The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Fecal Microbiota Profiling
2.3. Microbiota Data Processing and Analysis
2.4. Fecal Short Chain Fatty Acid Analysis
2.5. Measurement of Fecal Calprotectin, α1-Antitrypsin, and Zonulin
3. Results
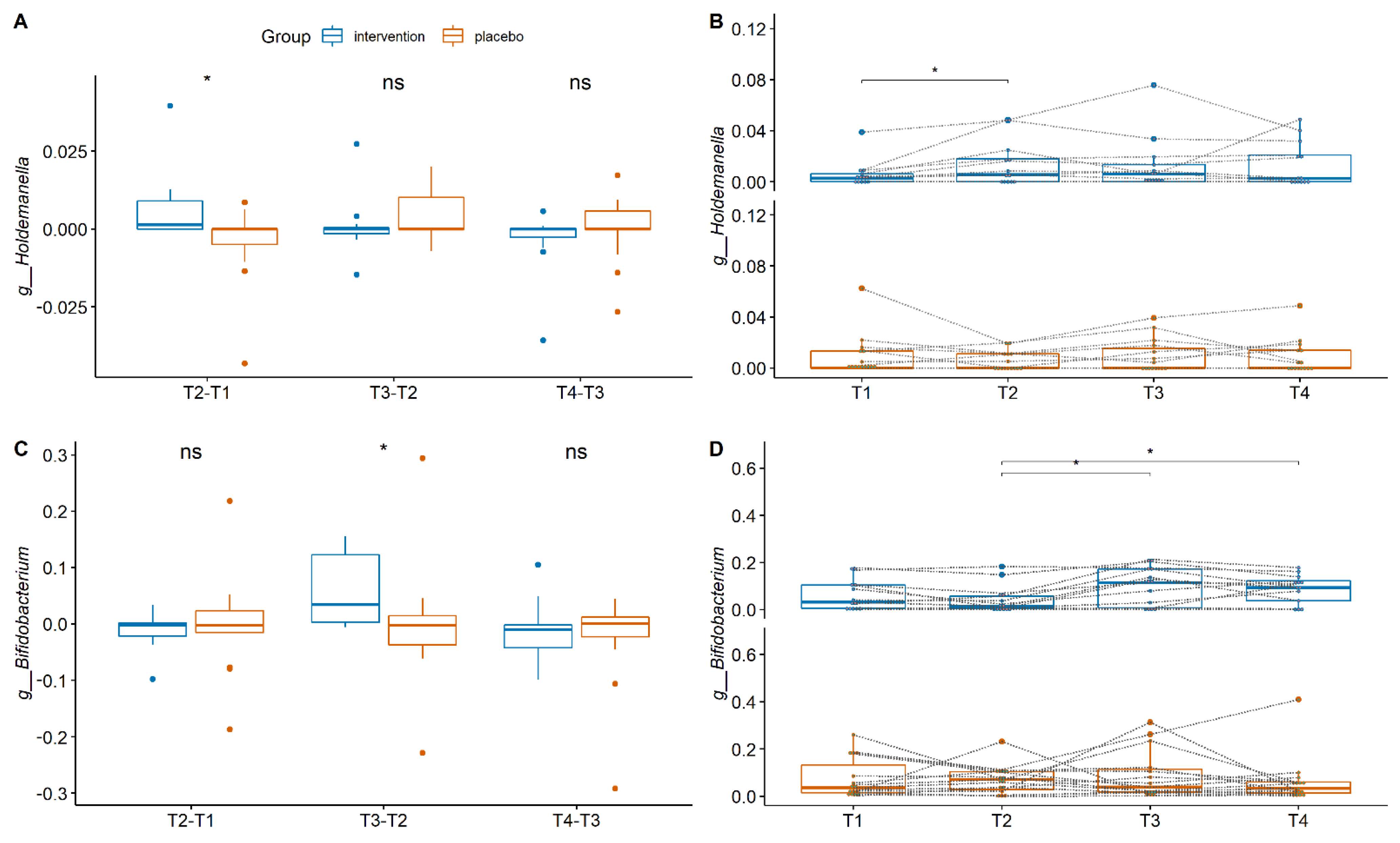
4. Gut Microbiota Composition between the Groups
4.1. Fecal Short Chain Fatty Acids
4.2. Intestinal Inflammatory Markers
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Hazra, N.C.; Rudisill, C.; Gulliford, M.C. Determinants of health care costs in the senior elderly: Age, comorbidity, impairment, or proximity to death? Eur. J. Health Econ. 2018, 19, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91. [Google Scholar] [PubMed]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatrics 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Van Splunter, M.; Perdijk, O.; Fick-Brinkhof, H.; Feitsma, A.L.; Floris-Vollenbroek, E.G.; Meijer, B.; Brugman, S.; Savelkoul, H.F.; van Hoffen, E.; van Neerven, R. Bovine lactoferrin enhances TLR7-mediated responses in plasmacytoid dendritic cells in elderly women: Results from a nutritional intervention study with bovine lactoferrin, GOS and vitamin D. Front. Immunol. 2018, 9, 2677. [Google Scholar] [CrossRef]
- Abd El Hafez, S.M.; Ismael, A.B.; Mahmoud, M.B.; Elaraby, A.-K.A. Development of new strategy for non-antibiotic therapy: Bovine lactoferrin has a potent antimicrobial and immunomodulator effects. Adv. Infect. Dis. 2013, 3, 36303. [Google Scholar]
- Okada, S.; Tanaka, K.; Sato, T.; Ueno, H.; Saito, S.; Okusaka, T.; Sato, K.; Yamamoto, S.; Kakizoe, T. Dose-response trial of lactoferrin in patients with chronic hepatitis C. Jpn. J. Cancer Res. 2002, 93, 1063–1069. [Google Scholar] [CrossRef]
- Jegasothy, H.; Weerakkody, R.; Selby-Pham, S.; Bennett, L.E. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. Biometals 2014, 27, 1371–1382. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Cleary, T.G. Effect of lactoferrin on enteric pathogens. Biochimie 2009, 91, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Dryja, D.; Leavens, A.; Neiswander, R.L.; Qiao, H.; DiRienzo, D.; Ogra, P. In vitro growth responses of bifidobacteria and enteropathogens to bovine and human lactoferrin. Dig. Dis. Sci. 2003, 48, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, M.I.; Groenink, J.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Amerongen, A.V.N. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Jaśkiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Masclee, A.A.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Logtenberg, M.J.; van Trijp, M.P.; Schols, H.A.; Masclee, A.A.; Smidt, H.; Jonkers, D.M.; Zoetendal, E.G. In vitro metabolic capacity of carbohydrate degradation by intestinal microbiota of adults and pre-frail elderly. ISME Commun. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Wright, O. Bioavailability of a novel form of microencapsulated bovine lactoferrin and its effect on inflammatory markers and the gut microbiome: A pilot study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Smolinska, A.; Hermes, G.D.; Masclee, A.A.; de Vos, P.; Schols, H.A.; van Schooten, F.J.; Smidt, H.; Jonkers, D.M. Sugar beet pectin supplementation did not alter profiles of fecal microbiota and exhaled breath in healthy young adults and healthy elderly. Nutrients 2019, 11, 2193. [Google Scholar] [CrossRef]
- Ramiro-Garcia, J.; Hermes, G.D.; Giatsis, C.; Sipkema, D.; Zoetendal, E.G.; Schaap, P.J.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2016, 5, 1791. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S.A. Tools for Microbiome Analysis in R. 2018. Available online: https://doi.org/10.18129/B9.bioc.microbiome (accessed on 16 May 2022).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Software 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.; Huuskonen, L.; Korpela, K.; Salojärvi, J.; Aalvink, S.; de Vos, W.M.; D’Haens, G.R. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef]
- De Maesschalck, C.; van Immerseel, F.; Eeckhaut, V.; de Baere, S.; Cnockaert, M.; Croubels, S.; Haesebrouck, F.; Ducatelle, R.; Vandamme, P. Faecalicoccus acidiformans gen. nov. sp. nov. isolated from the chicken caecum, and reclassification of Streptococcus pleomorphus (Barnes et al. 1977), Eubacterium biforme (Eggerth 1935) and Eubacterium cylindroides (Cato et al. 1974) as Faecalicoccus pleomorphus comb. nov. Holdemanella biformis gen. nov. comb. nov. and Faecalitalea cylindroides gen. nov. comb. nov. respectively, within the family Erysipelotrichaceae. Int. J. Syst. Evol. Microbiol. 2014, 64, 3877–3884. [Google Scholar]
- Pujo, J.; Petitfils, C.; le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; van Hul, M.; Barreau, F.; Blanpied, C. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; López-Almela, I.; Bullich-Vilarrubias, C.; Rueda-Ruzafa, L.; del Pulgar, E.M.G.; Benítez-Páez, A.; Liebisch, G.; Lamas, J.A.; Sanz, Y. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 2021, 35, e21734. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Saris, W.H.; Brummer, R.-J.M. Orally ingested human lactoferrin is digested and secreted in the upper gastrointestinal tract in vivo in women with ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.; Goossens, G.H.; Jocken, J.W.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G. Supplementation of diet with galacto-oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. Gastroenterology 2017, 153, 87–97.e3. [Google Scholar] [CrossRef]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar]
- Davis, L.; Martinez, I.; Walter, J.; Hutkins, R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int. J. Food Microbiol. 2010, 144, 285–292. [Google Scholar] [CrossRef]
- Aadland, E.; Fagerhol, M.K. Faecal calprotectin: A marker of inflammation throughout the intestinal tract. Eur. J. Gastroenterol. Hepatol. 2002, 14, 823–825. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Däbritz, J.; Musci, J.; Foell, D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 363. [Google Scholar] [CrossRef]
- Laudisi, F.; di Fusco, D.; Dinallo, V.; Stolfi, C.; di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef]



| Total Group | Intervention Group | Placebo Group | |
|---|---|---|---|
| Number of subjects | n = 25 | n = 12 | n = 13 |
| Age: median (range) 74.5 (69–85) 74 (70–84) 76 (69–85) | 74 (69–85) | 74 (70–84) | 74 (69–85) |
| BMI: median (range) | 24.5(20.3–29.4) | 23.2 (20.3–29.0) | 24.7 (20.8–29.4) |
| Reported arthrosis: number Y/N | 6/25 | 3/12 | 3/13 |
| Use of vitamin D before the study: | 9/25 | 5/12 | 4/13 |
| Medication blood pressure/cholesterol: | 10/25 | 5/12 | 5/13 |
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| Age | R2 = 0.035 | R2 = 0.028 | R2 = 0.018 | R2 = 0.017 |
| p-value = 0.47 | p-value = 0.67 | p-value = 0.86 | p-value = 0.87 | |
| BMI | R2 = 0.033 | R2 = 0.036 | R2 = 0.024 | R2 = 0.011 |
| p-value = 0.51 | p-value = 0.47 | p-value = 0.71 | p-value = 0.97 | |
| Medication | R2 = 0.032 | R2 = 0.046 | R2 = 0.061 | R2 = 0.064 |
| p-value = 0.58 | p-value = 0.31 | p-value = 0.93 | p-value = 0.17 | |
| Osteoarthritis | R2 = 0.074 | R2 = 0.086 | R2 = 0.064 | R2 = 0.059 |
| p-value = 0.09 | p-value = 0.07 | p-value = 0.67 | p-value = 0.2 | |
| Use of vitamin D before the study | R2 = 0.017 | R2 = 0.024 | R2 = 0.025 | R2 = 0.063 |
| p-value = 0.91 | p-value = 0.75 | p-value = 0.69 | p-value = 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstanti, P.; van Splunter, M.; van den Brink, E.; Belzer, C.; Nauta, A.; van Neerven, R.J.J.; Smidt, H. The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women. Nutrients 2022, 14, 2468. https://doi.org/10.3390/nu14122468
Konstanti P, van Splunter M, van den Brink E, Belzer C, Nauta A, van Neerven RJJ, Smidt H. The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women. Nutrients. 2022; 14(12):2468. https://doi.org/10.3390/nu14122468
Chicago/Turabian StyleKonstanti, Prokopis, Marloes van Splunter, Erik van den Brink, Clara Belzer, Arjen Nauta, R. J. Joost van Neerven, and Hauke Smidt. 2022. "The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women" Nutrients 14, no. 12: 2468. https://doi.org/10.3390/nu14122468
APA StyleKonstanti, P., van Splunter, M., van den Brink, E., Belzer, C., Nauta, A., van Neerven, R. J. J., & Smidt, H. (2022). The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women. Nutrients, 14(12), 2468. https://doi.org/10.3390/nu14122468








