Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strains
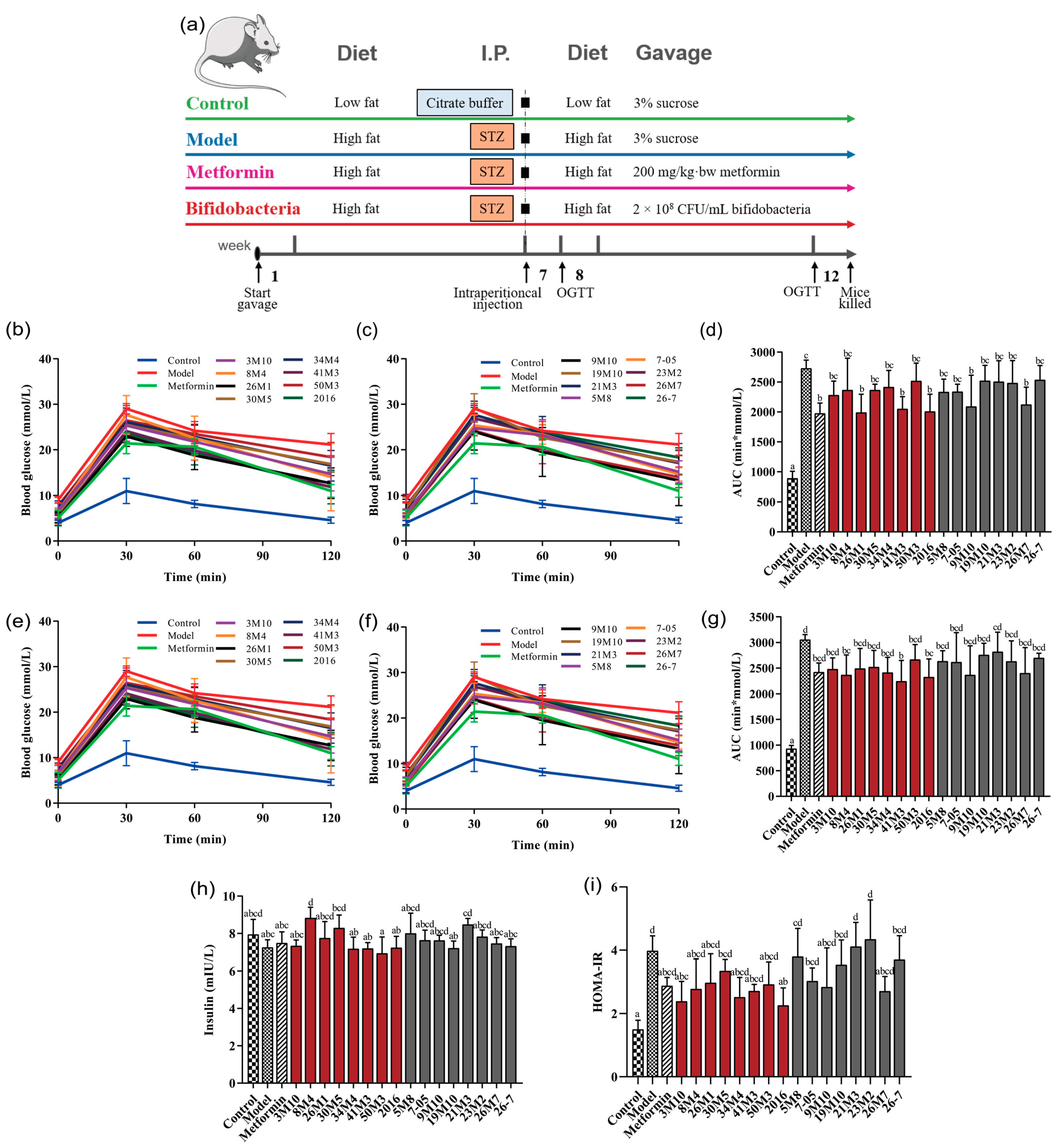
2.2. Animal Experiments
2.3. Sample Collection and Processing
2.4. Biochemical Analysis
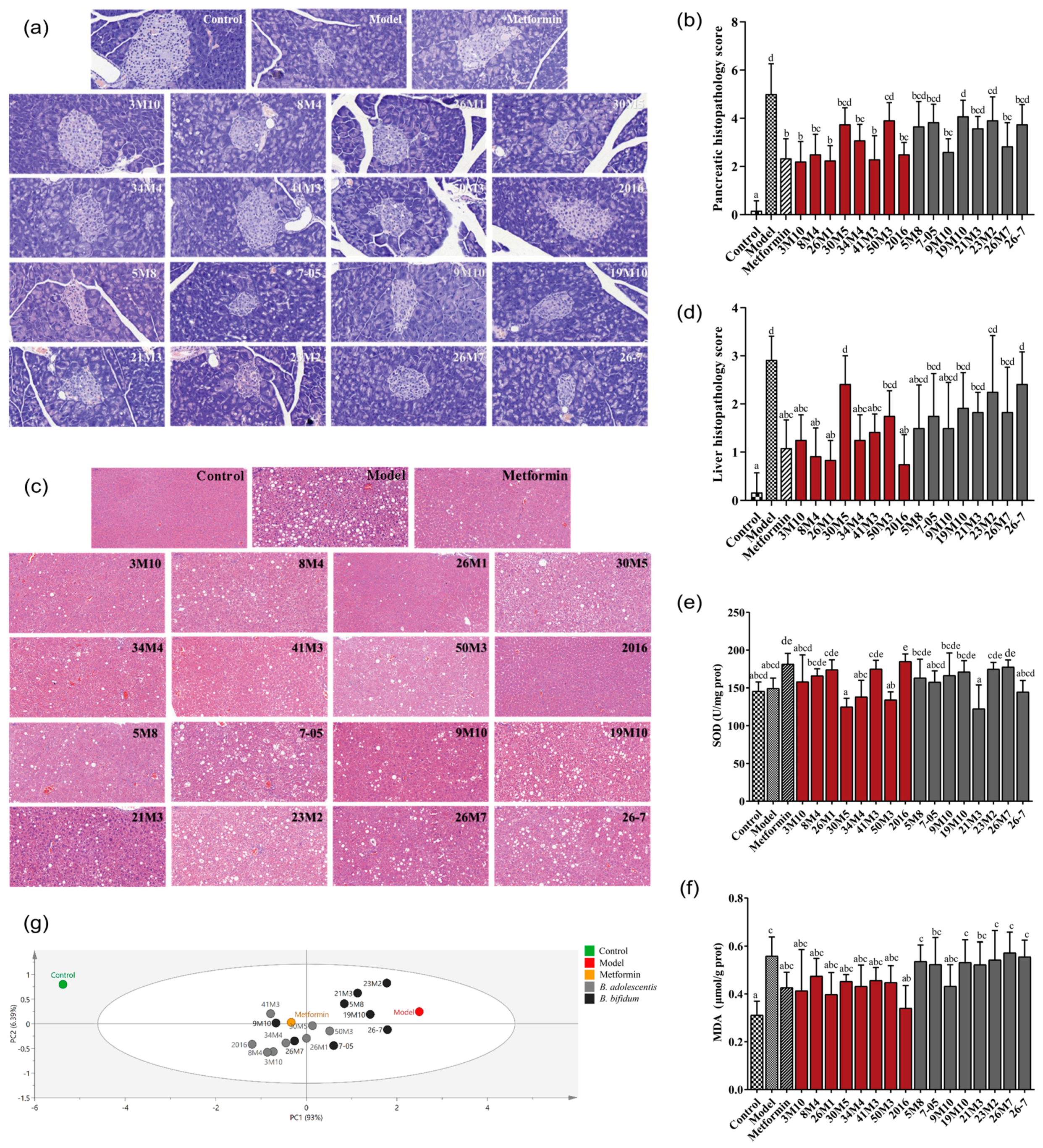
2.5. Histopathological Analysis
2.6. SCFA Analysis
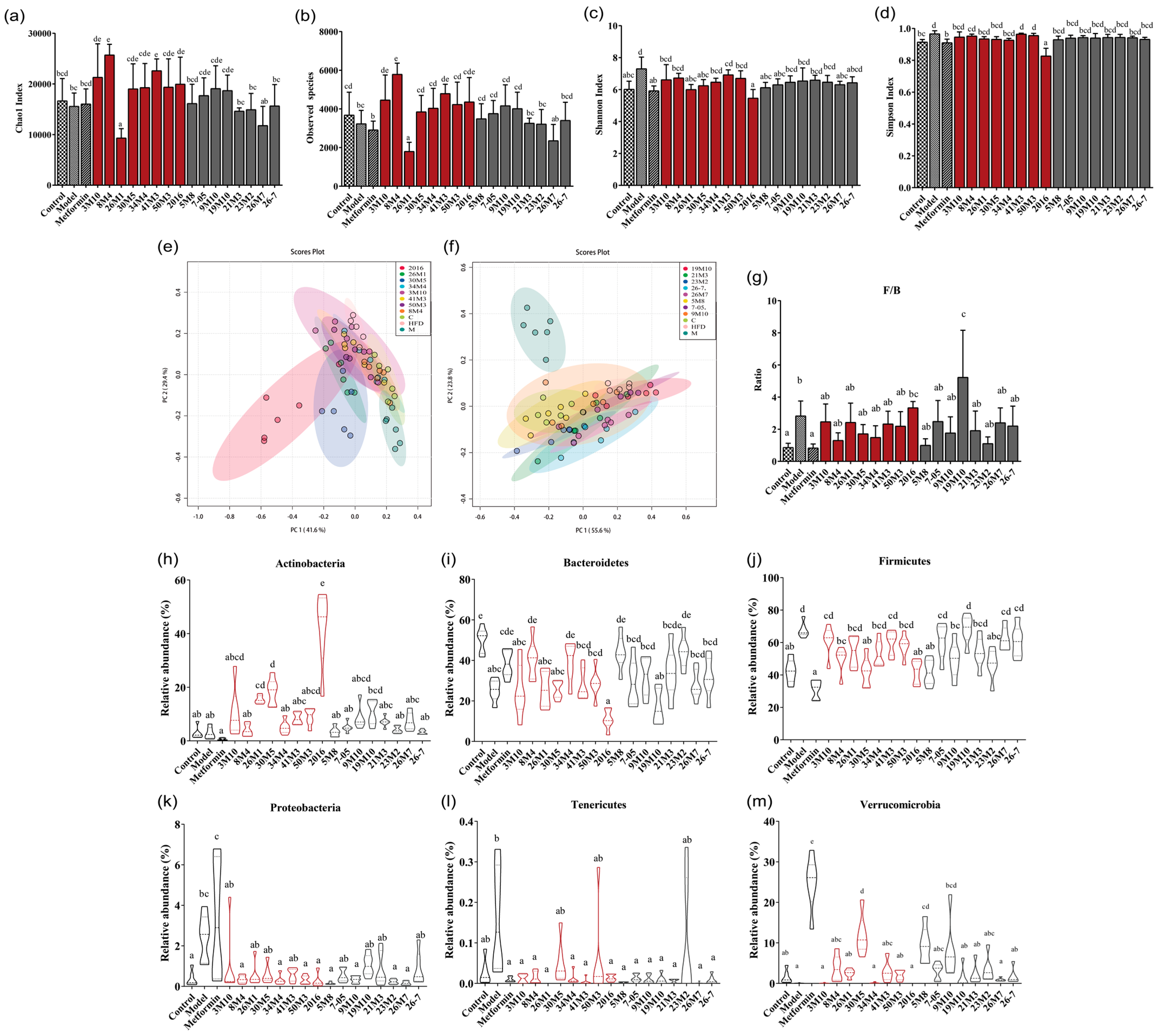
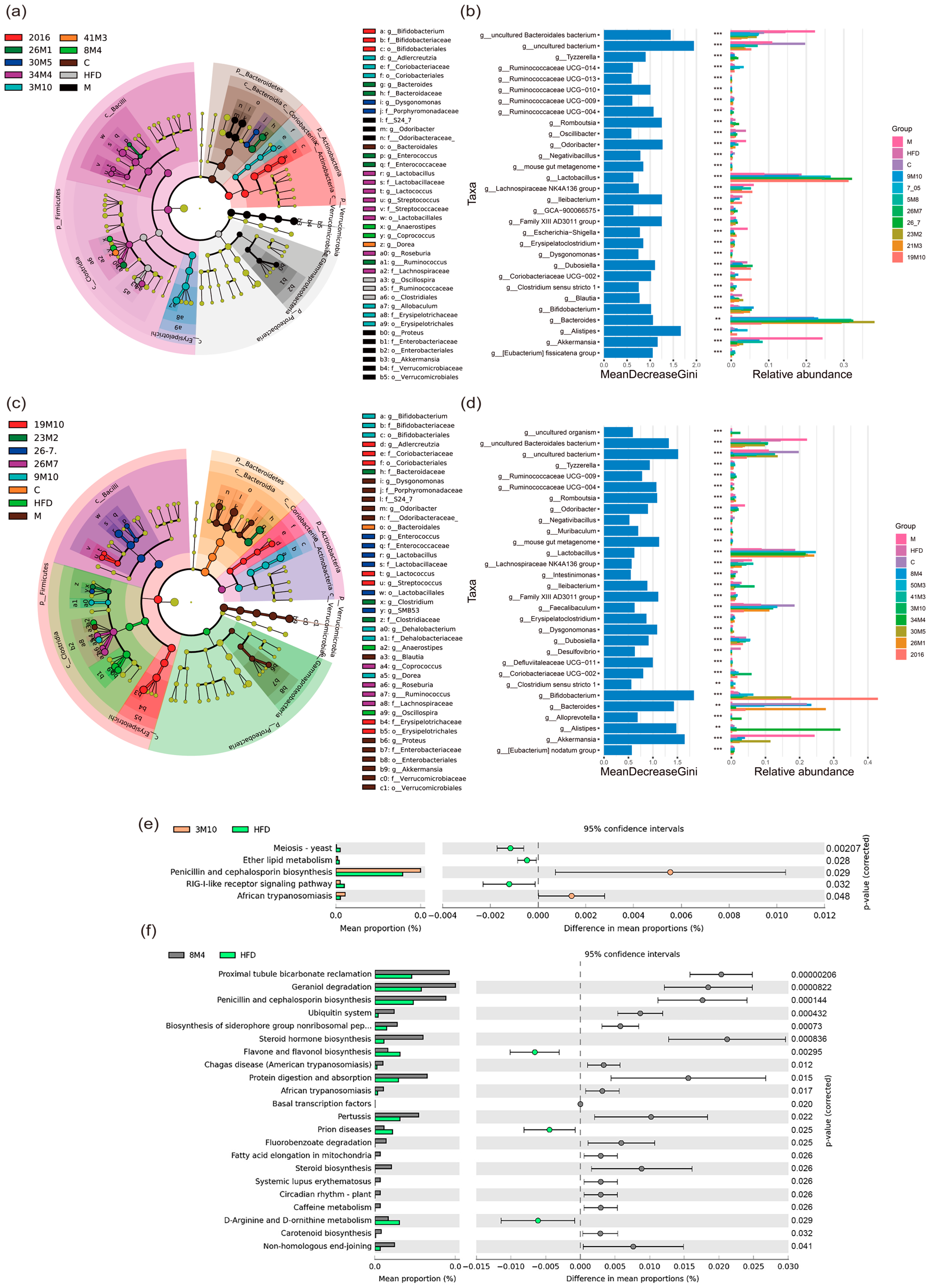
2.7. Gut Microbiota Profiling and Bioinformatical Analysis of Genome
2.8. Statistical Analysis
3. Results
3.1. B. adolescentis and B. bifidum Show Differential Effectiveness in Regulating Glucose and Lipid Metabolism Disorders in T2D Mice
3.2. B. adolescentis Strains Showed Superior Potential Than B. bifidum Strains in Attenuating Pancreatic and Liver Damage in T2D Mice
3.3. Bifidobacterium Strains Restore Gut Microbiota Homeostasis in Mice with T2D and Alleviate Symptoms
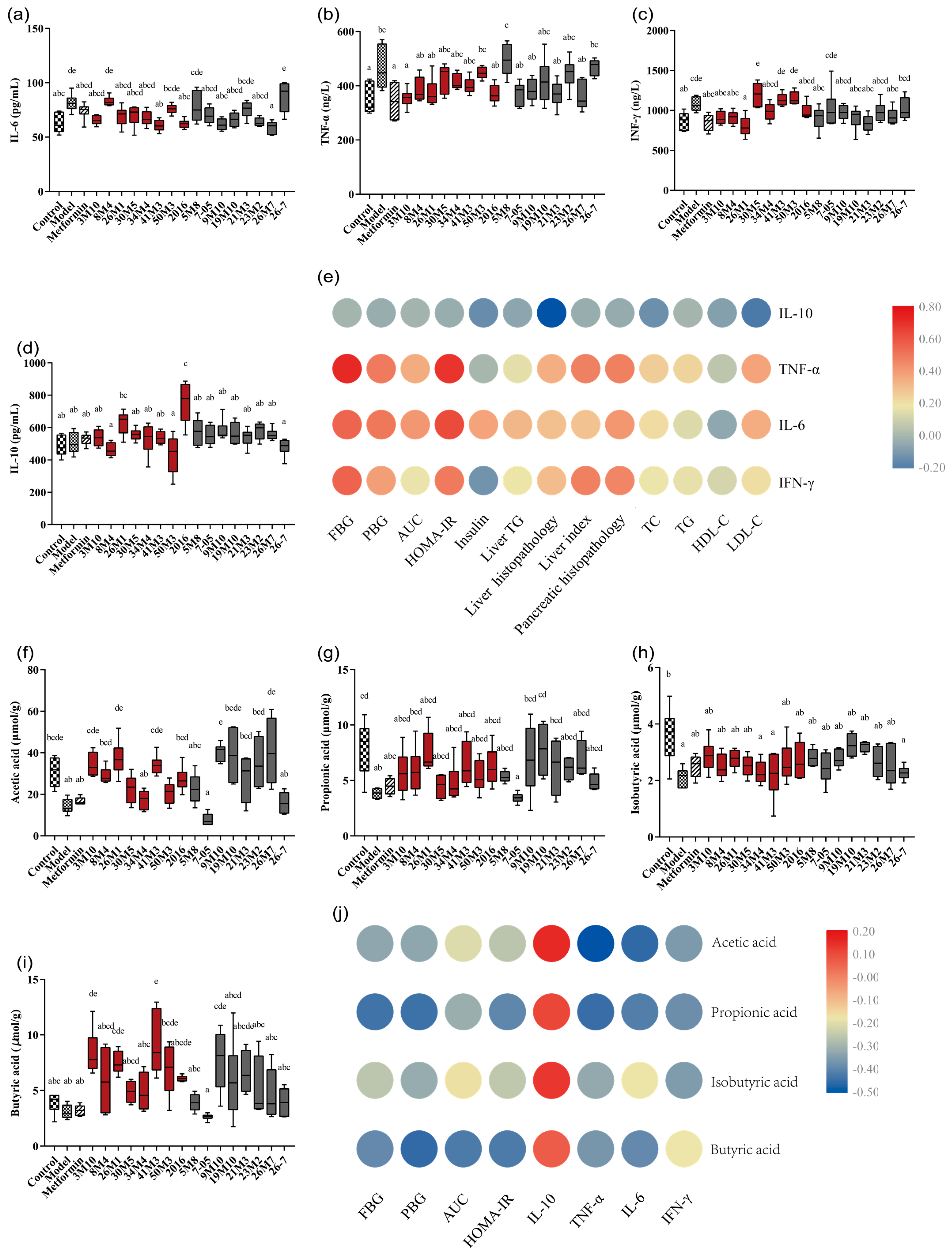
3.4. Bifidobacterium Strains Alleviates the Symptoms of T2D via the Gut Microbiota-SCFA-Inflammation Axis
3.5. The Greater Number and Stability of Core Genes, and a Unique Blood Sugar Regulation Gene May Give B. adolescentis an Advantage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ezzati, M.; Vander Hoorn, S.; Lopez, A.D.; Danaei, G.; Rodgers, A.; Mathers, C.D.; Murray, C.J.L. Comparative quantification of mortality and burden of disease attributable to selected risk factors. In Global Burden of Disease & Risk Factors; Oxford University Press: New York, NY, USA, 2006; Volume 369, pp. 241–396. [Google Scholar]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9 th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covington, P.; Christopher, R.; Davenport, M.; Fleck, P.; Mekki, Q.A.; Wann, E.R.; Karim, A. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: A randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin. Ther. 2008, 30, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Screening for potential novel probiotics with dipeptidyl peptidase iv-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front. Microbiol. 2019, 10, 2855. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H. Microbiota associated with type 2 diabetes and its related complications. Food Sci. Hum. Wellness 2013, 2, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Duranti, S.; Turroni, F.; Milani, C.; Foroni, E.; Bottacini, F.; Dal Bello, F.; Ferrarini, A.; Delledonne, M.; van Sinderen, D.; Ventura, M. Exploration of the genomic diversity and core genome of the bifidobacterium adolescentis phylogenetic group by means of a polyphasic approach. Appl. Environ. Microbiol. 2013, 79, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Ventura, M.; Butto, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A lactobacillus and bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Pulavendran, S.; Prasanthi, M.; Ramachandran, A.; Grant, R.; Snider, T.A.; Chow, V.T.; Malayer, J.R.; Teluguakula, N. Production of Neutrophil Extracellular Traps Contributes to the Pathogenesis of Francisella tularemia. Front. Immunol. 2020, 11, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Li, D.; Zhao, J.; Liu, X.; Gu, Z.; Chen, Y.Q.; Zhang, H.; Chen, W. Metagenomic insights into the effects of fructo-oligosaccharides (fos) on the composition of fecal microbiota in mice. J. Agric. Food Chem. 2015, 63, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Garidou, L.; Pomié, C.; Klopp, P.; Waget, A.; Charpentier, J.; Aloulou, M.; Giry, A.S.; Serino, M.; Stenman, L.; Lahtinen, S. The gut microbiota regulates intestinal cd4t cells expressing rorγt and controls metabolic disease. Cell Metab. 2015, 22, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, K.; Deng, Y.; Chen, R.; Liang, S.; Xie, H.; He, Y.; Chen, Y.; Yang, Q. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced nafld rats. Biomed. Pharmacother. 2018, 102, 1025–1036. [Google Scholar] [CrossRef]
- Sara, C.P.; David, P.; María, V.G.M.; Susana, M.F.; María, J.F. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Liang, X.; Feng, Q.; Li, J.; Pan, X.; Xie, P.; Jiang, Z.; Yang, Z. Ergosterol peroxide suppresses influenza a virus-induced pro-inflammatory response and apoptosis by blocking rig-i signaling. Eur. J. Pharmacol. 2019, 860, 172543. [Google Scholar] [CrossRef]
- Park, Y.; Jin, H.S.; Aki, D.; Lee, J.; Liu, Y.C. The ubiquitin system in immune regulation. Adv. Immunol. 2014, 124, 17–66. [Google Scholar] [CrossRef]
- Corsini, E.; Ruffo, F.; Racchi, M. Steroid hormones, endocrine disrupting compounds and immunotoxicology. Curr. Opin. Toxicol. 2018, 10, 69–73. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Gao, H.; Fan, L.; Chen, H.; Nie, S. Polysaccharide from plantago asiatica l. Attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocoll. 2017, 86, 34–42. [Google Scholar] [CrossRef]
- Tang, W.J.; Zhou, B.J. Imbalance of intestinal flora: A new target for nonalcoholic fatty liver disease treatment. World Chin. J. Dig. 2017, 25, 2000. [Google Scholar] [CrossRef]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhao, Y.; Xia, N.; Zhang, W.; Tang, Z.; Wang, C.; Zhu, X.; Cui, S. Kpnβ1 promotes palmitate-induced insulin resistance via nf-κb signaling in hepatocytes. J. Physiol. Biochem. 2015, 71, 763–772. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ho, K.T.; Lu, S.H.; Huang, C.N.; Shiau, M.Y. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef] [Green Version]
- Alipourfard, I.; Datukishvili, N.; Mikeladze, D. Tnf-α downregulation modifies insulin receptor substrate 1 (irs-1) in metabolic signaling of diabetic insulin-resistant hepatocytes. Mediat. Inflamm. 2019, 2019, 3560819. [Google Scholar] [CrossRef] [Green Version]
- Garland, S.H. Short chain fatty acids may elicit an innate immune response from preadipocytes: A potential link between bacterial infection and inflammatory diseases. Med. Hypotheses 2011, 76, 881–883. [Google Scholar] [CrossRef]
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of lactobacillus casei ccfm419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef. Microbes 2017, 8, 421–432. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, R.K.; Malhotra, S.; Pothuraju, R.; Shandilya, U.K. Lactobacillus rhamnosus ncdc17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef. Microbes 2017, 8, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, E.C.; Leonel, A.J.; Pelaez, J.M.N.; Lemos, V.S.; Silva, A.R. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing nfκb activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.W.; Sun, W.L.; Yu, N.; Sun, J.; Yu, X.X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.Z.; Xiu, Z.L.; et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.N.; et al. Roseburia spp. Abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell Host Microbe 2020, 27, 25–40.e26. [Google Scholar] [CrossRef]
- Faraz, B.; Phillip, E.; Nailliw, P.; Yunus, T.; Ankur, N.; Maliha, S.; Marco, R.; Sherry, W.; Stefan, G.; Bruce, H. Dietary fiber treatment corrects the composition of gut microbiota, promotes scfa production, and suppresses colon carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.X.; He, J.F.; Luo, X.G.; Zhang, T.C. Hypoglycemic effect of hypericum attenuatum choisy extracts on type 2 diabetes by regulating glucolipid metabolism and modulating gut microbiota. J. Funct. Foods 2019, 52, 479–491. [Google Scholar] [CrossRef]
- Ratajczak, W.; Ryl, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczynska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (scfas). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Liao, Z.; Lu, B.; Wang, M.; Lin, L.; Zhang, S.; Li, Y.; Liu, D.; Liao, Q.; Xie, Z. Huang-lian-jie-du-decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota modulation. Front. Microbiol. 2018, 9, 2380. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Casajus, N.; Dauvillee, D.; Viale, A.M.; Munoz, F.J.; Baroja-Fernandez, E.; Moran-Zorzano, M.T.; Eydallin, G.; Ball, S.; Pozueta-Romero, J. Glycogen phosphorylase, the product of the glgp gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J. Bacteriol. 2006, 188, 5266–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Species | Strain | Number | Source | Culture Conditions |
|---|---|---|---|---|
| B. adolescentis | FJSSZ3M10 | 3M10 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FGSZY8M4 (CCFM1108) | 8M4 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FHNFQ26M1 | 26M1 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FGSYC30M5 | 30M5 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FXJKS34M4 | 34M4 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FHNFQ41M3 | 41M3 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | FXJCJ50M3 | 50M3 | Human faeces | 37 °C, MRS, anaerobic |
| B. adolescentis | HuNan112 (CCFM1261) | 2016 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | FJSSZ5M8 | 5M8 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | FSDJN705 | 7-05 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | FXJCJ9M10 | 9M10 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | JSWX19M5 | 19M10 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | AHWH21M3 | 21M3 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | FHNFQ23M2 | 23M2 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | FHNFQ26M7 (CCFM1165) | 26M7 | Human faeces | 37 °C, MRS, anaerobic |
| B. bifidum | JSWX267 | 26-7 | Human faeces | 37 °C, MRS, anaerobic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Si, Q.; Lin, G.; Zhu, M.; Lu, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients 2022, 14, 2479. https://doi.org/10.3390/nu14122479
Qian X, Si Q, Lin G, Zhu M, Lu J, Zhang H, Wang G, Chen W. Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients. 2022; 14(12):2479. https://doi.org/10.3390/nu14122479
Chicago/Turabian StyleQian, Xin, Qian Si, Guopeng Lin, Minmin Zhu, Jingyu Lu, Hao Zhang, Gang Wang, and Wei Chen. 2022. "Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity" Nutrients 14, no. 12: 2479. https://doi.org/10.3390/nu14122479
APA StyleQian, X., Si, Q., Lin, G., Zhu, M., Lu, J., Zhang, H., Wang, G., & Chen, W. (2022). Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients, 14(12), 2479. https://doi.org/10.3390/nu14122479






