Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Digestion
2.3. Analysis of Toxic Elements
2.3.1. As, Cd, Pb
2.3.2. Hg
2.3.3. Certified Reference Materials
2.4. Risk Assessment
2.5. Statistical Analysis
3. Results
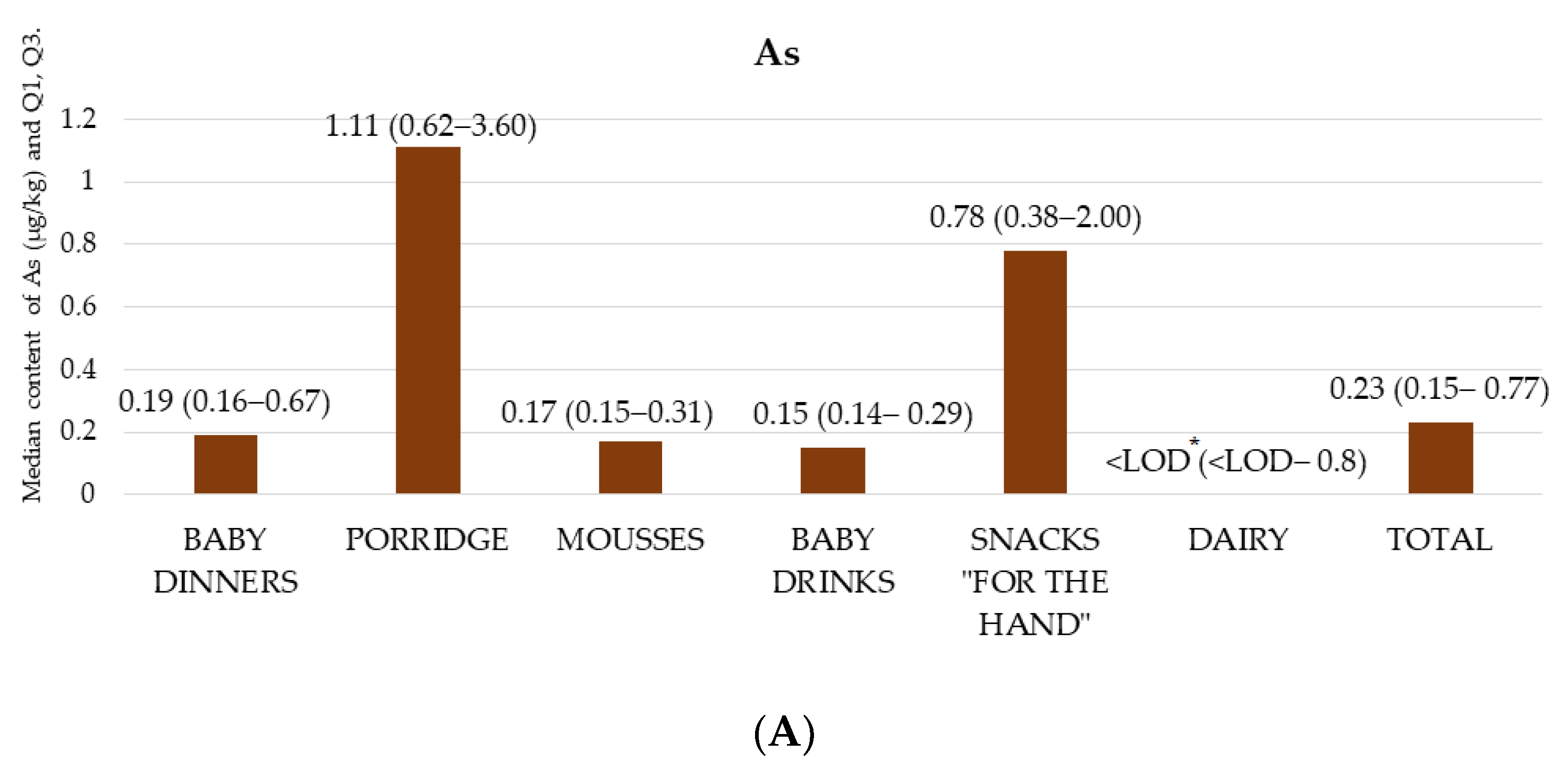
3.1. Content of As, Cd, Hg, and Pb
3.2. Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patriarca, M.; Menditto, A.; Rossi, B.; Lyon, T.D.B.; Fell, G.S. Environmental exposure to metals of newborns, infants and young children. Microchem. J. 2000, 67, 351–361. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres, and Dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 11–465. [Google Scholar]
- Gundert-Remy, U.; Damm, G.; Foth, H. High exposure to inorganic arsenic by food: The need for risk reduction. Arch. Toxicol. 2015, 89, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, J.; Chen, Y.; Shi, C.; Zhang, Q.; Ning, Z.; Yu, Y.; Li, Y. Urinary cadmium in relation to bone damage: Cadmium exposure threshold dose and health-based guidance value estimation. Ecotoxicol. Environ. Saf. 2021, 226, 112824. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E.; Bice, S.M. Concentrations and Health Risk of Polycyclic Aromatic Hydrocarbons in Some Brands of Biscuits in the Nigerian Market. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 338–357. [Google Scholar] [CrossRef]
- Llop, S.; Murcia, M.; Aguinagalde, X.; Vioque, J.; Rebagliato, M.; Cases, A.; Iñiguez, C.; Lopez-Espinosa, M.J.; Amurrio, A.; Navarrete-Muñoz, E.M.; et al. Exposure to mercury among Spanish preschool children: Trend from birth to age four. Environ. Res. 2014, 132, 83–92. [Google Scholar] [CrossRef]
- Young, E.C.; Davidson, P.W.; Wilding, G.; Myers, G.J.; Shamlaye, C.; Cox, C.; de Broeck, J.; Bennett, C.M.; Reeves, J.S. Association between prenatal dietary methyl mercury exposure and developmental outcomes on acquisition of articulatory-phonologic skills in children in the Republic of Seychelles. Neurotoxicology 2020, 81, 353–357. [Google Scholar] [CrossRef]
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Mercury and Prenatal Growth: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7140. [Google Scholar] [CrossRef]
- Etchevers, A.; Bretin, P.; Lecoffre, C.; Bidondo, M.L.; Le Strat, Y.; Glorennec, P.; Le Tertre, A. Blood lead levels and risk factors in young children in France, 2008–2009. Int. J. Hyg. Environ. Health 2014, 217, 528–537. [Google Scholar] [CrossRef]
- Bodeau-Livinec, F.; Glorennec, P.; Cot, M.; Dumas, P.; Durand, S.; Massougbodji, A.; Ayotte, P.; Le Bot, B. Elevated Blood Lead Levels in Infants and Mothers in Benin and Potential Sources of Exposure. Int. J. Environ. Res. Public Health 2016, 13, 316. [Google Scholar] [CrossRef] [Green Version]
- Téllez-Rojo, M.; Bellinger, D.; Arroyo-Quiroz, C.; Lamadrid-Figueroa, H.; Mercado-Garcia, A.; Schnaas-Arrieta, L.; Wright, R.; Hernandez-Avila, M.; Hu, H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 2006, 118, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) of the European Parliament and of the Council 852/2004 of 29 April 2004 on the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004R0852 (accessed on 31 May 2022).
- Gu, Z.; de Silva, S.; Reichman, S.M. Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia. Int. J. Environ. Res. Public Health 2020, 17, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardener, H.; Bowen, J.; Callan, S.P. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci. Total Environ. 2019, 651, 822–827. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/73 of 16 January 2018 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Mercury Compounds in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32018R0073&from=PL (accessed on 30 January 2022).
- Commission Regulation (EU) 2021/1323 of 10 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2021/1323/oj (accessed on 31 May 2022).
- World Health Organization; Food and Agriculture Organization of the United Nations & Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: Eightieth report of the Joint FAO/WHO Expert Committee on Food Additives. In Proceedings of the 80th Meeting, Rome, Italy, 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Weker, H.; Barańska, M.; Riahi, A.; Strucińska, M.; Więch, M.; Rowicka, G.; Dyląg, H.; Klemarczyk, W.; Bzikowska, A.; Socha, P. Nutrition of infants and young children in Poland—Pitnuts 2016. Dev. Period. Med. 2017, 21, 13–28. [Google Scholar]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 30 January 2022).
- Environmental Protection Agency. A Review of the Reference Dose and Reference Concentration Processes; Risk Assessment Forum United States Environmental Protection Agency, United States Environmental Protection Agency: Washington, DC, USA, 2002.
- GB 2762-2012; Polish National Food Safety Standard, Maximum Contamination Levels in Foodstuffs. National Commission for Health and Family Planning. General Veterinary Inspectorate: Warsaw, Poland, 2014.
- Škrbić, B.; Živančev, J.; Jovanović, G.; Farre, M. Essential and toxic elements in commercial baby food on the Spanish and Serbian market. Food Addit. Contam. Part B Surveill. 2017, 10, 27–38. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Jackson, B.P.; Carly McCalla, G.; Donohue, A.; Emmons, A.M. Co-exposure to methylmercury and inorganic arsenic in baby rice cereals and rice-containing teething biscuits. Environ. Res. 2017, 159, 639–647. [Google Scholar] [CrossRef]
- Ljung, K.; Palm, B.; Grandér, M.; Vahter, M. High concentrations of essential and toxic elements in infant formula and infant foods—A matter of concern. Food Chem. 2011, 127, 943–951. [Google Scholar] [CrossRef]
- Igweze, Z.N.; Ekhator, O.C.; Nwaogazie, I.; Orisakwe, O.E. Public Health and Paediatric Risk Assessment of Aluminium, Arsenic and Mercury in Infant Formulas Marketed in Nigeria. Sultan Qaboos Univ. Med. J. 2020, 20, e63–e70. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Woo, H.D.; Joo, J.; Park, K.S.; Oh, S.Y.; Kwon, H.J.; Park, J.D.; Hong, Y.S.; Sohn, S.J.; Yoon, H.J.; et al. Estimated long-term dietary exposure to lead, cadmium, and mercury in young Korean children. Eur. J. Clin. Nutr. 2014, 68, 1322–1326. [Google Scholar] [CrossRef]
- De Castro, C.S.; Arruda, A.F.; Da Cunha, L.R.; De Souza, J.R.; Braga, J.W.; Dórea, J.G. Toxic metals (Pb and Cd) and their respective antagonists (Ca and Zn) in infant formulas and milk marketed in Brasilia, Brazil. Int. J. Environ. Res. Public Health 2010, 7, 4062–4077. [Google Scholar] [CrossRef]
- Spungen, J.H. Children’s exposures to lead and cadmium: FDA total diet study 2014-16. Food Addit. Contam. 2019, 36, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kiczorowska, B. Determining the content of lead and cadmium in infant food from the Polish market. Int. J. Food Sci. Nutr. 2012, 63, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, J.; Sun, S.; Shi, Q.; Feng, H.; Zhang, Y.; Cao, S. Health risk assessment of total exposure from cadmium in South China. Chemosphere 2021, 269, 128673. [Google Scholar] [CrossRef] [PubMed]
- Meshref, A.M.S.; Moselhy, W.A.; Hassan, N.E.H.Y. Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014, 8, 381–388. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Chafey, C.; Testu, C.; Hulin, M.; Noël, L. Mercury in foods from the first French total diet study on infants and toddlers. Food Chem. 2018, 239, 920–925. [Google Scholar] [CrossRef]
- Martins, C.; Vasco, E.; Paixão, E.; Alvito, P. Total mercury in infant food, occurrence and exposure assessment in Portugal. Food Addit. Contam. Part B Surveill. 2013, 6, 151–157. [Google Scholar] [CrossRef]
- Junqué, E.; Garí, M.; Arce, A.; Torrent, M.; Sunyer, J.; Grimalt, J.O. Integrated assessment of infant exposure to persistent organic pollutants and mercury via dietary intake in a central western Mediterranean site (Menorca Island). Environ. Res. 2017, 156, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Misic, I.D.R.; Tosic, S.B.; Pavlovic, A.N.; Pecev-Marinkovic, E.T.; Mrmosanin, J.M.; Mitic, S.S.; Stojanovic, G.S. Trace element content in commercial complementary food formulated for infants and toddlers: Health risk assessment. Food Chem. 2022, 378, 132113. [Google Scholar] [CrossRef]
- Guérin, T.; Le Calvez, E.; Zinck, J.; Bemrah, N.; Sirot, V.; Leblanc, J.C.; Chekri, R.; Hulin, M.; Noël, L. Levels of lead in foods from the first French total diet study on infants and toddlers. Food Chem. 2017, 237, 849–856. [Google Scholar] [CrossRef]
- Ohtsu, M.; Mise, N.; Ikegami, A.; Mizuno, A.; Kobayashi, Y.; Nakagi, Y.; Nohara, K.; Yoshida, T.; Kayama, F. Oral exposure to lead for Japanese children and pregnant women, estimated using duplicate food portions and house dust analyses. Environ. Health Prev. Med. 2019, 24, 72. [Google Scholar] [CrossRef] [PubMed]
- Weker, H.; Barańska, M.; Dyląg, H.; Riahi, A.; Więch, M.; Strucińska, M.; Kurpińska, P.; Rowicka, G.; Klemarczyk, W. Analysis of nutrition of children aged 13–36 months in Poland: A nation-wide study. Med. Wieku Rozwoj 2011, 15, 224–231. [Google Scholar]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabeka, R.W.; McKenzie, A.D. Survey of total mercury in infant formulae and oral electrolytes sold in Canada. Food Addit. Contam. Part B Surveill. 2012, 5, 65–69. [Google Scholar] [CrossRef] [PubMed]

| Type of Products | n |
|---|---|
| BABY DINNERS | 102 |
| -with poultry | 24 |
| -with beef | 16 |
| -with pork | 13 |
| -with fish | 18 |
| -with rabbit | 11 |
| -vegetarian | 20 |
| PORRIDGE | 50 |
| -with milk | 8 |
| -with milk and fruit | 15 |
| -cereal gluten | 12 |
| -gluten-free cereal | 15 |
| FRUIT AND VEGETABLE MOUSSES | 58 |
| -fruit and vegetables | 9 |
| -fruit | 33 |
| -fruit and cereal | 6 |
| -fruit and dairy | 6 |
| -vegetables | 4 |
| BABY DRINKS | 64 |
| -fruit drinks and water | 22 |
| -fruit juices | 42 |
| SNACKS “FOR THE HAND” | 62 |
| -waffle/crisps | 30 |
| -biscuits/cookies | 17 |
| -fruit bars | 15 |
| DAIRY | 60 |
| -yellow cheese | 28 |
| -yogurt | 32 |
| TOTAL | 397 |
| Element | Declared Concentration in CRM (µg/kg) | Recovery (%) | Precision (%) |
|---|---|---|---|
| CORN FLOUR (INCT-CF-3) | |||
| As | 10 * | 101.3 | 4.5 |
| Cd | 7 * | 99.1 | 4.1 |
| Hg | 1.5 * | 101.7 | 4.6 |
| Pb | 52 * | 98.8 | 2.3 |
| SKIM MILK POWDER (CRM 63R) | |||
| Hg | 0.19 ± 0.02 | 98.3 | 3.8 |
| Pb | 18.5 ± 2.7 | 100.9 | 4.3 |
| SIMULATED DIET D | |||
| As | <50 * | - | 3.9 |
| Cd | 478 ± 26 | 98.4 | 2.4 |
| Hg | 52 * | 102.0 | 3.2 |
| Pb | 218 ± 13 | 99.6 | 2.2 |
| Baby Food Samples | Maximum Allowable Content (mg/kg Product Weight) | Exceeding Maximum Allowable Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Hg | Pb | |||||||||
| As | Cd | Hg | Pb | n | % | n | % | n | % | n | % | |
| Dinners (n = 102) | - | 0.04 | 0.01 | 0.02 | - | - | 0 | 0 | 3 | 0.75 | 1 | 0.25 |
| Porridges (n = 50) | 0.5 | 0.04 | 0.01 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0.00 | 2 | 0.50 |
| Mousses (n = 58) | 0.5 | 0.02 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 2 | 0.50 | 1 | 0.25 |
| Drinks (n = 64) | - | 0.02 | 0.02 | 0.02 | - | - | 0 | 0 | 1 | 0.25 | 7 | 1.76 |
| Baby snacks (n = 62) | - | 0.04 | 0.01 | 0.02 | - | - | 0 | 0 | 0 | 0.00 | 6 | 1.50 |
| Dairy (n = 60) | - | - | 0.01 | 0.02 | - | - | - | - | 0 | 0.00 | 1 | 0.25 |
| TOTAL (n = 397) | - | - | - | - | 6 | 1.50 | 18 | 4.53 | ||||
| Toxic Elements | Analyzed Food Group | p-Value |
|---|---|---|
| As | Dinners—drinks | <0.01 |
| Porridges—drinks | <0.01 | |
| Mousses—drinks | <0.01 | |
| Baby snacks—drinks | <0.01 | |
| Dairy—drinks | <0.01 | |
| Poultry dinners—fish dinners | <0.001 | |
| Beef dinners—fish dinners | <0.001 | |
| Pork dinners—fish dinners | <0.001 | |
| Rabbit dinners—fish dinners | <0.001 | |
| Vegetarian dinners—fish dinners | <0.001 | |
| Milk porridge—gluten-free cereal | <0.001 | |
| Hg | Mousses—dairy | <0.05 |
| Pork dinners—vegetarian dinners | <0.05 | |
| Pb | Fruit drinks and water—fruit juices | <0.01 |
| Type of Products | Elements | EDI (µg/Day) | EWI (µg/Week) | PTWI (%PTWI) (µg/kg/BW/Week) | BMDL (%BMDL) (µg/kg/BW/Day) |
|---|---|---|---|---|---|
| Baby dinners (n = 102) | As | 0.419 ± 0.909 | 2.938 ± 6.368 | NA | 0.042 ± 0.091 (1.40%) |
| Cd | 0.515 ± 0.744 | 3.607 ± 5.205 | 0.362 ± 0.523 (5.17%) | NA | |
| Hg | 0.544 ± 0.579 | 3.804 ± 4.049 | 0.382 ± 0.407 (23.89%) | NA | |
| Pb | 1.651 ± 0.922 | 11.563 ± 6.458 | NA | 0.166 ± 0.092 (33.2%) | |
| Porridges (n = 50) | As | 0.051 ± 0.054 | 0.362 ± 0.380 | NA | 0.005 ± 0.005 (0.17%) |
| Cd | 0.064 ± 0.065 | 0.449 ± 0.459 | 0.045 ± 0.046 (0.64%) | NA | |
| Hg | 0.097 ± 0.057 | 0.681 ± 0.402 | 0.068 ± 0.040 (4.28%) | NA | |
| Pb | 0.187 ± 0.114 | 1.313 ± 0.804 | NA | 0.018 ± 0.011 (3.77%) | |
| Mousses (n = 58) | As | 0.021 ± 0.017 | 0.148 ± 0.119 | NA | 0.002 ± 0.001 (0.07%) |
| Cd | 0.159 ± 0.226 | 1.0113 ± 1.583 | 0.111 ± 0.159 (1.59%) | NA | |
| Hg | 0.381 ± 0.239 | 2.669 ± 1.677 | 0.268 ± 0.168 (16.76%) | NA | |
| Pb | 0.892 ± 2.008 | 6.245 ± 14.056 | NA | 0.08 ± 0.02 (17.93%) | |
| Baby drinks (n = 64) | As | 0.037 ± 0.086 | 0.264 ± 0.607 | NA | 0.003 ± 0.008 (0.12%) |
| Cd | 0.078 ± 0.165 | 0.548 ± 1.551 | 0.055 ± 0.116 (0.78%) | NA | |
| Hg | 0.122 ± 0.080 | 0.858 ± 0.560 | 0.086 ± 0.056 (5.39%) | NA | |
| Pb | 0.553 ± 0.320 | 3.874 ± 2.242 | NA | 0.055 ± 0.032 (11.12%) | |
| Snacks (n = 62) | As | 0.242 ± 0.870 | 1.697 ± 6.091 | NA | 0.024 ± 0.087 (0.81%) |
| Cd | 0.252 ± 0.226 | 1.767 ± 1.585 | 0.177 ± 0.159 (2.53%) | NA | |
| Hg | 0.192 ± 0.136 | 1.348 ± 0.958 | 0.135 ± 0.096 (8.46%) | NA | |
| Pb | 1.053 ± 0.610 | 7.375 ± 4.276 | NA | 0.105 ± 0.061 (21.17%) | |
| Dairy (n = 60) | As | 0.007 ± 0.034 | 0.054 ± 0.233 | NA | 0.000 ± 0.003 (0.02%) |
| Cd | 0.063 ± 0.081 | 0.436 ± 0.563 | 0.044 ± 0.056 (0.63%) | NA | |
| Hg | 0.391 ± 0.668 | 2.726 ± 4.641 | 0.275 ± 0.470 (17.21%) | NA | |
| Pb | 1.168 ± 1.491 | 8.047 ± 10.397 | NA | 0.117 ± 0.149 (23.47%) | |
| TOTAL (n = 397) | As | 0.162 ± 0.598 | 1.139 ± 4.196 | NA | 0.016 ± 0.060 (0.54%) |
| Cd | 0.224 ± 0.439 | 1.571 ± 3.076 | 0.157 ± 0.309 (2.25%) | NA | |
| Hg | 0.314 ± 0.438 | 2.204 ± 3.070 | 0.221 ± 0.308 (13.84%) | NA | |
| Pb | 1.006 ± 1.192 | 7.046 ± 8.348 | NA | 0.101 ± 0.119 (20.23%) |
| THQ X ± SD (Min–Max) | ||||
|---|---|---|---|---|
| Type of Products | As | Cd | Hg | Pb |
| Baby dinners (n = 102) | 1.41 × 10−7 ± 3.04 × 10−7 (9.61 × 10−7–1.26 × 10−6) | 2.26 × 10−8 ± 4.41 × 10−8 (1.75 × 10−9–1.56 × 10−6) | 1.01 × 10−7 ± 1.19 × 10−7 (1.36 × 10−10–8.56 × 10−7) | 1.06 × 10−7 ± 1.46 × 10−7 (1.75 × 10−9–1.56 × 10−6) |
| Porridges (n = 50) | 1.82 × 10−8 ± 1.82 × 10–8 (1.23 × 10−8–2.44 × 10−6) | 1.31 × 10−8 ± 2.01 × 10−8 (1.82 × 10−8–2.31 × 10−6) | 1.06 × 10−7 ± 1.46 × 10−7 (7.66 × 10−10–8.65 × 10−7) | 1.01 × 10−7 ± 1.19 × 10−7 (1.88 × 10−8–8.65 × 10−7) |
| Mousses (n = 58) | 7.11 × 10−9 ± 5.70 × 10−9 (1.31 × 10−8–2.79 × 10−6) | 2.13 × 10−8± 2.44 × 10−8 (1.14 × 10−11–8.78 × 10−7) | 1.06 × 10−7 ± 1.19 × 10−7 (1.36 × 10−10–8.65 × 10−7) | c3.21 × 10−7 ± 2.88 × 10−7 (7.03 × 10−9–8.55 × 10−6) |
| Baby drinks (n = 64) | 1.29 × 10−8± 2.92 × 10−8 (1.31 × 10−8–1.47 × 10−6) | 3.06 × 10−8 ± 4.40 × 10−8 (8.54 × 10−11–4.46 × 10−7) | 1.98 × 10−7 ± 1.70 × 10−7 (2.06 × 10−10–4.50 × 10−6) | 2.98 × 10−7 ± 3.17 × 10−7 (1.05 × 10−9 –1.26 × 10−6) |
| Snacks (n = 62) | 8.12 × 10−8 ± 2.91 × 10−7 (5.95 × 10−9–2.31 × 10−6) | 4.99 × 10−8 ± 1.10 × 10−8 (8.52 × 10−11–9.89 × 10−7) | 1.76 × 10−7 ± 4.46 × 10−7 (6.11 × 10−11–1.05 × 10−7) | 4.51 × 10−7 ± 1.19 × 10−7 (1.46 × 10−10–1.16 × 10−6) |
| Dairy (n = 60) | 2.66 × 10−9 ± 1.127 × 10−8 (28.84 × 10−10–2.01 × 10−7) | 2.63 × 10−9 ± 2.33 × 10−8 (1.47 × 10−11–1.46 × 10−7) | 1.14 × 10−7 ± 6.18 × 10−7 (1.99 × 10−10–8.21 × 10−7) | 1.91 × 10−7 ± 1.19 × 10−7 (1.75 × 10−9–1.56 × 10−7) |
| TOTAL (n = 397) | 5.44 × 10−8 ± 2.01 × 10−8 (28.84 × 10−10–1.26 × 10−6) | 2.61 × 10−8 ± 4.42 × 10−8 (1.47 × 10−11–1.56 × 10−6) | 1.65 × 10−7 ± 1.47 × 10−7 (6.11 × 10−11–4.50 × 10−6) | 2.82 × 10−7 ± 2.21 × 10−7 (1.46 × 10−10–1.16 × 10−6) |
| Mean CR | 2.44 × 10−7 ± 8.97 × 10−7 (2.07 × 10−7–1.03 × 10−5) | 1.41 × 10−6 ± 2.77 × 10−6 (5.34 × 10−6–2.79 × 10−5) | NA | 8.56 × 10−9 ± 1.01 × 10−8 (1.48 × 10−10–1.32 × 10−7) |
| Average Content of Analyzed Parameters | Statistical Differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Products | Elements | n | X ± SD (µg/kg) | Min–Max (µg/kg) | Me (µg/kg) | Q1–Q3 (µg/kg) | Toxic Elements | Analyzed Food Group | p-Value |
| for children 6–12 months (a) | As Cd Hg Pb | 107 | 2.01 ± 3.67 2.22 ± 3.14 3.20 ± 2.86 7.82 ± 4.76 | 0.00–16.97 0.31–20.15 0.01–9.88 0.52–32.28 | 0.44 1.33 2.05 6.70 | 0.17–1.72 0.68–2.02 0.83–5.28 5.25–9.89 | As | a/b b/c | <0.01 <0.001 |
| for children under 12 months (b) | As Cd Hg Pb | 104 | 1.01 ± 2.54 2.17 ± 2.78 3.09 ± 2.22 7.74 ± 13.55 | 0.00–14.63 0.16–13.34 0.17–9.12 0.77–134.00 | 0.19 1.15 2.53 5.81 | 0.16–0.50 0.64–2.20 1.240–4.47 4.72–7.45 | Cd | a/c b/c | <0.001 <0.01 |
| without an age declaration (c) | As Cd Hg Pb | 186 | 1.35 ± 6.42 1.99 ± 3.23 2.68 ± 1.86 10.95 ± 7.02 | 0.00–84.71 0.14–18.54 0.00–9.12 0.460–48.18 | 0.20 0.63 2.11 9.38 | 0.00–0.66 0.27–2.24 1.27–3.74 7.18–12.09 | Pb | a/b a/c b/c | <0.05 <0.001 <0.001 |
| Type of Products | As (μg/kg) | Cd (μg/kg) | Hg (μg/kg) | Pb (μg/kg) | Reference |
|---|---|---|---|---|---|
| Baby food * | NA |
Me = 2.8
Max = 103.9 | NA | Max = 183.6 | [15] |
| Baby desserts | NA | Me = 0.71 Max = 14.90 | NA | Me = 2.14 | [30] |
| Baby juices | NA | Me = 0.80 Max = 1.34 | NA | Max = 5.11 | |
| Baby dinners | NA | Me = 8.31 Max = 28.2 | NA | Me = 20.6 Max = 41.0 | |
| Baby food | <LOD | <LOD | NA | <LOD | [23] |
| Max = 0.89 | Max < LOD | NA | <LOD | ||
| Baby dinners | NA | NA | Me = 0.80 Max = 7.40 | NA | [33] |
| Cereals food | NA | NA | Me = 0.58 Max = 1.0 | NA | |
| Rise baby food | Me = 0.088 | NA | Me = 0.062 | NA | [24] |
| Max = 0.150 | NA | Max = 0.3 | NA | ||
| Rise baby food | NA | NA | X = 0.94 ± 0.47 | NA | [14] |
| Milk-based food | X = 0.23 ± 0.05 | X = 0.23 ± 0.05 | NA | X = 1.2 ± 0.19 | [25] |
| Spelt based food | X = 3.8 ± 0.05 | X = 2.4 ± 0.11 | NA | X = 1.8 ± 0.12 | |
| Oat-based food | X = 2.4 ± 0.19 | X = 3.3 ± 0.14 | NA | X = 3.1 ± 0.23 | |
| Rice-based food | X = 1.7 ± 0.04 | X = 33 ± 0.56 | NA | X = 1.2 ± 0.12 | |
| Baby food | NA | X = 0.38 ± 0.2 | X = 0.22 ± 0.08 | X = 0.47 ± 0.14 | [27] |
| Cereal-based food | NA | NA | Me = 0.50 | NA | [34] |
| Baby food | NA | NA | Me = 0.40 | NA | |
| Baby food | NA | Me = 33.0 | NA | Me = 109 | [28] |
| Milk-based food | NA | X = 0.05 ± 0.005 | NA | X = 0.21 ± 0.02 | [32] |
| Milk-based food | NA | NA | Me = 0.03 | NA | [41] |
| Fruit-based food | <LOD | <LOD | NA | X = 12.0 | [36] |
| Vegetable-based food | X = 29.0 | ||||
| Meat based food | X = 34.50 | ||||
| Cereal-based food | X = 0.68 ± 0.67 | NA | NA | NA | [26] |
| Baby food | NA | NA | NA | X = 3.4 ± 2.01 | [37] |
| Milk-based food | X = 1.11 ± 0.21 | ||||
| Cereal-based food | X = 1.40 ± 1.95 | ||||
| Baby mouses | X = 2.15 ± 2.08 | ||||
| Baby drinks | X = 3.72 ± 2.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudzińska, A.; Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Soroczyńska, J.; Mielcarek, K.; Socha, K. Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market. Nutrients 2022, 14, 2325. https://doi.org/10.3390/nu14112325
Żmudzińska A, Puścion-Jakubik A, Bielecka J, Grabia M, Soroczyńska J, Mielcarek K, Socha K. Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market. Nutrients. 2022; 14(11):2325. https://doi.org/10.3390/nu14112325
Chicago/Turabian StyleŻmudzińska, Anita, Anna Puścion-Jakubik, Joanna Bielecka, Monika Grabia, Jolanta Soroczyńska, Konrad Mielcarek, and Katarzyna Socha. 2022. "Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market" Nutrients 14, no. 11: 2325. https://doi.org/10.3390/nu14112325
APA StyleŻmudzińska, A., Puścion-Jakubik, A., Bielecka, J., Grabia, M., Soroczyńska, J., Mielcarek, K., & Socha, K. (2022). Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market. Nutrients, 14(11), 2325. https://doi.org/10.3390/nu14112325









