Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diet Analysis
2.3. Chicken Breast Extract Intake
2.4. Body Composition
2.5. Incremental Exercise Test and Maximal Oxygen Uptake
2.6. Blood Sampling
2.7. Haematological Variables
2.8. Skeletal Muscle Damage and Lactate
2.9. Oxi-Antioxidant and Inflammatory Variables
2.10. Statistical Analysis
3. Results
3.1. Diet Analysis
3.2. Haematological Variables
3.3. Skeletal Muscle Damage and Lactate
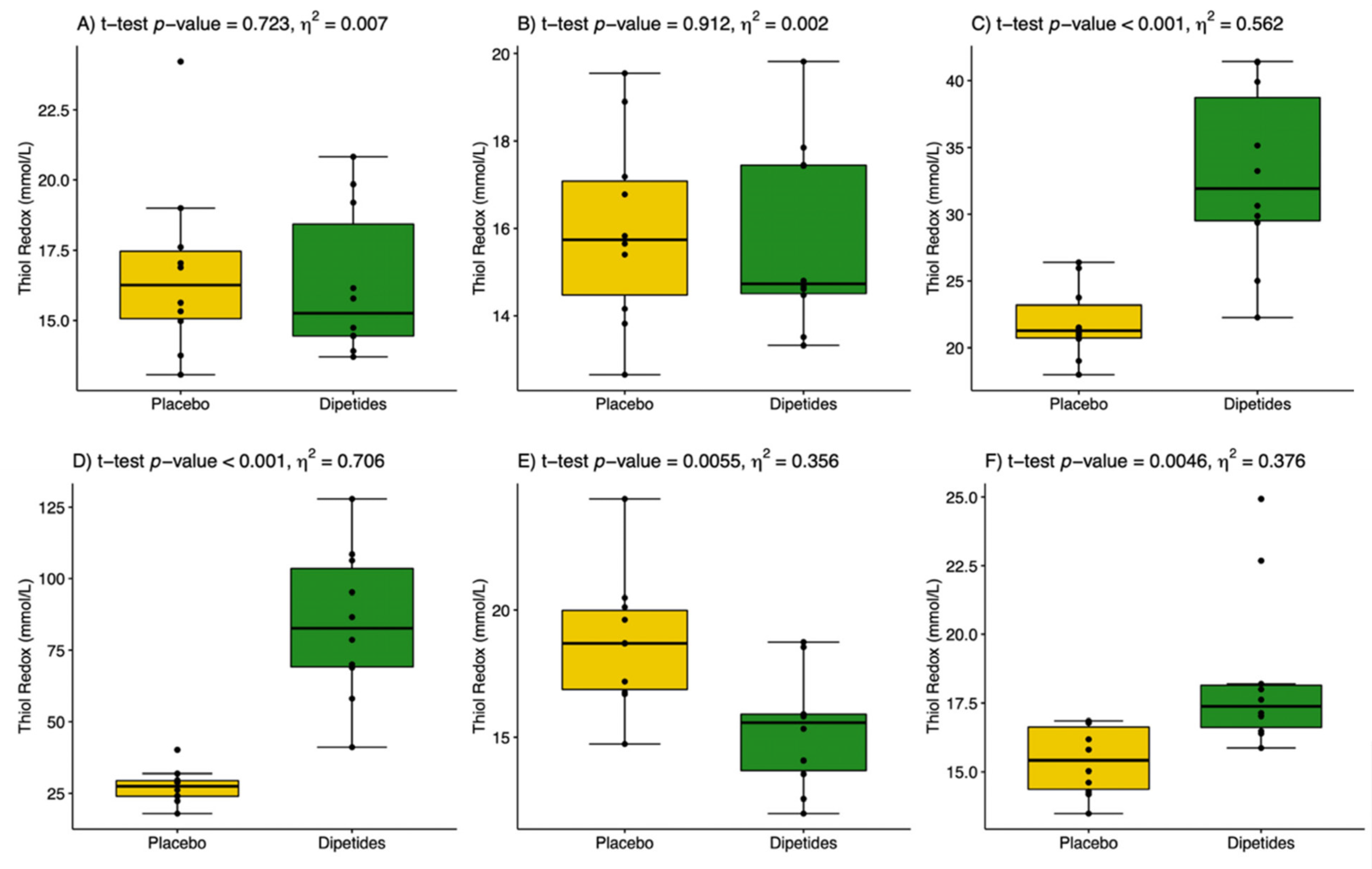
3.4. Oxi-Antioxidant and Inflammatory Variables
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagasawa, T.; Yonekura, T.; Nishizawa, N.; Kitts, D.D. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol. Cell. Biochem. 2001, 225, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Favero, G.; Ferroni, M.; Lonati, C.; Moghadasian, M.H. A carnosine analog with therapeutic potentials in the treatment of disorders related to oxidative stress. PLoS ONE 2019, 14, e0215170. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Reviews. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Jackson, M.C.; Lenney, J.F. The distribution of carnosine and related dipeptides in rat and human tissues. Inflamm. Res. 1996, 45, 132–135. [Google Scholar] [CrossRef]
- Hong, H.; Johnson, P. Histidine dipeptide levels in exercised and hypertensive rat muscles. Biochem. Soc. Trans. 1995, 23, 542. [Google Scholar] [CrossRef]
- Di Pasquale, M. Amino Acids and Proteins for Athlete; CRC Press, Taylor and Francis Group: ON, Ontario, Canada, 2008. [Google Scholar]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, S.; Xie, X.N.; Tan, Z.R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 2017, 11, 3511–3517. [Google Scholar] [CrossRef]
- Sato, M.; Karasawa, N.; Shimizu, M.; Morimatsu, F.; Yamada, R. Safety evaluation of chicken breast extract containing carnosine and anserine. Food Chem. Toxicol. 2008, 46, 480–489. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakao, T.; Maemura, H.; Sato, M.; Kamahara, K.; Morimatsu, F.; Takamatsu, K. Carnosine and anserine ingestion enhances contribution of nonbicarbonate buffering. Med. Sci. Sports Exerc. 2006, 38, 334–338. [Google Scholar] [CrossRef]
- Maemura, H.; Goto, K.; Yoshioka, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Takamatsu, K. Effects of carnosine and anserine supplementation on relatively high intensity endurance performance. Int. J. Sport Health Sci. 2006, 4, 86–94. [Google Scholar] [CrossRef][Green Version]
- Blancquaert, L.; Everaert, I.; Baguet, A.; Bex, T.; Barbaresi, S.; de Jager, S.; Lievens, E.; Stautemas, J.; De Smet, S.; Baron, G.; et al. Acute preexercise supplementation of combined carnosine and anserine enhances initial maximal power of Wingate tests in humans. J. Appl. Physiol. 2021, 130, 1868–1878. [Google Scholar] [CrossRef]
- MacFarlane, N.; McMurray, J.; O’Dowd, J.J.; Dargie, H.J.; Miller, D.J. Synergism of histidyl dipeptides as antioxidants. J. Mol. Cell. Cardiol. 1991, 23, 1205–1207. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kwon, D.; Kwon, D.-A.; Paik, I.K.; Auh, J.-H. Optimizing carnosine containing extract preparation from chicken breast for anti-glycating agents. Korean J. Food Sci. Anim. Resour. 2014, 34, 127. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine CCL24 in peripheral blood mononuclear cells from elderly people. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef]
- Alkhatib, A.; Feng, W.H.; Huang, Y.J.; Kuo, C.H.; Hou, C.W. Anserine reverses exercise-induced oxidative stress and preserves cellular homeostasis in healthy men. Nutrients 2020, 12, 1146. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i Ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego: Warszawa, Poland, 2020; ISBN 978–83–65870–28–5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 9 March 2022).
- Zembron-Lacny, A.; Gramacki, A.; Wawrzyniak-Gramacka, E.; Tylutka, A.; Hertmanowska, N.; Kasperska, A.; Czuba, M. Intermittent hypoxic exposure with high dose of arginine impact on circulating mediators of tissue regeneration. Nutrients 2020, 12, 1933. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.C.; Yee, C.W.; Shing, W.K.; Lai, T.P.; Ching, W.K.; Kei, K.K. The enhancing effects of a chicken-meat extract on serum Ig concentrations in normal and scalded animals. Br. J. Nutr. 2005, 94, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Kita, S.; Ono, H.; Kiso, Y.; Tanaka, T. Preventive effect of a chicken extract on the development of hypertension in stroke-prone spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2002, 66, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Geissler, C.; Boroumand-Naini, M.; Harada, M.; Iino, T.; Hirai, K.; Suwa, Y.; Tanaka, T.; Iwata, S. Chicken extract stimulates haemoglobin restoration in iron deficient rats. Int. J. Food Sci. Nutr. 1996, 47, 351–360. [Google Scholar] [CrossRef]
- Wu, T.; Watanabe, H.; Hong, L.K.; Abe, K.; Ni, Y.; Fu, Z. Effect of BRAND’s essence of chicken on the resetting process of circadian clocks in rats subjected to experimental jet lag. Mol. Biol. Rep. 2011, 38, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.C.; Lin, S.H. Effects of chicken extract on antioxidative status and liver protection under oxidative stress. J. Nutr. Sci. Vitaminol. 2004, 50, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; He, R.R.; Zhai, Y.J.; Abe, K.; Kurihara, H. Effects of carnosine on cyclophosphamide-induced hematopoietic suppression in mice. Am. J. Chin. Med. 2014, 42, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, R.R.; Tsoi, B.; Kurihara, H. Bioactivities of chicken essence. J. Food Sci. 2012, 77, R105–R110. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar] [CrossRef]
- Filippin, L.I.; Cuevas, M.J.; Lima, E.; Marroni, N.P.; Gonzalez-Gallego, J.; Xavier, R.M. The role of nitric oxide during healing of trauma to the skeletal muscle. Inflamm. Res. 2011, 60, 347–356. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Tylutka, A.; Zeromska, A.; Kasperska, A.; Wolny-Rokicka, E. Does high volume of exercise training increase aseptic vascular inflammation in male athletes? Am. J. Men’s Health 2019, 13, 1557988319858838. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lin, C.-I.; Chiu, C.-C.; Lin, Y.-T.; Huang, W.-K.; Huang, H.-Y.; Huang, C.-C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef]
- Huang, S.-W.; Hsu, Y.-J.; Lee, M.-C.; Li, H.-S.; Yeo, P.C.W.; Lim, A.L.; Huang, C.-C. In vitro and in vivo functional characterization of essence of chicken as an ergogenic aid. Nutrients 2018, 10, 1943. [Google Scholar] [CrossRef]
- Aydın, A.F.; Bingül, İ.; Küçükgergin, C.; Doğan-Ekici, I.; Doğru Abbasoğlu, S.; Uysal, M. Carnosine decreased oxidation and glycation products in serum and liver of high-fat diet and low-dose streptozotocin-induced diabetic rats. Int. J. Exp. Pathol. 2017, 98, 278–288. [Google Scholar] [CrossRef]
- Sakurai, T.; Kashimura, O.; Kano, Y.; Ohno, H.; Ji, L.L.; Izawa, T.; Best, T.M. Role of nitric oxide in muscle regeneration following eccentric muscle contractions in rat skeletal muscle. J. Physiol. Sci. 2013, 63, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Tylutka, A.; Wacka, E.; Wawrzyniak-Gramacka, E.; Hiczkiewicz, D.; Kasperska, A.; Czuba, M. Intermittent hypoxic exposure reduces endothelial dysfunction. BioMed Res. Int. 2020, 2020, 6479630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Chen, Y.-J.; Lin, L.-H.; Nakao, Y.; Lim, A.L.; Wang, M.-F.; Yong, S.M. Protective effects of hydrolyzed chicken extract (Probeptigen®/Cmi-168) on memory retention and brain oxidative stress in senescence-accelerated mice. Nutrients 2019, 11, 1870. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Williamson-Reisdorph, C.M.; Quindry, T.S.; Tiemessen, K.G.; Cuddy, J.; Hailes, W.; Slivka, D.; Ruby, B.C.; Quindry, J.C. Blood oxidative stress and post-exercise recovery are unaffected byhypobaric and hypoxic environments. J. Sports Sci. 2021, 39, 1356–1365. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Lomaestro, B.M.; Malone, M. Glutathione in health and disease: Pharmacotherapeutic issues. Ann. Pharmacother. 1995, 29, 1263–1273. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Gajewski, M.; Naczk, M.; Dziewiecka, H.; Siatkowski, I. Physical activity and alpha-lipoic acid modulate inflammatory response through changes in thiol redox status. J. Physiol. Biochem. 2013, 69, 397–404. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Gajewski, M.; Naczk, M.; Siatkowski, I. Effect of shiitake (Lentinus edodes) extract on antioxidant and inflammatory response to prolonged eccentric exercise. J. Physiol. Pharmacol. 2013, 64, 249–254. [Google Scholar] [PubMed]
- Slowinska-Lisowska, M.; Zembron-Lacny, A.; Rynkiewicz, M.; Rynkiewicz, T.; Kopec, W. Influence of L-carnosine on pro-antioxidant status in elite kayakers and canoeists. Acta Physiol. Hung. 2014, 101, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Slowinska-Lisowska, M.; Szygula, Z.; Witkowski, Z.; Szyszka, K. Modulatory effect of N-acetylcysteine on pro-antioxidant status and haematological response in healthy men. J. Physiol. Biochem. 2010, 66, 15–21. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- López-Mirabal, H.R.; Winther, J.R. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta 2008, 1783, 629–640. [Google Scholar] [CrossRef]
- Elokda, A.S.; Nielsen, D.H. Effects of exercise training on the glutathione antioxidant system. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 630–637. [Google Scholar] [CrossRef]
- Wawrzyniak-Gramacka, E.; Hertmanowska, N.; Tylutka, A.; Morawin, B.; Wacka, E.; Gutowicz, M.; Zembron-Lacny, A. The association of anti-inflammatory diet ingredients and lifestyle exercise with inflammaging. Nutrients 2021, 13, 3696. [Google Scholar] [CrossRef]
- Wu, D.; Yang, C.-C.; Chen, K.-Y.; Lin, Y.-C.; Wu, P.-J.; Hsieh, P.-H.; Nakao, Y.; Ow, M.Y.; Hsieh, Y.-C.; Hu, C.-J. Hydrolyzed chicken extract (Probeptigen®) on cognitive function in healthy middle-aged people: A randomized double-blind trial. Nutrients 2020, 12, 1362. [Google Scholar] [CrossRef]
- Ni, Y.; Ni, L.; Ma, L.; Wang, Z.; Zhao, Y.; Hu, L.; Zheng, L.; Fu, Z. Neuroprotective effects of ProBeptigen/CMI-168 on aging-induced cognitive decline and neuroinflammation in mice: A comparison with essence of chicken. Acta Biochim. Biophys. Sin. 2021, 53, 419–429. [Google Scholar] [CrossRef]


| Placebo n = 10 | Dipeptides n = 10 | p-Value | |
|---|---|---|---|
| Age (year) | 21.40 ± 2.12 | 20.25 ± 0.46 | 0.367 |
| Height (cm) | 175.50 ± 12.78 | 180.40 ± 11.97 | 0.388 |
| Weight (kg) | 71.18 ± 11.71 | 75.43 ± 18.84 | 0.482 |
| BMI (kg/m2) | 23.32 ± 2.68 | 23.13 ± 3.09 | 0.887 |
| FM (kg) | 14.71 ± 6.39 | 11.81 ± 8.41 | 0.307 |
| FFM (kg) | 62.27 ± 15.36 | 64.65 ± 13.12 | 0.720 |
| VO2max (mL/kg/min) | 54.79 ± 9.87 | 55.95 ± 2.48 | 0.751 |
| Variables | Reference Values | Placebo n = 10 | Dipeptides n = 10 | p-Value |
|---|---|---|---|---|
| RBC (106/µL) | 4.2–6.5 | 5.10 ±0.06 | 5.03 ± 0.37 | 0.577 |
| HB (g/dL) | 12.0–18.0 | 15.01 ± 0.64 | 14.64 ± 0.55 | 0.181 |
| HCT% | 38.0–54.0 | 46.05 ± 2.38 | 45.32 ± 2.51 | 0.513 |
| MCV fL | 80.0–97.0 | 88.98 ± 2.85 | 90.20 ± 2.77 | 0.345 |
| MCH (pg/RBC) | 26.0–32.0 | 29.01 ± 0.91 | 29.18 ± 1.55 | 0.768 |
| MCHC (g/dL) | 31.0–36.0 | 32.60 ± 0.32 | 32.35 ± 1.22 | 0.546 |
| RDW% | 11.5–14.8 | 13.75 ±0.76 | 13.73 ± 0.61 | 0.790 |
| WBC (103/µL) | 4.0–10.2 | 6.06 ± 0.36 | 5.77 ± 1.06 | 0.431 |
| PLT (103/µL) | 140–420 | 227 ± 12 | 226 ± 48 | 0.352 |
| NO (µmol/L) | H2O2 (µmol/L) | 8-Isoprostanes (pg/mL) | TAS (mmol/L) | GSHt (mmol/L) | GSSG (mmol/L) | CRP (mg/L) | |||
|---|---|---|---|---|---|---|---|---|---|
| Initial level | Placebo | 13.99 ± 0.74 | 16.31 ± 3.53 | 77.88 ± 10.95 | 19.48 ± 2.03 | 1271± 118 | 65.28 ± 4.72 | 0.11 ± 0.04 | |
| Dipeptides | 12.70 ± 1.57 | 16.34 ± 3.53 | 79.67 ± 11.74 | 19.13 ± 2.21 | 1191 ± 162 | 65.30 ± 6.06 | 0.01 ± 0.04 | ||
| p-value | 0.049 | 0.983 | 0.595 | 0.717 | 0.238 | 0.713 | 0.653 | ||
| After 14-day placebo or dipeptide intake | Before exercise | Placebo | 13.79 ± 0.85 | 14.91 ± 1.41 | 72.85 ± 21.05 | 14.98 ± 0.87 | 1368 ± 197 | 76.28 ± 8.46 | 0.12 ± 0.07 |
| Dipeptides | 15.33 ± 2.60 | 14.46 ± 0.41 | 81.46 ± 20.55 | 22.51 ± 2.15 | 1474 ± 143 | 83.61 ± 9.70 | 0.11 ± 0.04 | ||
| p-value | 0.106 | 0.382 | 0.384 | p < 0.001 | 0.179 | 0.090 | 0.745 | ||
| 1st min after exercise | Placebo | 18.27 ± 1.33 | 28.79 ± 5.93 | 93.36 ± 23.56 | 16.42 ± 1.58 | 1123 ± 92 | 47.29 ± 3.22 | 0.23 ± 0.06 | |
| Dipeptides | 19.07 ± 2.32 | 15.33 ± 4.01 | 74.42 ± 29.72 | 20.52 ± 1.74 | 1091 ± 61 | 30.35 ± 2.78 | 0.24 ± 0.07 | ||
| p-value | 0.376 | p < 0.001 | 0.161 | p < 0.001 | 0.435 | p < 0.001 | 0.519 | ||
| 30th min after exercise | Placebo | 17.27 ± 1.51 | 22.88 ± 5.49 | 96.34 ± 46.26 | 16.99 ± 1.93 | 1335 ± 126 | 46.47 ± 6.51 | 0.71 ± 0.16 | |
| Dipeptides | 14.03 ± 1.49 | 13.55 ± 0.93 | 73.17 ± 15.51 | 17.00 ± 0.87 | 1370 ± 163 | 15.41 ± 2.67 | 0.36 ± 0.12 | ||
| p-value | p < 0.001 | p < 0.001 | 0.910 | 0.987 | 0.592 | p < 0.001 | p < 0.001 | ||
| 24th h after exercise | Placebo | 14.66 ± 1.79 | 17.87 ± 2.99 | 166.82 ± 39.48 | 12.95 ± 2.05 | 1222 ±76 | 58.10 ± 4.31 | 0.30 ± 0.09 | |
| Dipeptides | 15.45 ± 3.00 | 18.92 ± 5.73 | 175.43 ± 43.30 | 18.76 ± 1.63 | 1223 ± 103 | 71.60 ± 7.67 | 0.05 ± 0.008 | ||
| p-value | 0.505 | 0.629 | 0.472 | p < 0.001 | 0.992 | p < 0.001 | p < 0.001 | ||
| 48th h after exercise | Placebo | 13.99 ± 1.23 | 21.29 ± 2.78 | 107.11 ± 13.89 | 11.05 ± 0.53 | 1345 ± 93 | 77.42 ± 4.91 | 0.47 ± 0.12 | |
| Dipeptides | 13.87 ± 1.57 | 17.48 ± 5.41 | 94.73 ± 35.81 | 17.88 ± 1.91 | 1374 ± 83 | 71.37 ± 3.35 | 0.07 ± 0.02 | ||
| p-value | 0.862 | 0.090 | 0.371 | p < 0.001 | 0.479 | p < 0.001 | p < 0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zembron-Lacny, A.; Wawrzyniak-Gramacka, E.; Książek, A.; Zagrodna, A.; Kopeć, W.; Słowińska-Lisowska, M. Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise. Nutrients 2022, 14, 2402. https://doi.org/10.3390/nu14122402
Zembron-Lacny A, Wawrzyniak-Gramacka E, Książek A, Zagrodna A, Kopeć W, Słowińska-Lisowska M. Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise. Nutrients. 2022; 14(12):2402. https://doi.org/10.3390/nu14122402
Chicago/Turabian StyleZembron-Lacny, Agnieszka, Edyta Wawrzyniak-Gramacka, Anna Książek, Aleksandra Zagrodna, Wiesław Kopeć, and Małgorzata Słowińska-Lisowska. 2022. "Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise" Nutrients 14, no. 12: 2402. https://doi.org/10.3390/nu14122402
APA StyleZembron-Lacny, A., Wawrzyniak-Gramacka, E., Książek, A., Zagrodna, A., Kopeć, W., & Słowińska-Lisowska, M. (2022). Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise. Nutrients, 14(12), 2402. https://doi.org/10.3390/nu14122402









