagia. J. Hum. Nutr. Diet. 2009, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.M.; Kjaersgaard, A.; Hansen, T.; Poulsen, I. Systematic review and evidence based recommendations on texture modified foods and thickened liquids for adults (above 17 years) with oropharyngeal dysphagia-An updated clinical guideline. Clin. Nutr. 2018, 37, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Painter, V.; Le Couteur, D.G.; Waite, L.M. Texture-modified food and fluids in dementia and residential aged care facilities. Clin. Interv. Aging 2017, 12, 1193–1203. [Google Scholar] [CrossRef]
- Cichero, J.A. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013, 12, 54. [Google Scholar] [CrossRef]
- Ortega, O.; Martín, A.; Clavé, P. Diagnosis and Management of Oropharyngeal Dysphagia Among Older Persons, State of the Art. J. Am. Med. Dir. Assoc. 2017, 18, 576–582. [Google Scholar] [CrossRef]
- Almirall, J.; Rofes, L.; Serra-Prat, M.; Icart, R.; Palomera, E.; Arreola, V.; Clavé, P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur. Respir. J. 2013, 41, 923–928. [Google Scholar] [CrossRef]
- Logemann, J.A. Oropharyngeal dysphagia and nutritional management. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 611–614. [Google Scholar] [CrossRef]
- Begum, M.N.; Johnson, C.S. A review of the literature on dehydration in the institutionalized elderly. e-SPEN Eur. E-J. Clin. Nutr. Metab. 2010, 5, e47–e53. [Google Scholar] [CrossRef]
- Mange, K.; Matsuura, D.; Cizman, B.; Soto, H.; Ziyadeh, F.N.; Goldfarb, S.; Neilson, E.G. Language guiding therapy: The case of dehydration versus volume depletion. Ann. Intern. Med. 1997, 127, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Stanga, Z.; Aubry, E. Dehydration in Dysphagia. In Dysphagia: Diagnosis and Treatment; Ekberg, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 859–871. [Google Scholar]
- Xiao, H.; Barber, J.; Campbell, E.S. Economic burden of dehydration among hospitalized elderly patients. Am. J. Health Syst. Pharm. 2004, 61, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Bacon, W.E.; Harris, T.; McBean, A.M.; Foley, D.J.; Phillips, C. The burden and outcomes associated with dehydration among US elderly, 1991. Am. J. Public Health 1994, 84, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Stookey, J.D.; Pieper, C.F.; Cohen, H.J. Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+ years. Public Health Nutr. 2005, 8, 1275–1285. [Google Scholar] [CrossRef]
- Bennett, J.A.; Thomas, V.; Riegel, B. Unrecognized chronic dehydration in older adults: Examining prevalence rate and risk factors. J. Gerontol. Nurs. 2004, 30, 22–53. [Google Scholar] [CrossRef]
- Yilmaz, K.; Karaböcüoglu, M.; Citak, A.; Uzel, N. Evaluation of laboratory tests in dehydrated children with acute gastroenteritis. J. Paediatr. Child Health 2002, 38, 226–228. [Google Scholar] [CrossRef]
- Snyder, N.A.; Feigal, D.W.; Arieff, A.I. Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann. Intern. Med. 1987, 107, 309–319. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Kavouras, S.A.; Walsh, N.P.; Roberts, W.O. Diagnosing dehydration? Blend evidence with clinical observations. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 434–438. [Google Scholar] [CrossRef]
- Seoane, F.; Abtahi, S.; Abtahi, F.; Ellegård, L.; Johannsson, G.; Bosaeus, I.; Ward, L.C. Mean Expected Error in Prediction of Total Body Water: A True Accuracy Comparison between Bioimpedance Spectroscopy and Single Frequency Regression Equations. Biomed. Res. Int. 2015, 2015, 656323. [Google Scholar] [CrossRef]
- Mulasi, U.; Kuchnia, A.J.; Cole, A.J.; Earthman, C.P. Bioimpedance at the bedside current applications, limitations, and opportunities. Nutr. Clin. Pract. 2015, 30, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Vilardell, N.; Rofes, L.; Arreola, V.; Speyer, R.; Clavé, P. A Comparative Study Between Modified Starch and Xanthan Gum Thickeners in Post-Stroke Oropharyngeal Dysphagia. Dysphagia 2016, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; White, C.; McKenzie, S.; Belafsky, P.C. Effects of bolus rheology on aspiration in patients with Dysphagia. J. Acad. Nutr. Diet. 2014, 114, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.; Chen, J.; Cichero, J.A.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Mentes, J. Oral hydration in older adults: Greater awareness is needed in preventing, recognizing, and treating dehydration. Am. J. Nurs. 2006, 106, 40–49, quiz 50. [Google Scholar] [CrossRef]
- Kayser-Jones, J.; Schell, E.S.; Porter, C.; Barbaccia, J.C.; Shaw, H. Factors contributing to dehydration in nursing homes: Inadequate staffing and lack of professional supervision. J. Am. Geriatr. Soc. 1999, 47, 1187–1194. [Google Scholar] [CrossRef]
- Lavizzo-Mourey, R.; Johnson, J.; Stolley, P. Risk factors for dehydration among elderly nursing home residents. J. Am. Geriatr. Soc. 1988, 36, 213–218. [Google Scholar] [CrossRef]
- Jefatura del Estado. Ley 32/2014, de 22 de diciembre, de Metrología. BOE 2014, 309. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2014-13359 (accessed on 10 May 2022).
- Bolívar-Prados, M.; Tomsen, N.; Arenas, C.; Ibáñez, L.; Clavé, P. A bit thick: Hidden risks in thickening products’ labelling for dysphagia treatment. Food Hydrocoll. 2022, 123, 106960. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015, 349, g7647. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Plaisier, A.; Maingay-de Groof, F.; Mast-Harwig, R.; Kalkman, P.M.; Wulkan, R.W.; Verwers, R.; Neele, M.; Hop, W.C.; Groeneweg, M. Plasma water as a diagnostic tool in the assessment of dehydration in children with acute gastroenteritis. Eur. J. Pediatr. 2010, 169, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Çelik, T.; Altekin, E.; İşgüder, R.; Kenesari, Y.; Duman, M.; Arslan, N. Evaluation of neutrophil gelatinase-associated lipocalin in pediatric patients with acute rotavirus gastroenteritis and dehydration. Ital. J. Pediatr. 2013, 39, 52. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.A.; Waltzman, M.; Monuteaux, M.C.; Bachur, R.G. Value of point-of-care ketones in assessing dehydration and acidosis in children with gastroenteritis. Acad. Emerg. Med. 2013, 20, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
| Study | OD Etiology | Assessment for OD | Hydration Assessment Parameter | Number of Participants | Conclusion | QA (%) 1 |
|---|---|---|---|---|---|---|
| Bioimpedance Studies | ||||||
| Carrión S. [9] | Geriatric, neurologic, other. | VFS, V-VST | ICW, ECW, TBW | 133 (A = 95 older with OD (chronic NRL or aging) + B = 23 older with OD (CAP) + C = 15 healthy older people) | Older patients with OD presented a significant reduction in ICW compared to healthy older people. | 75.00 |
| Tomsen N. [10] | Geriatric | VFS | ECW, ICW, TBW, ECW/ICW, PA° | 43 (A = 15 young healthy + B = 14 healthy older + C = 14 older with OD) | Both older groups showed a significant reduction in ICW, ECW, ECW/ICW ratio and PA° compared to young healthy people. | 81.25 |
| Goldberg LR. [28] | Stroke | SLP clinical assessment and FOIS | TBW (kg) | 19 | The mean levels of TBW for both groups were lower, indicating risk for inadequate hydration in OD. | 80.00 |
| Sezgin B. [29] | Maxillary carcinoma | EAT-10, MDADI, FOSS, FOIS | ECW, ICW, TBW | 10 | After total maxillectomy, the prevalence of OD increased and hydration worsened (significant decrease in TBW, ICW and ECW). | 96.15 |
| Ramos-Vázquez AG. [30] | Neurodegenerative, stroke, head and neck, autoimmune disease, infectious disease, other. | EAT-10 and V-VST | PA° | 79 (Nectar = 27 Spoon-thick = 27 Exclusive tube feeding = 25) | More severe OD patients showed a lower PA°, which was related to an alteration of cell integrity and permeability. | 87.50 |
| Biochemical studies | ||||||
| Lee A. [42] | Geriatric | Bedside swallowing test and/or SLP | Not specified | 211 | Patients with swallowing impairments were at increased risk of dehydration. | 72.22 |
| Churchill M. [36] | Stroke | BDST, MBS | BUN/Cr ratio, BUN, serum sodium | 296 | Dysphagia was a marker for increased risk of dehydration. | 85.00 |
| Crary MA. [31] | Stroke | MASA, FOIS | BUN/Cr ratio | 67 | Ischemic stroke patients with OD were at risk for dehydration on admission to the hospital. | 72.73 |
| Crary MA. [13] | Stroke | MASA, FOIS | BUN/Cr ratio | 64 | Poor hydration status among acute ischemic stroke with a decrease in hydration specific to patients with dysphagia. | 60.00 |
| Schmidt J. [37] | Stroke | MBS technique | Serum sodium, BUN | 59 | No significant differences in dehydration were observed between aspirators and non-aspirators. | 40.00 |
| Smithard DG. [41] | Stroke | VFS | Hematocrit, plasma sodium, urea and osmolality | 121 | Patients with swallowing difficulties were more likely to use parenteral fluids (p < 0.001) and for longer times (p < 0.0001). | 68.18 |
| Botigué T. [32] | Geriatric | V-VST | BUN/Cr ratio, blood osmolarity, serum sodium | 53 | No significant differences were observed in hydration status between OD and non-OD patients. | 75.00 |
| Buoite SA. [39] | Acute stroke | V-VST | Urine osmolality | 95 (OD = 18 nOD = 56 Not evaluated = 21) | OD was not significantly associated with a higher risk of dehydration on discharge. | 90.00 |
| Murray J. [35] | Stroke | VFS | BUN/Cr ratio | 100 | Dysphagia was not a significant predictor of any of the outcomes measured. | 72.73 |
| Goroff H. [34] | Stroke | Previous records. If needed FEES or VFS. | BUN/Cr ratio, BUN, serum sodium | 712 (Liquid = 675 Nectar or honey = 33 Honey = 4) | Mild dehydration on discharge from the acute care hospital. | 90.90 |
| Sala R. [40] | CVA | Standardized test of dysphagia | Urea | 187 | Mild dehydration in the whole group demonstrated by increased serum urea. | 81.80 |
| Kim KL. [43] | Geriatric | VFS | BUN/Cr ratio | 52 | No significant differences were observed in the BUN/Cr ratio between the two groups. | 65.00 |
| Leibovitz A. [14] | Geriatric | FOSS (SLP evaluation) | BUN/Cr ratio, BUN, serum osmolarity, urine sodium, urine osmolality, serum creatinine, serum sodium, urine/creatinine, urine/serum osmolality | 95 | The mean number of dehydration markers was significantly higher in the FOSS-2 group compared with NGT-fed patients. | 87.50 |
| Howard MM. [38] | CVA, TBI | FOIS, PenAsp Scale, MBS | BUN, creatinine, serum sodium | 20 (CVA = 19 TBI = 1) | BUN, creatinine and serum sodium levels were high, indicating signs of dehydration in the initial stage of the study. | 63.64 |
| Murray J. [35] | Stroke | VFS | BUN/Cr ratio | 14 | Most participants were classified as dehydrated on entry to the study. | 80.77 |
| Study | Study Design | OD Etiology | Assessment for OD | Number of Participants | TF or Product Therapy | Effect on Hydration Status | QA (%) 1 |
|---|---|---|---|---|---|---|---|
| Bioimpedance Studies | |||||||
| Sezgin B. [29] | RCT | Total maxillectomy | FOSS, FOIS, EAT-10, MDADE | 12 | Use of xanthan-gum for 3 months post-total maxillectomy | Using xanthan-gum-based liquid thickener helped maintain ICW, ECW and TBW. | 96.15 |
| Biochemical studies | |||||||
| Goroff H. [34] | Cohort | Ischemic stroke | Previous records; if needed, FEES or VFS. | 712 (Safe swallow = 675 Nectar = 33 Honey = 4) | Modified cornstarch and maltodextrin | After an active hydration intervention, there was an improvement in hydration on discharge and a decrease in the need for intravenous hydration. | 90.90 |
| DePippo KL. [48] | RCT | Stroke | BDST and MBS | 115 | 3 groups: (A = formal intervention B = formal intervention + reevaluation C = active intervention) TP not specified | Intensity of the treatment (diet alteration and compensatory swallowing techniques) did not affect the development of post-stroke complications. | 88.45 |
| Whelan K. [47] | RCT | Acute stroke | SLP or VFS | 24 | Powder thickened (maize starch) Pre-thickened drink | The results of the study showed no correlation between the traditional biochemical markers of hydration and daily fluid intake or fluid balance. | 80.77 |
| Murray J. [35] | RCT | Stroke | VFS | 14 (G1:6 G2:8) | Xanthan-gum | Those who were permitted water had improved hydration levels compared to those on TF alone, suggesting even a small amount of water per day made a difference to hydration levels. | 80.77 |
| Crary M. [13] | Case-control | Ischemic stroke | MASA, FOIS | 64 (38% OD) | TP not specified | Any modification of regular liquids and solid diets contributed to reduced hydration on discharge. | 60.00 |
| Howard MM. [38] | Cohort | CVA and TBI | FOIS and PenAsp Scale | 20 | Pre-packaged TF (starch-based) | Patients receiving a higher viscosity fluid had poorer hydration status compared to those receiving a thin textured fluid. | 63.64 |
| Study | Number of Participants Studied | QA (%) 1 | PICO QUESTIONS | ||
|---|---|---|---|---|---|
| Effect of TF Therapy on Fluid Intake and Hydration Status | TF Therapy Depended on OD Severity or the Type of Thickening Product Used | TF Improved or Aggravated Dehydration | |||
| Goroff H. [34] | 712 | 90.90 | POSITIVE EFFECT | NOT ANSWERED | POSITIVE EFFECT |
| Sezgin B. [29] | 22 | 96.15 | POSITIVE EFFECT | NOT ANSWERED | POSITIVE EFFECT |
| DePippo K. [48] | 115 | 88.45 | NEUTRAL | NOT ANSWERED | NEUTRAL |
| Murray J. [35] | 14 | 80.77 | NEUTRAL | NOT ANSWERED | NEUTRAL |
| Whelan K. [47] | 24 | 80.77 | NEUTRAL | NOT ANSWERED | NEUTRAL |
| Crary M. [13] | 64 | 60.00 | NEGATIVE EFFECT | NOT ANSWERED | NEGATIVE EFFECT |
| Howard MM. [38] | 20 | 65.00 | NEGATIVE EFFECT | NOT ANSWERED | NEGATIVE EFFECT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viñas, P.; Bolivar-Prados, M.; Tomsen, N.; Costa, A.; Marin, S.; Riera, S.A.; Barcons, N.; Clavé, P. The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews. Nutrients 2022, 14, 2497. https://doi.org/10.3390/nu14122497
Viñas P, Bolivar-Prados M, Tomsen N, Costa A, Marin S, Riera SA, Barcons N, Clavé P. The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews. Nutrients. 2022; 14(12):2497. https://doi.org/10.3390/nu14122497
Chicago/Turabian StyleViñas, Paula, Mireia Bolivar-Prados, Noemi Tomsen, Alicia Costa, Sergio Marin, Stephanie A. Riera, Núria Barcons, and Pere Clavé. 2022. "The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews" Nutrients 14, no. 12: 2497. https://doi.org/10.3390/nu14122497
APA StyleViñas, P., Bolivar-Prados, M., Tomsen, N., Costa, A., Marin, S., Riera, S. A., Barcons, N., & Clavé, P. (2022). The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews. Nutrients, 14(12), 2497. https://doi.org/10.3390/nu14122497







