Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development
Abstract
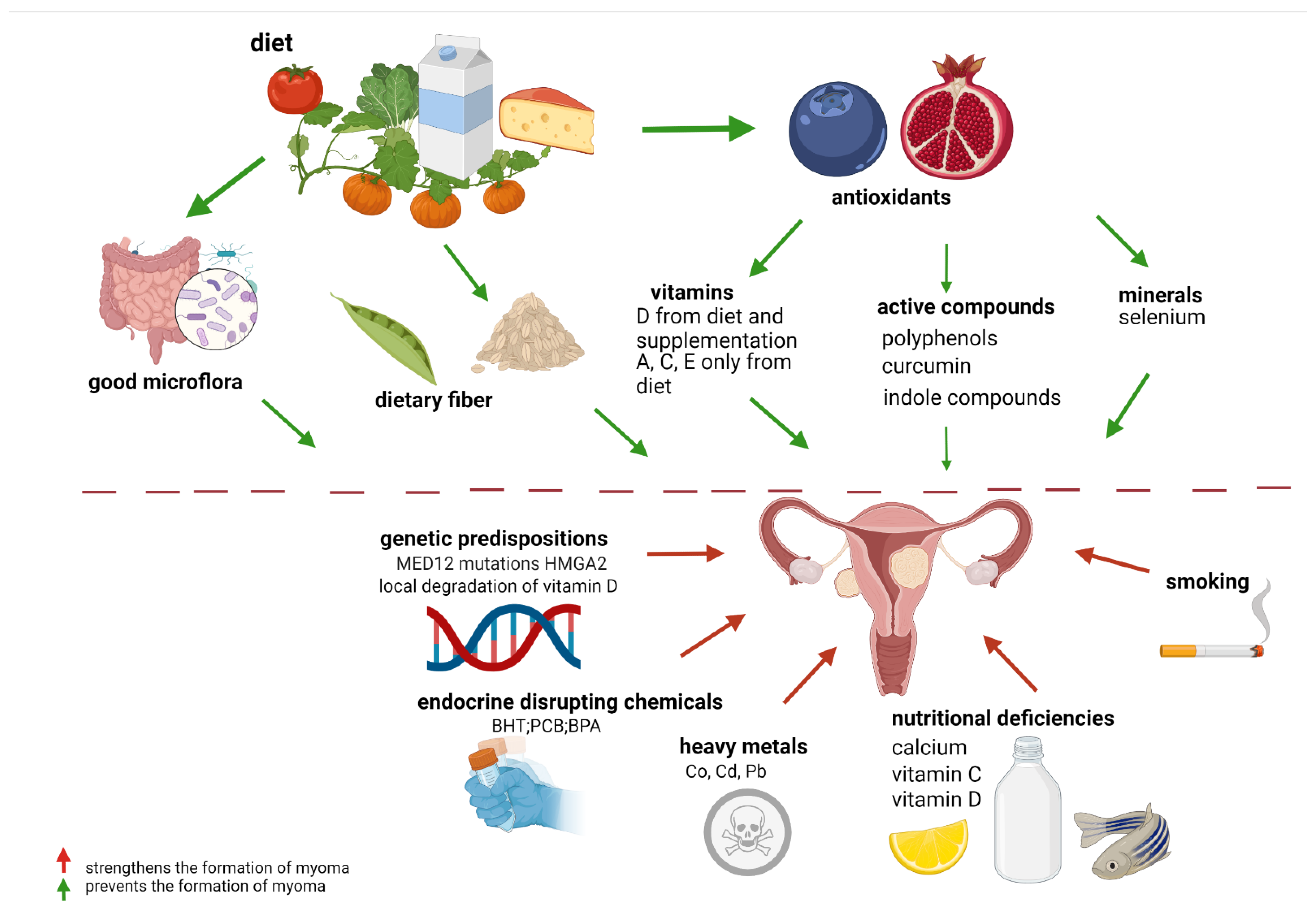
1. Introduction
1.1. Nutrients and Their Deficiencies
1.2. The Influence of Intestinal Dysbiosis
2. Vitamins
2.1. Carotenoids, such as Lycopene
2.2. Vitamin A. Retinoids
2.3. Vitamin E
2.4. Vitamin D
2.5. Vitamin C
2.6. Other Vitamins
3. The Active Compounds from Plants
3.1. Green Tea—Polyphenols
3.2. Curcumin/Turmeric
3.3. Quercetin and Indole-3-Carbinol
4. Micro- and Macro Elements
4.1. Selenium
4.2. Other Trace Elements
5. Endocrine-Disrupting Chemicals
6. Conclusions
7. Methods
Search Strategy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galindo, L.J.; Hernández-Beeftink, T.; Salas, A.; Jung, Y.; Reyes, R.; de Oca, F.M.; Hernández, M.; Almeida, T.A. HMGA2 and MED12 alterations frequently co-occur in uterine leiomyomas. Gynecol. Oncol. 2018, 150, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Machado-Lopez, A.; Simón, C.; Mas, A. Molecular and Cellular Insights into the Development of Uterine Fibroids. Int. J. Mol. Sci. 2021, 22, 8483. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, E.; Qiang, W.; Zhang, Q.; Espona-Fiedler, M.; Druschitz, S.A.; Liu, Y.; Mittal, K.; Kong, B.; Kurita, T.; Wei, J.-J. MED12 and HMGA2 mutations: Two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod. Pathol. 2014, 27, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, H.-R.; Mehine, M.; Mäkinen, N.; Pasanen, A.; Pitkänen, E.; Karhu, A.; Sarvilinna, N.S.; Sjöberg, J.; Heikinheimo, O.; Bützow, R.; et al. Global metabolomic profiling of uterine leiomyomas. Br. J. Cancer 2017, 117, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Markowski, D.N.; Bartnitzke, S.; Löning, T.; Drieschner, N.; Helmke, B.M.; Bullerdiek, J. MED12 mutations in uterine fibroids-their relationship to cytogenetic subgroups. Int. J. Cancer 2012, 131, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Jelinsky, S.A.; Harris, H.A.; Choe, S.E.; Cotreau, M.M.; Kimberland, M.L.; Wilson, E.; Saraf, K.A.; Liu, W.; McCampbell, A.S.; et al. Comparison of Human and Rat Uterine Leiomyomata: Identification of a Dysregulated Mammalian Target of Rapamycin Pathway. Cancer Res. 2009, 69, 6171–6178. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Abusamaan, M.S.; Memaj, I.; Saed, M.G.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Oxidative stress: A key regulator of leiomyoma cell survival. Fertil. Steril. 2017, 107, 1387–1394.e1. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Saed, M.G.; Abu-Soud, H.M.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Uterine fibroids are characterized by an impaired antioxidant cellular system: Potential role of hypoxia in the pathophysiology of uterine fibroids. J. Assist. Reprod. Genet. 2013, 30, 969–974. [Google Scholar] [CrossRef]
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine Fibroids: Pathogenesis and Interactions with Endometrium and Endomyometrial Junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Biagini, A.; Lucarini, G.; Carpini, G.D.; Berretta, A.; Di Primio, R.; Ciavattini, A. Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells. Stem Cells Int. 2018, 2018, 1716246. [Google Scholar] [CrossRef]
- Protic, O.; Toti, P.; Islam, S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Wesselink, A.K.; Bethea, T.N.; Brasky, T.M.; Wegienka, G.; Harmon, Q.; Block, T.; Baird, D.D. Intake of Lycopene and other Carotenoids and Incidence of Uterine Leiomyomata: A Prospective Ultrasound Study. J. Acad. Nutr. Diet. 2021, 121, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Orta, O.R.; Terry, K.L.; Missmer, S.A.; Harris, H.R. Dairy and related nutrient intake and risk of uterine leiomyoma: A prospective cohort study. Hum. Reprod. 2020, 35, 453–463. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, Q.; Dong, S.; Qin, L.; Li, G.; Wang, P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: A case-control study in China. Asia Pac. J. Clin. Nutr. 2013, 22, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Y.; Lu, Q.; Ren, M. Vegetarian diet and reduced uterine fibroids risk: A case-control study in Nanjing, China. J. Obstet. Gynaecol. Res. 2016, 42, 87–94. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Boggs, D.A.; Rosenberg, L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am. J. Clin. Nutr. 2011, 94, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhai, Y.; Wang, C.; Liu, T.; Tian, S. Association of dietary diversity with uterine fibroids among urban premenopausal women in Shijiazhuang, China: A cross-sectional study. Asia Pac. J. Clin. Nutr. 2020, 29, 771–781. [Google Scholar] [CrossRef]
- Martin, C.L.; Huber, L.R.B.; Thompson, M.E.; Racine, E.F. Serum Micronutrient Concentrations and Risk of Uterine Fibroids. J. Women’s Health 2011, 20, 915–922. [Google Scholar] [CrossRef]
- Terry, K.L.; Missmer, S.A.; Hankinson, S.E.; Willett, W.C.; De Vivo, I. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am. J. Obstet. Gynecol. 2008, 198, 37.e1–37.e8. [Google Scholar] [CrossRef]
- Wise, L.A.; Palmer, J.R.; Ruiz-Narvaez, E.; Reich, D.E.; Rosenberg, L. Is the Observed Association Between Dairy Intake and Fibroids in African Americans Explained by Genetic Ancestry? Am. J. Epidemiology 2013, 178, 1114–1119. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.; Palmer, J.; Kumanyika, S.K.; Rosenberg, L. A Prospective Study of Dairy Intake and Risk of Uterine Leiomyomata. Am. J. Epidemiology 2010, 171, 221–232. [Google Scholar] [CrossRef]
- Makwe, C.C.; Soibi-Harry, A.P.; Rimi, G.S.; Ugwu, O.A.; Ajayi, A.T.; Adesina, T.A.; Okunade, K.S.; Oluwole, A.A.; Anorlu, R.I. Micronutrient and Trace Element Levels in Serum of Women With Uterine Fibroids in Lagos. Cureus 2021, 13, e18638. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Akhtar, M.M.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Molecular targets of dietary phytochemicals for possible prevention and therapy of uterine fibroids: Focus on fibrosis. Crit. Rev. Food Sci. Nutr. 2017, 57, 3583–3600. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Zannotti, A.; Greco, S.; Pellegrino, P.; Giantomassi, F.; Carpini, G.D.; Goteri, G.; Ciavattini, A.; Ciarmela, P. Macrophages and Immune Responses in Uterine Fibroids. Cells 2021, 10, 982. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Chunwei, Z.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Torun, A.; Hupalowska, A.; Trzonkowski, P.; Kierkus, J.; Pyrzynska, B. Intestinal Microbiota in Common Chronic Inflammatory Disorders Affecting Children. Front. Immunol. 2021, 12, 642166. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Bahar, A.; Keikha, M.; Karbalaei, M.; Kobyliak, N.; Yousefi, B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocrine, Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Behrooz, M.; Gargari, B.P. Daily consumption effects of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clin. Nutr. ESPEN 2021, 41, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, N.; Gursu, M.F.; Khachik, F.; Sarkar, F.H.; Munkarah, A.; Ali-Fehmi, R.; Kmak, D.; et al. Lycopene Supplementation Prevents the Development of Spontaneous Smooth Muscle Tumors of the Oviduct in Japanese Quail. Nutr. Cancer 2004, 50, 181–189. [Google Scholar] [CrossRef]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, N.; Khachik, F.; Seren, S.; Kucuk, O. Dietary Tomato Powder Supplementation in the Prevention of Leiomyoma of the Oviduct in the Japanese Quail. Nutr. Cancer 2007, 59, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, R.R.; Xie, S.; Hsu, C.-J.; Wang, J.; Zhang, S.; Zou, C. The chemopreventive agents 4-HPR and DFMO inhibit growth and induce apoptosis in uterine leiomyomas. Am. J. Obstet. Gynecol. 2004, 190, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A. Retinoids regulate genes involved in retinoic acid synthesis and transport in human myometrial and fibroid smooth muscle cells. Hum. Reprod. 2008, 23, 1076–1086. [Google Scholar] [CrossRef]
- Lattuada, D.; Vigano, P.; Mangioni, S.; Sassone, J.; Di Francesco, S.; Vignali, M.; Di Blasio, A.M. Accumulation of Retinoid X Receptor-α in Uterine Leiomyomas Is Associated with a Delayed Ligand-Dependent Proteasome-Mediated Degradation and an Alteration of Its Transcriptional Activity. Mol. Endocrinol. 2007, 21, 602–612. [Google Scholar] [CrossRef][Green Version]
- Ben-Sasson, H.; Ben-Meir, A.; Shushan, A.; Karra, L.; Rojansky, N.; Klein, B.Y.; Levitzki, R.; Ben-Bassat, H. All-trans-retinoic acid mediates changes in PI3K and retinoic acid signaling proteins of leiomyomas. Fertil. Steril. 2011, 95, 2080–2086. [Google Scholar] [CrossRef]
- Malik, M.; Webb, J.; Catherino, W.H. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin. Endocrinol. 2008, 69, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga-Semeniuk, E.; Wołczyński, S. Dietary carotenoids in normal and pathological tissues of corpus uteri. Folia Histochem. Cytobiol. 2008, 46, 283–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A.W. Retinoic acid pathway genes show significantly altered expression in uterine fibroids when compared with normal myometrium. Mol. Hum. Reprod. 2007, 13, 577–585. [Google Scholar] [CrossRef]
- Catherino, W.H.; Malik, M. Uterine leiomyomas express a molecular pattern that lowers retinoic acid exposure. Fertil. Steril. 2007, 87, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Holdsworth-Carson, S.J.; Waldrip, L.; Nevzorova, J.; Martelotto, L.; Vollenhoven, B.J.; Rogers, P.A.W. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction 2013, 146, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Szymańska-Majchrzak, J.; Sentkowska, A.; Kilian, K.; Rogulski, Z.; Nowicka, G.; Jakiel, G.; Tomaszewski, P.; Włodarczyk, M. Alpha-Tocopherol Serum Levels Are Increased in Caucasian Women with Uterine Fibroids: A Pilot Study. BioMed Res. Int. 2018, 2018, 6793726. [Google Scholar] [CrossRef]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 Reduces Extracellular Matrix-Associated Protein Expression in Human Uterine Fibroid Cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef]
- Lima, M.S.O.; da Silva, B.B.; de Medeiros, M.L.; dos Santos, A.R.; Brazil, E.D.D.N.; Filho, W.M.N.E.; Ibiapina, J.O.; Brito, A.G.A.; Costa, P.V.L. Evaluation of vitamin D receptor expression in uterine leiomyoma and nonneoplastic myometrial tissue: A cross-sectional controlled study. Reprod. Biol. Endocrinol. 2021, 19, 67. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 2015, 100, E572–E582. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Halder, S.K.; Allah, A.S.A.; Roshdy, E.; Rajaratnam, V.; Sabry, M. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Women’s Health 2013, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the Risk of Uterine Fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Paffoni, A.; Somigliana, E.; Vigano, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D Status in Women With Uterine Leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The associations between serum vitamin D, calcium and uterine fibroids in Chinese women: A case-controlled study. J. Int. Med Res. 2020, 48, 300060520923492. [Google Scholar] [CrossRef]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef]
- Wise, L.A.; Ruiz-Narváez, E.A.; Haddad, S.A.; Rosenberg, L.; Palmer, J.R. Polymorphisms in vitamin D–related genes and risk of uterine leiomyomata. Fertil. Steril. 2014, 102, 503–510.e1. [Google Scholar] [CrossRef]
- Singh, V.; Barik, A.; Imam, N. Vitamin D3 Level in Women with Uterine Fibroid: An Observational Study in Eastern Indian Population. J. Obstet. Gynecol. India 2019, 69, 161–165. [Google Scholar] [CrossRef]
- Tunau, K.A.; Garba, J.A.; Panti, A.A.; Shehu, C.E.; Adamu, A.N.; AbdulRahman, M.B.; Ahmad, M.K. Low plasma vitamin D as a predictor of uterine fibroids in a nigerian population. Niger. Postgrad. Med J. 2021, 28, 181–186. [Google Scholar] [CrossRef]
- Xu, F.; Li, F.; Li, L.; Lin, D.; Hu, H.; Shi, Q. Vitamin D as a risk factor for the presence of asymptomatic uterine fibroids in premenopausal Han Chinese women. Fertil. Steril. 2021, 115, 1288–1293. [Google Scholar] [CrossRef]
- Bläuer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- ElHusseini, H.; Elkafas, H.; Abdelaziz, M.; Halder, S.; Atabiekov, I.; Eziba, N.; Ismail, N.; El Andaloussi, A.; Al-Hendy, A. Diet-induced vitamin D deficiency triggers inflammation and DNA damage profile in murine myometrium. Int. J. Women’s Health 2018, 10, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.R.; Ahmed, E.; Sayed, A.A.; Hussein, M.; Abdelaal, I.I.; Fetih, A.N.; Abou-Taleb, H.A.; Yousef, A.-E.A. Human uterine leiomyoma contains low levels of 1, 25 dihdroxyvitamin D3, and shows dysregulated expression of vitamin D metabolizing enzymes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Włodarczyk, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef]
- Islam, S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Updat. 2018, 24, 59–85. [Google Scholar] [CrossRef]
- Arici, A.; Sozen, I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Ciavattini, A.; Carpini, G.D.; Serri, M.; Vignini, A.; Sabbatinelli, J.; Tozzi, A.; Aggiusti, A.; Clemente, N. Hypovitaminosis D and “small burden” uterine fibroids: Opportunity for a vitamin D supplementation. Medicine 2016, 95, e5698. [Google Scholar] [CrossRef]
- Kaplan, Z.A.O.; Taşçi, Y.; Topçu, H.O.; Erkaya, S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol. Endocrinol. 2018, 34, 261–264. [Google Scholar] [CrossRef]
- Halder, S.K.; Sharan, C.; Al-Hendy, O.; Al-Hendy, A. Paricalcitol, a Vitamin D Receptor Activator, Inhibits Tumor Formation in a Murine Model of Uterine Fibroids. Reprod. Sci. 2014, 21, 1108–1119. [Google Scholar] [CrossRef]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 Treatment Shrinks Uterine Leiomyoma Tumors in the Eker Rat Model1. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Ogawa, K.; Mori, T.; Ogi, H.; Itoh, K.; Teramukai, S.; Kitawaki, J. Expression profiles of E/P receptors and fibrosis in GnRHa-treated and -untreated women with different uterine leiomyomas. PLoS ONE 2020, 15, e0242246. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, I.; Grabowska, M.; Nawrocka-Rutkowska, J.; Piasecka, M.; Starczewski, A. Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. J. Clin. Med. 2021, 10, 562. [Google Scholar] [CrossRef]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 Reduces TGF-β3-Induced Fibrosis-Related Gene Expression in Human Uterine Leiomyoma Cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef]
- Hajhashemi, M.; Ansari, M.; Haghollahi, F.; Eslami, B. The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Casp. J. Intern. Med. 2019, 10, 125–131. [Google Scholar] [CrossRef]
- Arjeh, S.; Darsareh, F.; Asl, Z.A.; Kutenaei, M.A. Effect of oral consumption of vitamin D on uterine fibroids: A randomized clinical trial. Complement. Ther. Clin. Pr. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Tanha, F.D.; Feizabad, E.; Farahani, M.V.; Amuzegar, H.; Moradi, B.; Sadeh, S.S. The Effect of Vitamin D Deficiency on Overgrowth of Uterine Fibroids: A Blinded Randomized Clinical Tria. Int. J. Fertil. Steril. 2021, 15, 95–100. [Google Scholar] [CrossRef]
- Suneja, A.; Faridi, F.; Bhatt, S.; Guleria, K.; Mehndiratta, M.; Sharma, R. Effect of Vitamin D3 supplementation on symptomatic uterine leiomyoma in women with Hypovitaminosis D. J. Midlife Health 2021, 12, 53–60. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Escrig, J.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Long-term vitamin D treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil. Steril. 2020, 113, 205–216.e4. [Google Scholar] [CrossRef]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Diamond, M.P.; Boyer, T.G.; Halder, S.K. Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells. J. Clin. Endocrinol. Metab. 2016, 101, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Corachán, A.; Trejo, M.G.; Carbajo-García, M.C.; Monleón, J.; Escrig, J.; Faus, A.; Pellicer, A.; Cervelló, I.; Ferrero, H. Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation. Fertil. Steril. 2021, 115, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol. Sin. 2019, 40, 957–970. [Google Scholar] [CrossRef]
- Elkafas, H.; Ali, M.; Elmorsy, E.; Kamel, R.; Thompson, W.E.; Badary, O.; Al-Hendy, A.; Yang, Q.; Yang, Q. Vitamin D3 Ameliorates DNA Damage Caused by Developmental Exposure to Endocrine Disruptors in the Uterine Myometrial Stem Cells of Eker Rats. Cells 2020, 9, 1459. [Google Scholar] [CrossRef]
- Xess, S.; Sahu, J. A study to correlate association between vitamin D with fibroid and its supplementation in the progression of the disease. Int. J. Reprod. Contracept. Obstet. Gynecol. 2020, 9, 1477–1481. [Google Scholar] [CrossRef][Green Version]
- Sheng, B.; Song, Y.; Liu, Y.; Jiang, C.; Zhu, X. Association between vitamin D and uterine fibroids: A study protocol of an open-label, randomised controlled trial. BMJ Open 2020, 10, e038709. [Google Scholar] [CrossRef]
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus epigallocatechin gallate: A novel promising approach for uterine myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar]
- Yılmaz, S.G.; Gul, T.; Attar, R.; Yıldırım, G.; Isbir, T. Association between fok1 polymorphism of vitamin D receptor gene with uterine leiomyoma in Turkish populations. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 128–131. [Google Scholar] [CrossRef]
- Fazeli, E.; Piltan, S.; Gholami, M.; Akbari, M.; Falahati, Z.; Yassaee, F.; Sadeghi, H.; Mirfakhraie, R. CYP24A1 expression analysis in uterine leiomyoma regarding MED12 mutation profile. Arch. Gynecol. Obstet. 2021, 303, 787–792. [Google Scholar] [CrossRef]
- Man, A.M.S.-D.; Elbers, P.; Straaten, H.M.O.-V. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef]
- Lee, B.; Kim, K.; Cho, H.Y.; Yang, E.J.; Suh, D.H.; No, J.H.; Lee, J.R.; Hwang, J.W.; Do, S.H.; Kim, Y.B. Effect of intravenous ascorbic acid infusion on blood loss during laparoscopic myomectomy: A randomized, double-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pourmatroud, E.; Hormozi, L.; Hemadi, M.; Golshahi, R. Intravenous ascorbic acid (vitamin C) administration in myomectomy: A prospective, randomized, clinical trial. Arch. Gynecol. Obstet. 2012, 285, 111–115. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green Tea Extract Inhibition of Human Leiomyoma Cell Proliferation Is Mediated via Catechol- O -Methyltransferase. Gynecol. Obstet. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef]
- Ozercan, I.H.; Sahin, N.; Akdemir, F.; Onderci, M.; Seren, S.; Sahin, K.; Kucuk, O. Chemoprevention of fibroid tumors by [−]-epigallocatechin-3-gallate in quail. Nutr. Res. 2008, 28, 92–97. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Women’s Health 2013, 5, 477–486. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Grandi, G.; Del Savio, M.C.; Melotti, C.; Feliciello, L.; Facchinetti, F. Vitamin D and green tea extracts for the treatment of uterine fibroids in late reproductive life: A pilot, prospective, daily-diary based study. Gynecol. Endocrinol. 2021, 38, 63–67. [Google Scholar] [CrossRef]
- Miriello, D.; Galanti, F.; Cignini, P.; Antonaci, D.; Schiavi, M.C.; Rago, R. Uterine fibroids treatment: Do we have new valid alternative? Experiencing the combination of vitamin D plus epigallocatechin gallate in childbearing age affected women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2843–2851. [Google Scholar] [CrossRef]
- Ahmed, R.S.I.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El Banna, H.; Foldes, R.; et al. Biological and Mechanistic Characterization of Novel Prodrugs of Green Tea Polyphenol Epigallocatechin Gallate Analogs in Human Leiomyoma Cell Lines. J. Cell. Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef]
- Biro, R.; Richter, R.; Ortiz, M.; Sehouli, J.; David, M. Effects of epigallocatechin gallate-enriched green tea extract capsules in uterine myomas: Results of an observational study. Arch. Gynecol. Obstet. 2021, 303, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef]
- Malik, M.; Mendoza, M.; Payson, M.; Catherino, W.H. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil. Steril. 2009, 91, 2177–2184. [Google Scholar] [CrossRef]
- Tsuiji, K.; Takeda, T.; Li, B.; Wakabayashi, A.; Kondo, A.; Kimura, T.; Yaegashi, N. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol. Endocrinol. 2011, 27, 512–517. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Li, Y.; Peng, T.; Kuang, Y.; Shi, X.; Wang, G.; Peng, F.; Yu, C. Inhibition of Fibroblast Activation in Uterine Leiomyoma by Components of Rhizoma Curcumae and Rhizoma Sparganii. Front. Public Health 2021, 9, 650022. [Google Scholar] [CrossRef]
- Yu, C.H.; Zhao, J.S.; Zhao, H.; Peng, T.; Shen, D.C.; Xu, Q.X.; Li, Y.; Webb, R.C.; Wang, M.H.; Shi, X.M.; et al. Transcriptional profiling of uterine leiomyoma rats treated by a traditional herb pair, Curcumae rhizoma and Sparganii rhizoma. Braz. J. Med Biol. Res. 2019, 52, e8132. [Google Scholar] [CrossRef]
- Greco, S.; Islam, S.; Zannotti, A.; Carpini, G.D.; Giannubilo, S.R.; Ciavattini, A.; Petraglia, F.; Ciarmela, P. Quercetin and indole-3-carbinol inhibit extracellular matrix expression in human primary uterine leiomyoma cells. Reprod. Biomed. Online 2020, 40, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, C.; Wang, X.; Liu, F.; Sung, C.J.; Quddus, M.R.; Lawrence, W.D. The expression of selenium-binding protein 1 is decreased in uterine leiomyoma. Diagn. Pathol. 2010, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, M.; Sahin, N.; Özercan, I.; Seren, S.; Sahin, K.; Kucuk, O. The Effects of Selenium Supplementation on the Spontaneously Occurring Fibroid Tumors of Oviduct, 8-Hydroxy-2′-Deoxyguanosine Levels, and Heat Shock Protein 70 Response in Japanese Quail. Nutr. Cancer 2010, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Nasiadek, M.; Krawczyk, T.; Sapota, A. Tissue levels of cadmium and trace elements in patients with myoma and uterine cancer. Hum. Exp. Toxicol. 2005, 24, 623–630. [Google Scholar] [CrossRef]
- Johnstone, E.B.; Louis, G.M.B.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32. [Google Scholar] [CrossRef]
- Elkafas, H.; Badary, O.A.; Elmorsy, E.; Kamel, R.; Yang, Q.; Al-Hendy, A. Endocrine-Disrupting Chemicals and Vitamin D Deficiency in the Pathogenesis of Uterine Fibroids. J. Adv. Pharm. Res. 2021, 5, 248–263. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Chen, H.-Y.; Ali, M.; Shieh, T.-M.; Huang, Y.-J.; Wang, K.-L.; Chang, H.-Y.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. The Role of Cell Proliferation and Extracellular Matrix Accumulation Induced by Food Additive Butylated Hydroxytoluene in Uterine Leiomyoma. Nutrients 2021, 13, 3074. [Google Scholar] [CrossRef]
- Wang, K.-H.; Kao, A.-P.; Chang, C.-C.; Lin, T.-C.; Kuo, T.-C. Bisphenol A at environmentally relevant doses induces cyclooxygenase-2 expression and promotes invasion of human mesenchymal stem cells derived from uterine myoma tissue. Taiwan. J. Obstet. Gynecol. 2013, 52, 246–252. [Google Scholar] [CrossRef]
- Neblett, M.F., 2nd; Curtis, S.W.; Gerkowicz, S.A.; Spencer, J.B.; Terrell, M.L.; Jiang, V.S.; Marder, M.E.; Barr, D.B.; Marcus, M.; Smith, A.K. Examining Reproductive Health Outcomes in Females Exposed to Polychlorinated Biphenyl and Polybrominated Biphenyl. Sci. Rep. 2020, 10, 3314–3319. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Henn, B.C.; Fruh, V.; Orta, O.R.; Weuve, J.; Hauser, R.; Williams, P.L.; McClean, M.D.; Sjodin, A.; Bethea, T.N.; et al. A Prospective Ultrasound Study of Plasma Polychlorinated Biphenyl Concentrations and Incidence of Uterine Leiomyomata. Epidemiology 2021, 32, 259–267. [Google Scholar] [CrossRef]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. 1,25 Dihydroxyvitamin D3 Enhances the Antifibroid Effects of Ulipristal Acetate in Human Uterine Fibroids. Reprod. Sci. 2019, 26, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Męczekalski, B.; Lukaszuk, K.; Jakiel, G. Potential synergism between ulipristal acetate and vitamin D3 in uterine fibroid pharmacotherapy—2 case studies. Gynecol. Endocrinol. 2019, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, I.; Nawrocka-Rutkowska, J.; Brodowska, A.; Marciniak, A.; Starczewski, A.; Szczuko, M. Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development. Nutrients 2022, 14, 734. https://doi.org/10.3390/nu14040734
Szydłowska I, Nawrocka-Rutkowska J, Brodowska A, Marciniak A, Starczewski A, Szczuko M. Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development. Nutrients. 2022; 14(4):734. https://doi.org/10.3390/nu14040734
Chicago/Turabian StyleSzydłowska, Iwona, Jolanta Nawrocka-Rutkowska, Agnieszka Brodowska, Aleksandra Marciniak, Andrzej Starczewski, and Małgorzata Szczuko. 2022. "Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development" Nutrients 14, no. 4: 734. https://doi.org/10.3390/nu14040734
APA StyleSzydłowska, I., Nawrocka-Rutkowska, J., Brodowska, A., Marciniak, A., Starczewski, A., & Szczuko, M. (2022). Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development. Nutrients, 14(4), 734. https://doi.org/10.3390/nu14040734







