The Role of a High-Fat, High-Fructose Diet on Letrozole-Induced Polycystic Ovarian Syndrome in Prepubertal Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatment
2.2. Sample Collection
2.3. Serum Biochemical Analysis
2.4. Calculation of the HOMA-IR, HOMA-β, and QUICKI Indices
2.5. Statistical Analysis
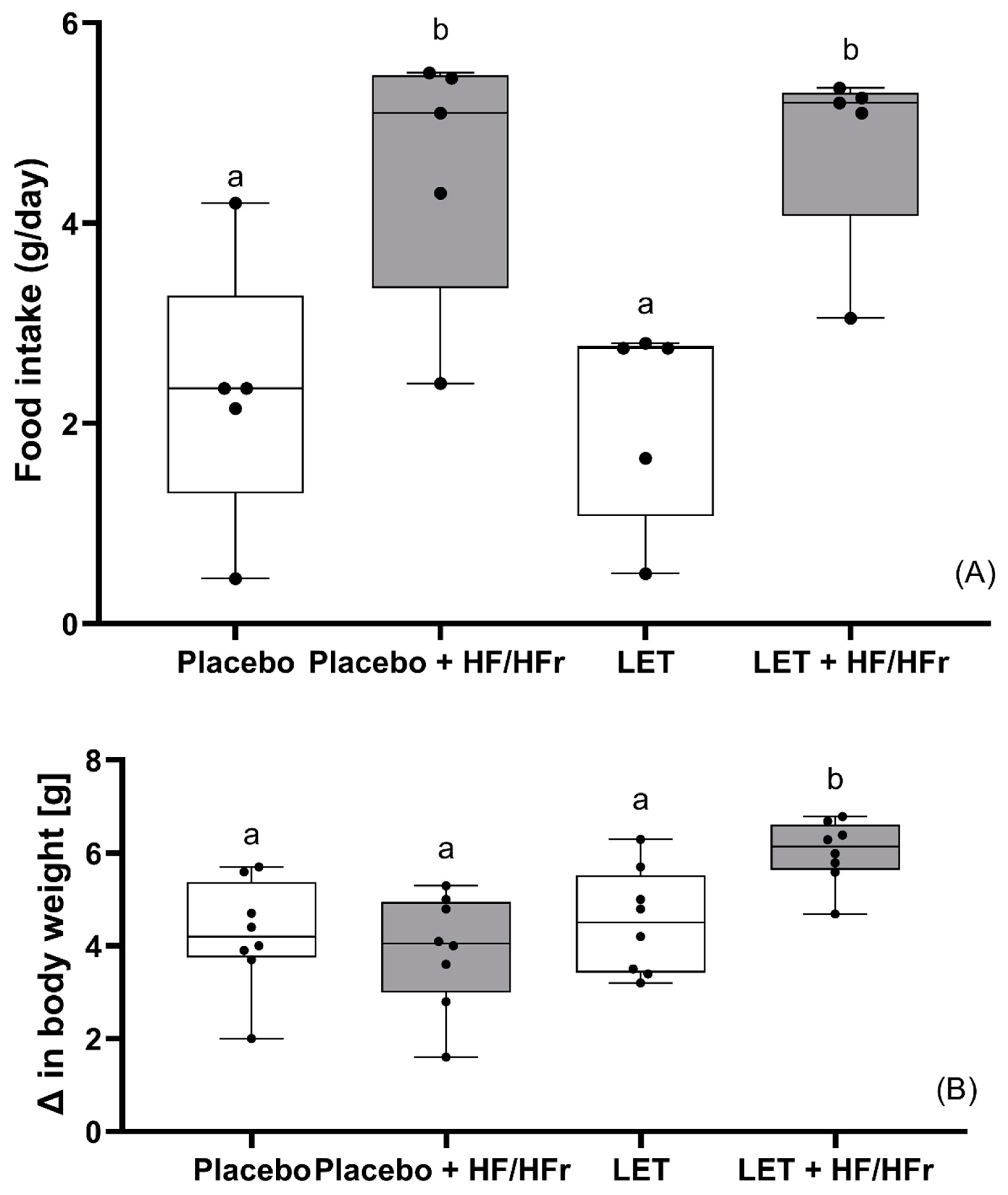
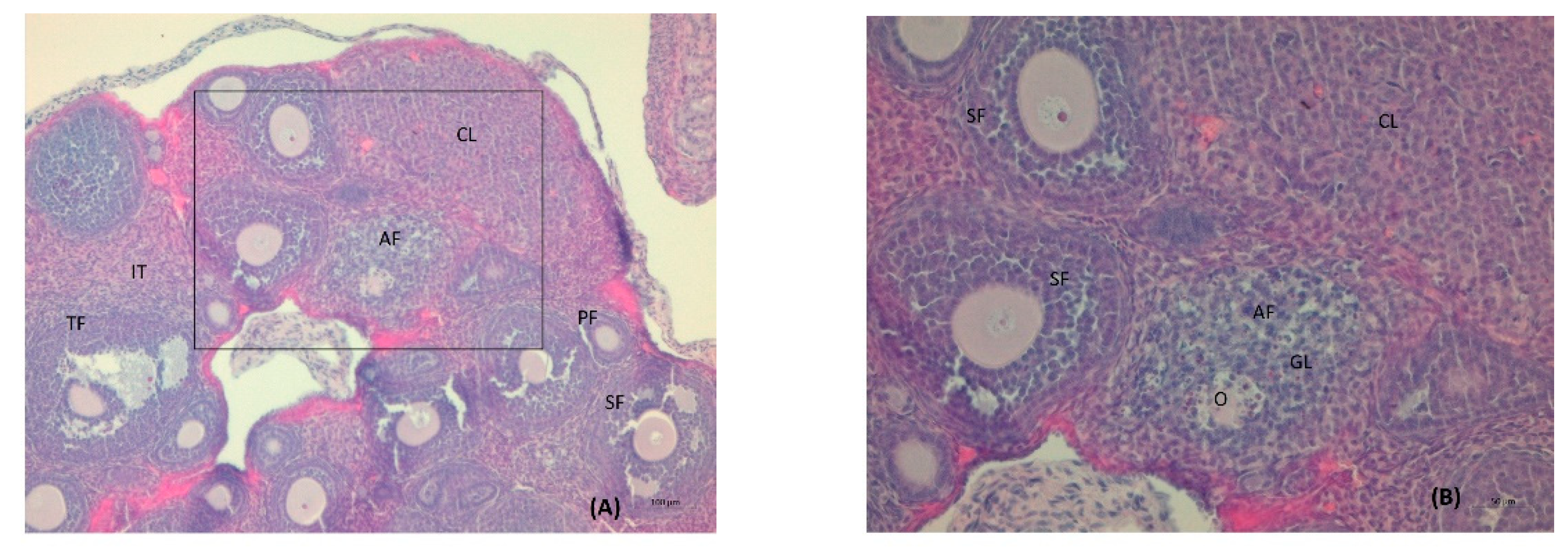
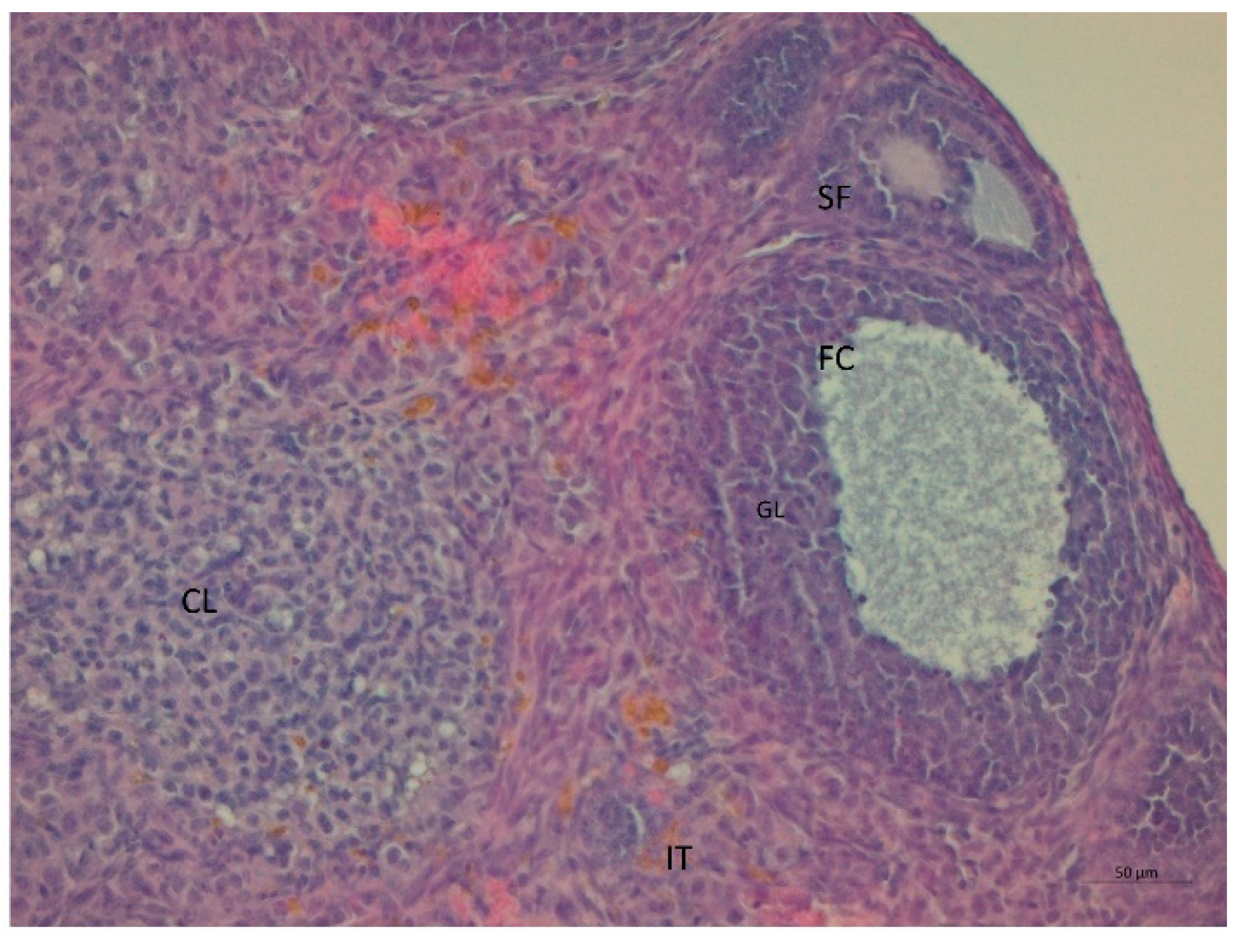
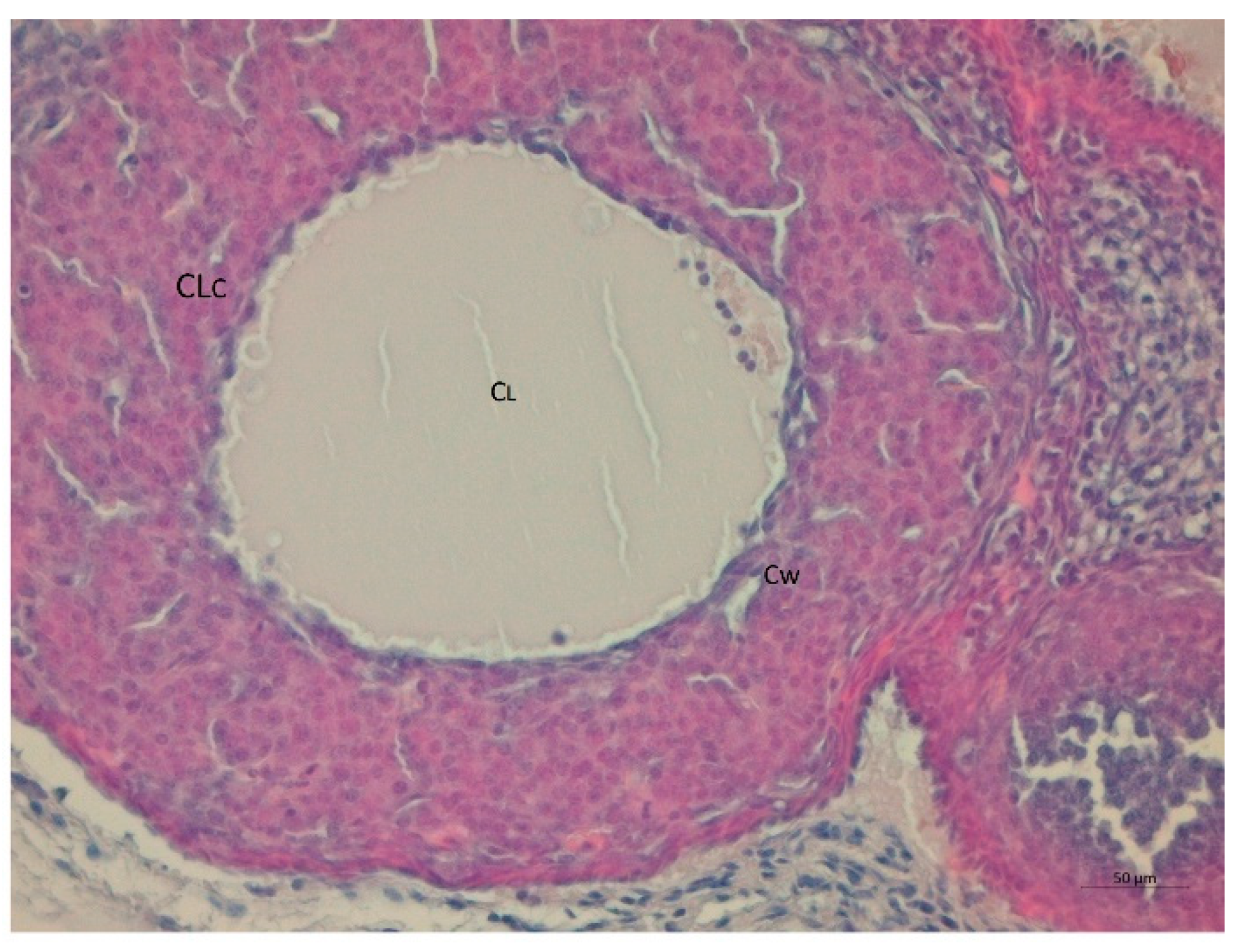
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Begum, N.; Manipriya, K.; Veeresh, B. Role of high-fat diet on letrozole-induced polycystic ovarian syndrome in rats. Eur. J. Pharmacol. 2022, 917, 174746. [Google Scholar] [CrossRef] [PubMed]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, P.J.; Skarra, D.V.; Ho, B.S.; Sau, L.; Anvar, A.R.; Kelley, S.T.; Thackray, V.G. Letrozole treatment of adult female mice results in a similar reproductive phenotype but distinct changes in metabolism and the gut microbiome compared to pubertal mice. BMC Microbiol. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfield, R.L. Identifying Children at Risk for Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt Solorzano, C.M.; McCartney, C.R.; Blank, S.K.; Knudsen, K.L.; Marshall, J.C. Hyperandrogenaemia in adolescent girls: Origins of abnormal gonadotropin-releasing hormone secretion. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2019, 502, 214–221. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Munt, A.E.; Partridge, S.R.; Allman-Farinelli, M. The barriers and enablers of healthy eating among young adults: A missing piece of the obesity puzzle: A scoping review: Barriers and enablers of healthy eating. Obes. Rev. 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Giussani, M.; Lieti, G.; Orlando, A.; Parati, G.; Genovesi, S. Fructose Intake, Hypertension and Cardiometabolic Risk Factors in Children and Adolescents: From Pathophysiology to Clinical Aspects. A Narrative Review. Front. Med. 2022, 9, 792949. [Google Scholar] [CrossRef]
- Tsan, L.; Décarie-Spain, L.; Noble, E.E.; Kanoski, S.E. Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Front. Neurosci. 2021, 15, 632312. [Google Scholar] [CrossRef]
- Pathak, G.; Nichter, M. Polycystic ovary syndrome in globalizing India: An ecosocial perspective on an emerging lifestyle disease. Soc. Sci. Med. 2015, 146, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ressler, I.B.; Grayson, B.E.; Ulrich-Lai, Y.M.; Seeley, R.J. Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. Am. J. Physiol. Metab. 2015, 308, E1076–E1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, P.; Ho, B.S.; Sau, L.; Kelley, S.T.; Thackray, V.G. Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome. PLoS ONE 2019, 14, e0223274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manneras, L.; Cajander, S.; Holmang, A.; Seleskovic, Z.; Lystig, T.; Lonn, M.; Stener-Victorin, E. A New Rat Model Exhibiting Both Ovarian and Metabolic Characteristics of Polycystic Ovary Syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, Y.; Kim, S.W.; Kim, Y.Y.; Ku, S.-Y. Animal Models for Human Polycystic Ovary Syndrome (PCOS) Focused on the Use of Indirect Hormonal Perturbations: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 2720. [Google Scholar] [CrossRef] [Green Version]
- Kafali, H.; Iriadam, M.; Ozardalı, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Thackray, V.G.; Ryan, G.E.; Tolson, K.; Glidewell-Kenney, C.A.; Semaan, S.J.; Poling, M.C.; Iwata, N.; Breen, K.M.; Duleba, A.J.; et al. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice1. Biol. Reprod. 2015, 93, 69. [Google Scholar] [CrossRef]
- Wang, H.-B.; Loh, D.H.; Whittaker, D.S.; Cutler, T.; Howland, D.; Colwell, C.S. Time-Restricted Feeding Improves Circadian Dysfunction as well as Motor Symptoms in the Q175 Mouse Model of Huntington’s Disease. Eneuro 2018, 5. [Google Scholar] [CrossRef]
- Huang, L.; Liang, A.; Li, T.; Lei, X.; Chen, X.; Liao, B. Mogroside V Improves Follicular Development and Ovulation in Young-Adult PCOS Rats Induced by Letrozole and High-Fat Diet Through Promoting Glycolysis. Front. Endocrinol. 2022, 13, 838204. [Google Scholar] [CrossRef]
- Varlamov, O.; Chu, M.P.; McGee, W.K.; Cameron, J.L.; O’Rourke, R.; Meyer, K.A.; Bishop, C.; Stouffer, R.L.; Roberts, C. Ovarian Cycle-Specific Regulation of Adipose Tissue Lipid Storage by Testosterone in Female Nonhuman Primates. Endocrinology 2013, 154, 4126–4135. [Google Scholar] [CrossRef] [Green Version]
- McGee, W.K.; Bishop, C.V.; Pohl, C.R.; Chang, R.J.; Marshall, J.C.; Pau, F.K.; Stouffer, R.L.; Cameron, J.L. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am. J. Physiol. Metab. 2014, 306, E1292–E1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.S.; Perets, R.A.; Sarfert, K.S.; Bowman, J.; Ozark, P.A.; Whitworth, G.B.; Blythe, S.N.; Toporikova, N. High-fat high-sugar diet induces polycystic ovary syndrome in a rodent model. Biol. Reprod. 2017, 96, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Yu, J.; Liang, C.; Li, S.; Wen, X.; Li, Y. Characterization on gut microbiome of PCOS rats and its further design by shifts in high-fat diet and dihydrotestosterone induction in PCOS rats. Bioprocess Biosyst. Eng. 2020, 44, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T. Baker MThe ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Boutron I, redaktor. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Margalioth, E.J.; Gal, M.; Ben-Chetrit, A.; Algur, N.; Zylber-Haran, E.; Brooks, B.; Huerta, M.; Spitz, I.M. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum. Reprod. 2005, 20, 1814–1819. [Google Scholar] [CrossRef]
- Xu, J.; Dun, J.; Yang, J.; Zhang, J.; Lin, Q.; Huang, M.; Ji, F.; Huang, L.; You, X.; Lin, Y. Letrozole Rat Model Mimics Human Polycystic Ovarian Syndrome and Changes in Insulin Signal Pathways. Med Sci. Monit. 2020, 26, e923073-1. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Alipour, B.; Roohelhami, E.; Rashidkhani, B.; Shahrdami, F.; Edalati, S. Evaluating indicators of oxidative stress and their relationship with Insulin Resistance in polycystic ovary syndrome. Prog. Nutr. 2019, 21, 178–183. [Google Scholar] [CrossRef]
- Skarra, D.V.; Hernández-Carretero, A.; Rivera, A.J.; Anvar, A.R.; Thackray, V.G. Hyperandrogenemia Induced by Letrozole Treatment of Pubertal Female Mice Results in Hyperinsulinemia Prior to Weight Gain and Insulin Resistance. Endocrinology 2017, 158, 2988–3003. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, A.K.; Campbell, E.R.; Benrick, A.; Giacobini, P.; Dumesic, A.D.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptiste, C.G.; Battista, M.-C.; Trottier, A.; Baillargeon, J.-P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2009, 122, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.S.; Shah, G. High-fat diet exposure from pre-pubertal age induces polycystic ovary syndrome (PCOS) in rats. Reproduction 2018, 155, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-P.; Ronveaux, C.C.; Knotts, T.A.; Rutkowsky, J.R.; Ramsey, J.J.; Raybould, H.E. Sex differences in response to short-term high fat diet in mice. Physiol. Behav. 2020, 221, 112894. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhu, Y.; Jiang, J.; Shi, Y.; Chen, Z. The clinical characteristics and etiological study of nonalcoholic fatty liver disease in Chinese women with PCOS. Iran. J. Reprod. Med. 2013, 11, 725–732. [Google Scholar]
- Basaranoglu, M. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [Green Version]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama CMN de, O.; Pereira, S.S.; de Oliveira, L.L. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef]





| Variables | Placebo | Letrozole | ||
|---|---|---|---|---|
| Control | HF/HFr | Control | HF/HFr | |
| Total cholesterol (mg/dL) | 131.9 ± 21.8 a | 188.4 ± 26.0 b | 137.6 ± 23.0 a | 191.7 ± 39.8 b |
| LDL cholesterol (mg/dL) | 51.6 ± 5.9 a | 77.1 ± 4.9 b | 49.5 ± 2.5 a | 74.5 ± 6.2 b |
| HDL cholesterol (mg/dL) | 30.8 ± 4.1 a | 40.0 ± 3.2 b | 28.9 ± 1.8 a | 38.3 ± 1.6 b |
| Non-HDL cholesterol (mg/dL) | 101.2 ± 22.0 a | 148.4 ± 28.3 b | 108.7 ± 23.7 a | 153.4 ± 40.6 b |
| Triglycerides (mg/dL) | 141.2 ± 16.4 | 135.9 ± 21.3 | 136.2 ± 16.6 | 128.7 ± 12.9 |
| TC/HDL ratio | 4.4 ± 0.9 | 4.8 ± 1.0 | 4.8 ± 0.9 | 5.0 ± 1.2 |
| NEFA (mmol/L) | 1.09 ± 0.05 a | 1.24 ± 0.06 b | 1.14 ± 0.11 a | 1.12 ± 0.06 a |
| Glucose (mg/dL) | 119.9 ± 14.0 ab | 140.1 ± 23.0 b | 113.9 ± 12.0 a | 136.3 ± 18.4 ab |
| Insulin (mU/L) | 2.9 ± 0.8 | 3.4 ± 1.5 | 2.6 ± 0.7 | 3.4 ± 0.7 |
| HOMA-IR | 0.9 ± 0.3 ab | 1.0 ± 0.2 ab | 0.7 ± 0.2 a | 1.2 ± 0.3 b |
| HOMA-β | 18.5 ± 2.9 | 17.7 ± 10.9 | 20.3 ± 9.3 | 20.0 ± 8.0 |
| QUICKI | 0.8 ± 0.1 ab | 0.7 ± 0.1 ab | 0.8 ± 0.1 a | 0.7 ± 0.1 b |
| CRP (mg/L) | 25.4 ± 4.2 | 27.2 ± 1.5 | 27.5 ± 2.9 | 29.4 ± 3.0 |
| TAC (μmol/L) | 6.5 ± 5.6 a | 2.8 ± 1.1 ab | 4.9 ± 3.5 ab | 1.6 ± 0.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczyńska, J.M.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.A.; Łukomska, A.; Bajerska, J. The Role of a High-Fat, High-Fructose Diet on Letrozole-Induced Polycystic Ovarian Syndrome in Prepubertal Mice. Nutrients 2022, 14, 2478. https://doi.org/10.3390/nu14122478
Pieczyńska JM, Pruszyńska-Oszmałek E, Kołodziejski PA, Łukomska A, Bajerska J. The Role of a High-Fat, High-Fructose Diet on Letrozole-Induced Polycystic Ovarian Syndrome in Prepubertal Mice. Nutrients. 2022; 14(12):2478. https://doi.org/10.3390/nu14122478
Chicago/Turabian StylePieczyńska, Joanna Maria, Ewa Pruszyńska-Oszmałek, Paweł Antoni Kołodziejski, Anna Łukomska, and Joanna Bajerska. 2022. "The Role of a High-Fat, High-Fructose Diet on Letrozole-Induced Polycystic Ovarian Syndrome in Prepubertal Mice" Nutrients 14, no. 12: 2478. https://doi.org/10.3390/nu14122478
APA StylePieczyńska, J. M., Pruszyńska-Oszmałek, E., Kołodziejski, P. A., Łukomska, A., & Bajerska, J. (2022). The Role of a High-Fat, High-Fructose Diet on Letrozole-Induced Polycystic Ovarian Syndrome in Prepubertal Mice. Nutrients, 14(12), 2478. https://doi.org/10.3390/nu14122478








