Abstract
Background: Additional metabolic indicators ought to be combined as outcome variables when exploring the impact of breastfeeding on obesity risk. Given the role of a healthy lifestyle in reducing obesity, we aimed to assess the effect of breastfeeding duration on different obesity phenotypes according to metabolic status in children and adolescents, and to explore the offsetting effect of healthy lifestyle factors on the associations between breastfeeding duration and obesity phenotypes. Methods: A total of 8208 eligible children and adolescents aged 7–18 years were recruited from a Chinese national cross-sectional study conducted in 2013. Anthropometric indicators were measured in the survey sites, metabolic indicators were tested from fasting blood samples, and breastfeeding duration and sociodemographic factors were collected by questionnaires. According to anthropometric and metabolic indicators, obesity phenotypes were divided into metabolic healthy normal weight (MHNW), metabolic unhealthy normal weight (MUNW), metabolic healthy obesity (MHO), and metabolic unhealthy obesity (MUO). Four common obesity risk factors (dietary consumption, physical activity, screen time, and sleep duration) were used to construct a healthy lifestyle score. Scores on the lifestyle index ranged from 0 to 4 and were further divided into unfavorable lifestyles (zero or one healthy lifestyle factor), intermediate lifestyles (two healthy lifestyle factors), and favorable lifestyle (three or four healthy lifestyle factors). Multinomial logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for the associations between breastfeeding duration and obesity phenotypes. Furthermore, the interaction terms of breastfeeding duration and each healthy lifestyle category were tested to explore the offsetting effect of lifestyle factors. Results: The prevalence of obesity among Chinese children and adolescents aged 7–18 years was 11.0%. Among the children and adolescents with obesity, the prevalence of MHO and MUO was 41.0% and 59.0%, respectively. Compared to the children and adolescents who were breastfed for 6–11 months, prolonged breastfeeding (≥12 months) increased the risks of MUNW (OR = 1.35, 95% CI: 1.19–1.52), MHO (OR = 1.61, 95% CI: 1.27–2.05), and MUO (OR = 1.46, 95% CI: 1.20–1.76). When stratified by healthy lifestyle category, there was a typical dose–response relationship between duration of breastfeeding over 12 months and MUNW, MHO, and MUO, with an increased risk of a favorable lifestyle moved to an unfavorable lifestyle. Conclusions: Prolonged breastfeeding (≥12 months) may be associated with increased risks of MUNW, MHO, and MUO, and the benefits of breastfeeding among children and adolescents may begin to wane around the age of 12 months. The increased risks may be largely offset by a favorable lifestyle.
1. Introduction
Recently, childhood obesity has been among the looming global public health issues, which could cause metabolic abnormalities, type 2 diabetes, cardiovascular diseases, and tumors in adulthood, thus representing a serious medical burden [1,2,3]. China has the largest number of children and adolescents with obesity in the world [4], where the prevalence of overweight and obesity among children and adolescents aged 6–17 years has exceeded 19% [5]. Body mass index (BMI) is a combination of weight and height, thus representing a typical way to identify obesity. However, it has been shown that individuals with the same BMI may have different status of metabolic components, including blood glucose, blood pressure, and blood lipid levels [6]. Therefore, the definition of obesity by BMI alone does not accurately reflect obesity-related metabolic status and susceptibility to metabolic diseases. There are two obesity phenotypes based on BMI combined with metabolic components. People with obesity and normal metabolic characteristics are considered as metabolic healthy obesity (MHO), while people with obesity and metabolically unhealthy status are defined as metabolic unhealthy obesity (MUO) [7,8]. MUO is associated with a higher risk of cardiovascular disease and mortality compared to MHO [9]. Given that different obesity phenotypes have varying disease risks, identifying the modifiable risks and protective factors of obesity phenotypes may contribute to future precise stratified management of obesity intervention.
Among modifiable risk factors for childhood obesity in the first 1000 days of life, breastfeeding was considered as an effective protective factor to reduce the risk of childhood obesity due to the bioactive compounds of breast milk [10,11,12,13,14,15]. However, breastfeeding as a measure to prevent overweight and obesity in children and adolescents has produced conflicting results. Some systematic reviews, most of which were conducted in European countries [16,17,18], as well as several studies from the United States [19,20,21] and Brazil [22], reported that breastfeeding duration was inversely related to the risk of obesity in children and adolescents. However, a large randomized controlled trial that promoted longer breastfeeding duration in Belarus did not show significant or meaningful changes in obesity, blood pressure, or cardiometabolic risk factors in children and adolescents [23,24,25]. Another cohort study in Japan had similar findings [26]. In addition, cohort studies in Finland and Sweden found a positive association between breastfeeding duration and obesity [27,28]. According to these findings, the association between breastfeeding duration and childhood obesity may vary by study design, ethnicities, confounding factors, and the definition of obesity itself, thus necessitating further exploration [29,30].
Previous studies that measured only BMI in children may have underestimated the true impact of breastfeeding on obesity risk [31], and more metabolic indicators need to be combined as outcome variables such as MUO with higher health damage to assess the effect of breastfeeding. More studies urgently ought to explore the associations between breastfeeding duration and obesity phenotypes. Studies have found that breastfeeding was associated with healthier eating behaviors in later childhood, such as higher consumption of vegetables and fruits and lower consumption of sugary drinks and ultra-processed foods [32,33,34]. Additionally, a large number of studies showed that a favorable lifestyle such as healthy diet, adequate sleeping duration, and physical activity established in later life would significantly decrease the risk of childhood obesity [35,36,37]. However, it remains unclear whether adherence to a healthy lifestyle could influence the associations between breastfeeding duration and obesity or obesity phenotypes.
We hypothesized that breastfeeding duration was related to obesity phenotypes, and that these associations might be affected by a healthy lifestyle. In this study, we aimed to assess the effect of breastfeeding duration on different obesity phenotypes and to investigate whether a healthy lifestyle would modify these associations among children and adolescents aged 7–18 years using the data from a Chinese national cross-sectional study in 2013.
2. Materials and Methods
2.1. Study Design and Population
Data were collected and maintained by a national cross-sectional study conducted in September 2013. Children and adolescents aged 7–18 years from seven provinces, namely, Tianjin, Shanghai, Chongqing, Liaoning, Hunan, Ningxia, and Guangdong, were selected by a multistage cluster random sampling method. The seven provinces in this study came from different economic levels and different geographical regions of China. The detailed description of the study design was reported in a previous study [38]. In brief, 3–4 districts were randomly selected from each province, with 12–16 schools randomly selected from each district, and 2–3 classes per grade randomly selected from each school. A total of 15,733 children and adolescents aged 7–18 years with a blood sample were included (Figure 1). In our study, children and adolescents with normal weight and obesity were extracted, and cases with missing data about systolic blood pressure (SBP), diastolic blood pressure (DBP), and breastfeeding were excluded. The remaining 8208 children and adolescents were included in final analysis.
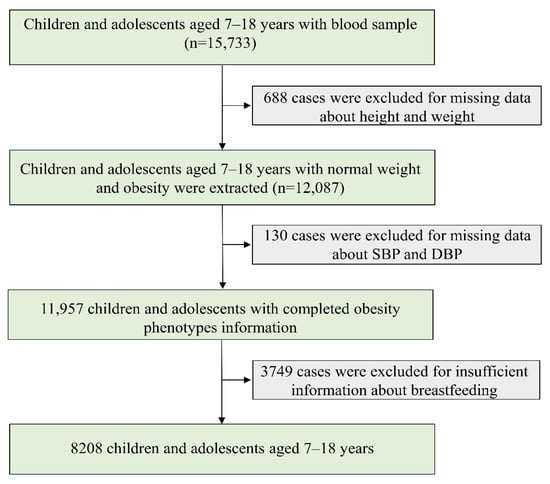
Figure 1.
Flow chart of data. SBP, systolic blood pressure; DBP, diastolic blood pressure.
2.2. Data Collection and Questionnaire Survey
2.2.1. Anthropometric Measurements
All anthropometric measurements were conducted using standardized instruments and procedures. Height (cm) was measured to the nearest 0.1 cm by a portable stadiometer (model TZG, China) where participants need to stand straight with light clothes and without shoes. Body weight (kg) was assessed by a lever-type weight scale (model RGT-140, China) to the nearest 0.1 kg. Body mass index (BMI) was then calculated as weight (kg) divided by the square of the height (m). Children and adolescents were required to sit quietly for at least 5 min prior to the first measurement of SBP (mmHg) and DBP (mmHg) using a mercury sphygmomanometer (model XJ1ID, Shanghai Medical Instruments Co., Ltd., Shanghai, China). Each indicator was measured twice, and the mean of the two measurements was calculated for the final analysis. Specifically, a 5 min break was allowed between the two measurements of blood pressure.
2.2.2. Blood Sample Collection and Detection
Venous blood was collected by venipuncture after fasting for 12 h. After centrifuging at 3000 rpm for 10 min, serum was collected and transported to the experimental center at low temperature (−80 °C). Blood biochemical analyses were performed by a biomedical analysis company certified by Peking University [39]. Fasting plasma glucose (FPG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were analyzed using the hexokinase method, enzymatic method, and clearance method, respectively.
2.2.3. Questionnaire Survey
A self-administered questionnaire of children and adolescents was used to obtain information on basic characteristics (e.g., age, sex, residence, and single-child status), dietary consumption (meat, sugar-sweetened beverage, fruit, and vegetable consumption), physical activity, screen time, and sleep duration. The questionnaires were revised in the early stage of our project and validated by experts, before being deemed feasible and acceptable for children and parents. Some questionnaires (3%) from the same participants were re-examined within a week. All questionnaires were also checked for logicality and integrity. Children aged 7–9 years were administered the questionnaire with the assistance of their parents. We gathered the frequency (day) and amount (servings) of each food over the course of a week. The average daily intake of a single food was calculated using the following equation: (day × quantity in each of those days)/7 [40].
A self-administered parent questionnaire was used to obtain information on breastfeeding duration, birth weight, delivery time, and delivery model for children and adolescents, education level and tobacco and alcohol consumption for parents, maternal age at delivery, and family household income. Family history of diseases was considered as having a family history of obesity, hypertension, diabetes mellitus, or cerebrovascular disease when either paternal or maternal diagnosis was self-reported.
2.3. Definition and Categorization of Indicators
2.3.1. Obesity Phenotypes
Obesity phenotypes included metabolic healthy normal weight (MHNW), metabolic unhealthy normal weight (MUNW), MHO, and MUO according to expert consensus on the definition of metabolically healthy obesity and screening metabolically healthy obesity in Chinese children [41]. Obesity phenotypes were evaluated on the basis of (1) BMI (normal weight was determined as BMI < 85th percentile and obesity was determined as BMI ≥ 95th percentile for sex- and age-specific group [42]) and (2) metabolic abnormalities ((a) hypertension: SBP and/or DBP ≥ 95th percentile for sex- and age-specific group; (b) elevated fasting glucose: FPG ≥ 5.6 mmol/L; (c) high TG: TG ≥ 1.70 mmol/L; (d) low HDL-C: HDL-C < 1.03 mmol/L). MHNW was defined as normal weight without metabolic abnormalities. MUNW was defined as normal weight with 1–4 metabolic abnormalities. MHO was defined as obesity without metabolic abnormalities. MUO was defined as obesity with 1–4 metabolic abnormalities.
2.3.2. Breastfeeding Duration
Children and adolescents were categorized into three subgroups of 0–5 month(s), 6–11 months, and ≥12 months according to breastfeeding duration (in month). Breastfeeding was not limited to exclusive breastfeeding.
2.3.3. Healthy Lifestyle
Four common obesity risk factors (dietary consumption, physical activity, screen time, and sleep duration) were used to construct a healthy lifestyle score [43,44]. A healthy diet was based on four regularly consumed foods (meat, sugar-sweetened beverage, fruit, and vegetable consumption) linked to childhood obesity [45,46]. In this study, dietary optimum components were defined as daily intake of fruits of ≥3 servings (one serving is about 100 g), vegetables of ≥4 servings (one serving is about 100 g), meat products of 2–3 servings (one serving is about 50 g), and weekly intake of sugar-sweetened beverage of <1 serving (one serving is about 250 mL) according to the dietary guidelines for school-age children in China (2016) [47]. Children and adolescents were considered to have a healthy diet if they consumed at least two of the four common foods in the required servings. Physical activity was categorized by the cutoff of moderate to vigorous physical activity for 1 h per day [48]. Screen time was defined as <2 h of screen time per day and ≥2 h per day. Sleep duration was defined as sufficient sleep and insufficient sleep according to the cutoff of 9 h for children and adolescents [49]. Children and adolescents scored one point for each of the four defined health behaviors. Scores on the lifestyle index ranged from 0–4, and they were further divided into unfavorable lifestyles (zero or one healthy lifestyle factor), intermediate lifestyles (two healthy lifestyle factors), and favorable lifestyles (three or four healthy lifestyle factors) [37].
2.3.4. Confounding Variables
In this study, age, sex, residence, single-child status, delivery model, delivery date, birth weight, family history of diseases, parental education level, parental tobacco and alcohol consumption, maternal age at delivery, and family household income were considered as confounding variables. Children and adolescents were categorized according to their birth weight into low birth weight (LBW, birth weight < 2500 g), normal birth weight (NBW, birth weight: 2500–3999 g), and high birth weight (HBW, birth weight ≥ 4000 g).
2.4. Statistics Analysis
Quantitative variables were shown as mean (standard deviation) or median (interquartile range) according to the normality of distribution, and categorical variables were shown as number (percentage). Nonparametric test or Bonferroni multiple comparison method and chi-squared test were used to compare difference of quantitative data and categorical data, respectively, between different breastfeeding duration groups. A multinomial logistic regression model was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for the associations between breastfeeding duration and obesity phenotypes, and MHNW was considered as the reference group. The interaction terms of breastfeeding duration and healthy lifestyle category were also tested. When interactions were significant, stratified analyses were performed. The final model was adjusted for age, sex, residence, single-child status, delivery model, delivery time, birth weight, family history of diseases, parental education level, parental tobacco and alcohol consumption, maternal age at delivery, and family household income. All statistical analyses were performed with IBM SPSS Statistics version 26.0 and GraphPad Prism 9. A two-tailed p-value <0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Participants
In the total population, the prevalence of obesity was 11.0%, and the prevalence of MHO and MUO was 4.4% and 6.6%, respectively. Table 1 shows the characteristics of children and adolescents, as well as their parents, by breastfeeding duration groups. A total of 8208 eligible children and adolescents, 2373 in the breastfeeding duration for 0–5 months group, 3184 in the breastfeeding duration for 6–11 months group, and 2651 in the breastfeeding duration for ≥12 months group, were included in the final analysis. Among the children and adolescents with obesity, the prevalence of MHO and MUO was 41.0% and 59.0% respectively.

Table 1.
Demographic characteristics of eligible children and adolescents and their parents, stratified by breastfeeding duration (n = 8208).
Compared to the breastfeeding duration for 6–11 months group, children and adolescents who breastfed for ≥12 months had a lower proportion of MHNW (62.0% vs. 69.5%) and a higher proportion of MUNW (38.0% vs. 27.2%); children and adolescents who breastfed for 0–5 months had a higher proportion of MHO (48.9% vs. 37.3%) and a lower proportion of MUO (51.1% vs. 62.7%). A lower proportion of children and adolescents with breastfeeding duration for ≥12 months engaged in a favorable (15.3% vs. 13.5%) and intermediate lifestyle (36.1% vs. 32.6%). Parents in the breastfeeding duration for 0–5 months group had the highest education level (36.2% and 36.4% for junior college or above); however, those in the breastfeeding duration for ≥12 months group had the lowest education level (16.0% and 15.1% for junior college or above). No significant difference was found in monthly household income between different breastfeeding duration groups.
3.2. Associations between Breastfeeding Duration and Obesity Phenotypes
Figure 2 and Supplementary Table S1 show the OR values for the associations between breastfeeding duration and obesity phenotypes. Compared to the children and adolescents who were breastfed for 6–11 months, participants who were in the longer breastfeeding duration group (≥12 months) had higher risks of MUNW (OR = 1.35, 95% CI: 1.19–1.52), MHO (OR = 1.61, 95% CI: 1.27–2.05), and MUO (OR = 1.46, 95% CI: 1.20–1.76) after adjusting for age, sex, residence, single-child status, delivery model, delivery date, birth weight, family history of diseases, parental education level, parental tobacco and alcohol consumption, maternal age at delivery, and family household income. In Model 1 (crude model, see Supplementary Table S1), participants who were in the breastfeeding duration for 0–5 months group had a higher risk of MHO (OR = 1.46, 95% CI: 1.16–1.84); however, after adjusting for confounders, these associations turned out to be nonsignificant (Model 3 in Supplementary Table S1).
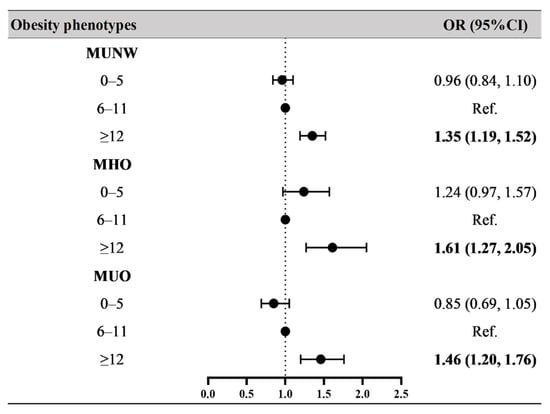
Figure 2.
Associations between breastfeeding duration and obesity phenotypes. Obesity phenotypes include metabolic healthy normal weight (MHNW, reference group), metabolic unhealthy normal weight (MUNW), metabolic healthy obesity (MHO), and metabolic unhealthy obesity (MUO). Bold values of OR (95% CI) are statistically significant (p < 0.05). Adjusted for age, sex, residence, single-child status, delivery model, delivery date, family history of diseases (obesity, hypertension, diabetes mellitus and cerebrovascular disease), parental education level, parental tobacco and alcohol consumption, maternal age at delivery, and family household income.
3.3. The Offsetting Effect of Healthy Lifestyle
When breastfeeding duration and healthy lifestyle category were evaluated as interaction variables for obesity phenotypes, it was discovered that healthy lifestyle category and breastfeeding duration for ≥12 months had a significant interaction effect on MUNW, MHO, and MUO (Supplementary Table S2). Figure 3 shows the adjusted OR values for associations between breastfeeding duration and obesity phenotypes stratified by healthy lifestyle category. Although the OR values in certain groups were not statistically significant, there was a typical dose–response relationship between breastfeeding duration for more than 12 months and obesity phenotypes, with increased risks of MUNW (favorable: OR = 1.08, 95% CI: 0.77–1.51; intermediate: OR = 1.35, 95% CI: 1.09–1.68; unfavorable: OR = 1.40, 95% CI: 1.18–1.66), MHO (favorable: OR = 1.25, 95% CI: 0.66–2.37; intermediate: OR = 1.81, 95% CI: 1.17–2.81; unfavorable: OR = 1.61, 95% CI: 1.15–2.23), and MUO (favorable: OR = 0.93, 95% CI: 0.53–1.61; intermediate: OR = 1.41, 95% CI: 1.03–1.94; unfavorable: OR = 1.68, 95% CI: 1.27–2.21) when a favorable lifestyle moved to an unfavorable lifestyle.
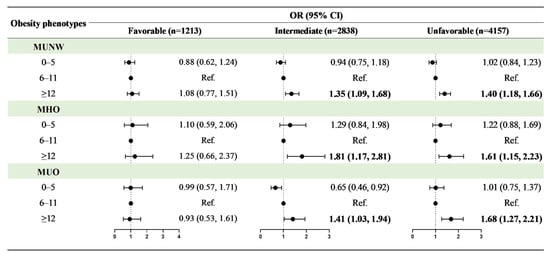
Figure 3.
Associations between breastfeeding duration and obesity phenotypes in different healthy lifestyle groups. Obesity phenotypes include metabolic healthy normal weight (MHNW, reference group), metabolic unhealthy normal weight (MUNW), metabolic healthy obesity (MHO), and metabolic unhealthy obesity (MUO). Adjusted for age, sex, residence, single-child status, delivery model, delivery date, birth weight, family history of diseases (obesity, hypertension, diabetes mellitus and cerebrovascular disease), parental education level, parental tobacco and alcohol consumption, maternal age at delivery, and family household income. Bold values of OR (95% CI) are statistically significant (p < 0.05).
4. Discussion
This study is the first to report the national prevalence of different obesity phenotypes in China. The prevalence of obesity among Chinese children and adolescents aged 7–18 years was 11.0%. Among the children and adolescents with obesity, the prevalence of MHO and MUO was 41.0% and 59.0%, respectively. On the basis of the different obesity phenotype outcomes, we found that prolonged breastfeeding (≥12 months) was associated with increased risks of MUNW, MHO, and MUO, while a favorable lifestyle may offset the negative effects to some extent.
Consistent with the findings of existing studies [50,51], our results support the conclusion that prolonged breastfeeding is detrimental. Adults of a birth cohort study in Finland who breastfed for 8 months or more reported an 8.8% increase in overweight compared to those who breastfed for a shorter period of time [51]. A prospective cohort study in Sweden found that obesity prevalence was slightly higher for children aged 4 years with breastfeeding for 12 months than those who were breastfed for 9 months [30]. We speculated that the benefits of breastfeeding might begin to wane around the age of 12 months. The physiological mechanism via which prolonged breastfeeding might increase the risk of obesity is chronic exposure to maternal hormones present in breast milk, which could theoretically alter infant lipid metabolism and increase body fat composition later in life [51]. For example, a cohort study in the United Kingdom showed that prolonged breastfeeding was associated with higher cholesterol concentrations in later life [31], while mothers who breastfed for longer than 12 months had increased free thyroxine concentrations [52], which were associated with the regulation of circulating lipoprotein concentrations [53]. In addition, there were U-shaped associations between breastfeeding duration and body fat percentage and blood pressure [31,51]. Infants in poorer families tend to be breastfed for longer [51,54]. For children older than 12 months with a better supply of complementary food in high-income countries, additional breast milk may increase fat intake since the primary nutrient in breast milk after 12 months for infant is fat [55]. These facts may be significant reasons for the higher risk of MHO and MUO in longer periods (≥12 months) of breastfeeding.
A previous study from the same database found that prolonged breastfeeding (≥12 months) was associated with low lipid level and reduced risk of abnormal blood lipids; however, the beneficial effects were mainly reflected in total cholesterol (TC) [56]. Although the OR values of HDL-C and TG were not statistically significant, the HDL-C levels in children and adolescents who were breastfed for more than 12 months were lower than those in the non-breastfeeding group (p < 0.01). Only HDL-C and TG overlapped with the metabolic indicators of obesity phenotypes in the present study. Therefore, the results of the two studies are not contradictory, but are presented from different perspectives due to the role of breastfeeding duration in different diseases. Actually, this is the first study in the world to examine the associations between different obesity phenotypes and breastfeeding duration. This study provides a standardized outcome and detailed extensive subgroup analysis, serving as a reference and a more specific association for future investigation.
Breastfeeding for shorter periods of time might be hazardous due to the negative effects of alternate feeding [51]. Nevertheless, in our study, we did not find a role for short-term breastfeeding. The present study discovered that more educated parents established a shorter breastfeeding duration. Highly educated parents may turn to other alternatives such as more intensive care and promoting a healthier lifestyle for their children in later life to prevent obesity. However, the present study did not collect specific information on artificial feeding and food availability. Formula-fed children had higher protein intakes than breastfed children, relative to exclusive breastfeeding [57]. Nevertheless, higher protein intake is thought to increase insulin and insulin-like growth factor-1 (IGF-1) secretion in early childhood, which is associated with early obesity rebound [57]. Breastfed children could automatically control their food intake according to their own requirements, while formula-fed babies were passive. In addition, introducing complementary foods as early as 4 months of age or before increased the risk of overweight in childhood [58]. Thus, the findings may only be applicable to areas with a diverse food market, such as China.
The World Health Organization (WHO) recommends exclusive breastfeeding for 6 months and continued breastfeeding until 2 years or more [59], which is a very ideal goal to reach [60,61], especially in those countries and regions with limited drinking water and food security and diversity [62]. In fact, many Western countries, including 65% of European members and the United States, choose not to fully follow this recommendation [63]. The American Academy of Pediatrics recommends exclusive breastfeeding for 6 months and continued breastfeeding until 1 year or more [64], while guidelines from the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition state that complementary foods could be introduced between the 17th and 26th weeks of life [65]. Moreover, Grummer-Strawn and Rollins emphasized the difficulty of conclusions from breastfeeding research due to the different conclusions of many studies, methodological weaknesses, and the varied mechanisms of breastfeeding affecting health [66]. Despite a systematic review which demonstrated that breastfeeding was linked to a 12% lower incidence of obesity when compared to never breastfeeding [67], this association might no longer be present after controlling for maternal BMI, smoking, and socioeconomic variables, as argued by several well-designed studies [23,27]. Given that varied settings with different social atmospheres, food supplies, and individual characteristics, the recommended breastfeeding duration from WHO might not be suitable for every country, which also need to establish individual policies according to their specific conditions.
Research has shown that, further away from infancy, there is a greater impact of other lifestyle factors (such as diet, exercise) on obesity [21]. When children adhered to a favorable lifestyle in later life, they could have a 50% lower risk of obesity than those who adhered to an unfavorable lifestyle [37]. These findings suggest that the increased risk of childhood obesity induced by prolonged breastfeeding duration might be largely offset by a favorable lifestyle later in life.
Despite these promising results, several limitations remained. Firstly, the associations observed in this study were from a cross-sectional study, leading to weak causal inference. Secondly, due to excessive sample deletion, there may have been some selection bias in the samples of this study. Thirdly, the reliability and validity information of the questionnaire was not provided in this study, and the validity of the questionnaire was not verified. In particular, breastfeeding was not collected according to the scale; thus, there might have been information bias. Fourthly, the information about breastfeeding was retrospectively investigated, and there was a certain recall bias, leading to overestimation or underestimation of effect values. In addition, the breastfeeding group and healthy lifestyle category were artificially divided; thus, misclassification bias existed. Fifthly, the role of formula milk powder and complementary foods in infant feeding was not excluded in this study. Additionally, despite the analysis of many variables that could have led to bias, residual confounding existed. Lastly, this study was not a large-scale national survey, and more regional participation is needed in the future.
5. Conclusions
Prolonged breastfeeding (≥12 months) may be associated with increased risks of MUNW, MHO, and MUO, and the benefits of breastfeeding might begin to wane around the age of 12 months among children and adolescents. The increased risks may be largely offset by a favorable lifestyle such as healthy diet, limited screen time, adequate physical activity, and sufficient sleep duration. Breastfeeding duration for no more than 12 months and the establishment of a healthy lifestyle are recommended to prevent different obesity phenotypes for children and adolescents in China. Breastfeeding duration until 2 years or more, as recommended by the WHO, may be amended on the basis of the actual situation in different countries, such as the addition of children’s complementary foods, and policies in the light of evidence-based studies should be formulated.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu14101999/s1: Table S1. Associations between breastfeeding duration and obesity phenotypes; Table S2. Risk of obesity phenotypes according to healthy lifestyle category and breastfeeding duration.
Author Contributions
Conceptualization, J.D. and Y.S.; data curation, T.C., N.M., Y.L., P.Z. and D.S.; formal analysis, J.D.; funding acquisition, Y.S.; investigation, Z.Z., Y.M. and J.M.; methodology, J.D. and T.C.; project administration, Y.D., Z.Z., Y.M., Y.S. and J.M.; resources, Y.D., Z.Z., Y.M., Y.S. and J.M.; software, J.D., Y.L. and P.Z.; supervision, Y.M., Y.S. and J.M.; validation, Y.D., Z.Z. and Y.S.; visualization, N.M. and D.S.; writing—original draft, J.D. and T.C.; writing—review and editing, T.C., N.M., Y.L., P.Z., D.S., Y.D., Z.Z., Y.M., J.M. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
The present research was supported by funding from the Natural Science Foundation of Beijing (Grant No. 7222247 to Y.S.)
Institutional Review Board Statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving students were approved by the Ethics Committee of Peking University (NO. IRB0000105213034).
Informed Consent Statement
All participants and their parents voluntarily signed informed consent forms.
Data Availability Statement
The data supporting the conclusions of this article can be made available from the corresponding author upon request.
Acknowledgments
The authors gratefully acknowledge all the schoolteachers and doctors for their assistance in data collection and all students and their guardians for participating in the study. We also would like to thank Patrick W.C. Lau from Hong Kong Baptist University for his contribution to the English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Boekholdt, S.M.; Wareham, N.; Luben, R.; Welch, A.; Bingham, S.; Buchan, I.; Day, N.; Khaw, K. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: A population-based prospective study. Circulation 2007, 116, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Kramer, C.K.; Zinman, B.; Retnakaran, R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 758–769. [Google Scholar] [CrossRef]
- Eftekharzadeh, A.; Asghari, G.; Serahati, S.; Hosseinpanah, F.; Azizi, A.; Barzin, M.; Mirmiran, P.; Azizi, F. Predictors of incident obesity phenotype in nonobese healthy adults. Eur. J. Clin. Investig. 2017, 47, 357–365. [Google Scholar] [CrossRef]
- Mirzaei, B.; Abdi, H.; Serahati, S.; Barzin, M.; Niroomand, M.; Azizi, F.; Hosseinpanah, F. Cardiovascular risk in different obesity phenotypes over a decade follow-up: Tehran Lipid and Glucose Study. Atherosclerosis 2017, 258, 65–71. [Google Scholar] [CrossRef]
- Rhee, E.-J.; Lee, M.K.; Kim, J.D.; Jeon, W.S.; Bae, J.C.; Park, S.E.; Park, C.-Y.; Oh, K.-W.; Park, S.-W.; Lee, W.-Y. Metabolic health is a more important determinant for diabetes development than simple obesity: A 4-year retrospective longitudinal study. PLoS ONE 2014, 9, e98369. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and breastfeeding: The strength of association. Women Birth 2015, 28, 81–86. [Google Scholar] [CrossRef]
- Cope, M.B.; Allison, D.B. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long-term effects of breastfeeding: Systematic reviews and meta-analysis’ with respect to obesity. Obes. Rev. 2008, 9, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Agosti, M. Nutrition in the First 1000 Days: Ten Practices to Minimize Obesity Emerging from Published Science. Int. J. Environ. Res. Public Health 2017, 14, 1491. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Arenz, S.; Rückerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. 2004, 28, 1247–1256. [Google Scholar] [CrossRef]
- Harder, T.; Bergmann, R.; Kallischnigg, G.; Plagemann, A. Duration of breastfeeding and risk of overweight: A meta-analysis. Am. J. Epidemiol. 2005, 162, 397–403. [Google Scholar] [CrossRef]
- Spatz, D.L. Preventing obesity starts with breastfeeding. J. Périnat. Neonatal Nutr. 2014, 28, 41–50. [Google Scholar] [CrossRef]
- Hawkins, S.S.; Baum, C.F.; Rifas-Shiman, S.L.; Oken, E.; Taveras, E.M. Examining Associations between Perinatal and Postnatal Risk Factors for Childhood Obesity Using Sibling Comparisons. Child. Obes. 2019, 15, 254–261. [Google Scholar] [CrossRef]
- Colen, C.G.; Ramey, D.M. Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc. Sci. Med. 2014, 109, 55–65. [Google Scholar] [CrossRef]
- Metzger, M.W.; McDade, T.W. Breastfeeding as obesity prevention in the United States: A sibling difference model. Am. J. Hum. Biol. 2010, 22, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Contarato, A.A.; Rocha, E.D.; Czarnobay, S.A.; Mastroeni, S.S.; Veugelers, P.J.; Mastroeni, M.F. Independent effect of type of breastfeeding on overweight and obesity in children aged 12–24 months. Cad. Saude Publ. 2016, 32, e00119015. [Google Scholar]
- Kramer, M.S.; Matush, L.; Vanilovich, I.; Platt, R.W.; Bogdanovich, N.; Sevkovskaya, Z.; Dzikovich, I.; Shishko, G.; Collet, J.; Martin, R.M.; et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. Am. J. Clin. Nutr. 2007, 86, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: A randomized trial. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; Thompson, J.; et al. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: A cluster-randomized, controlled trial. Circulation 2014, 129, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sata, M.; Yamagishi, K.; Sairenchi, T.; Irie, F.; Sunou, K.; Watanabe, H.; Iso, H.; Ota, H. Breastfeeding in Infancy in Relation to Subsequent Physical Size: A 20-year Follow-up of the Ibaraki Children’s Cohort Study (IBACHIL). J. Epidemiol. 2021; advance online publication. [Google Scholar]
- Wallby, T.; Lagerberg, D.; Magnusson, M. Relationship Between Breastfeeding and Early Childhood Obesity: Results of a Prospective Longitudinal Study from Birth to 4 Years. Breastfeed Med. 2017, 12, 48–53. [Google Scholar] [CrossRef]
- Fall, C.H.; Borja, J.B.; Osmond, C.; Richter, L.; Bhargava, S.K.; Martorell, R.; Stein, A.D.; Barros, F.C.; Victora, C.G.; the COHORTS Group. Infant-feeding patterns and cardiovascular risk factors in young adulthood: Data from five cohorts in low- and middle-income countries. Int. J. Epidemiol. 2011, 40, 47–62. [Google Scholar] [CrossRef]
- Taveras, E.M.; Gillman, M.W.; Kleinman, K.; Rich-Edwards, J.W.; Rifas-Shiman, S.L. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics 2010, 125, 686–695. [Google Scholar] [CrossRef]
- Kelly, Y.J.; Watt, R.G.; Nazroo, J.Y. Racial/ethnic differences in breastfeeding initiation and continuation in the United Kingdom and comparison with findings in the United States. Pediatrics 2006, 118, e1428–e1435. [Google Scholar] [CrossRef]
- Ma, J.; Qiao, Y.; Zhao, P.; Li, W.; Katzmarzyk, P.T.; Chaput, J.; Fogelholm, M.; Kuriyan, R.; Lambert, E.; Maher, C.; et al. Breastfeeding and childhood obesity: A 12-country study. Matern. Child Nutr. 2020, 16, e12984. [Google Scholar] [CrossRef]
- Hamulka, J.; Zielinska, M.A.; Jeruszka-Bielak, M.; Górnicka, M.; Głąbska, D.; Guzek, D.; Hoffmann, M.; Gutkowska, K. Analysis of Association between Breastfeeding and Vegetable or Fruit Intake in Later Childhood in a Population-Based Observational Study. Int. J. Environ. Res. Public Health 2020, 17, 3755. [Google Scholar] [CrossRef] [PubMed]
- Perrine, C.G.; Galuska, D.A.; Thompson, F.E.; Scanlon, K.S. Breastfeeding duration is associated with child diet at 6 years. Pediatrics 2014, 134 (Suppl. 1), S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.C.D.A.; Ribeiro, S.A.V.; Andreoli, C.S.; de Carvalho, C.A.; Pessoa, M.C.; de Novaes, J.F.; Priore, S.E.; Franceschini, S.D.C.C. Association of exclusive breastfeeding duration with consumption of ultra-processed foods, fruit and vegetables in Brazilian children. Eur. J. Nutr. 2019, 58, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yoo, J.E.; Kim, K.; Choi, S.; Park, S.M. Associations between birth weight, obesity, fat mass and lean mass in Korean adolescents: The Fifth Korea National Health and Nutrition Examination Survey. BMJ Open 2018, 8, e018039. [Google Scholar] [CrossRef]
- Vasylyeva, T.L.; Barche, A.; Chennasamudram, S.P.; Sheehan, C.; Singh, R.; Okogbo, M.E. Obesity in prematurely born children and adolescents: Follow up in pediatric clinic. Nutr. J. 2013, 12, 150. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zou, Z.Y.; Dong, Y.H.; Xu, R.B.; Yang, Y.D.; Ma, J. A Healthy Lifestyle Offsets the Increased Risk of Childhood Obesity Caused by High Birth Weight: Results from a Large-Scale Cross-Sectional Study. Front. Nutr. 2021, 8, 736900. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zou, Z.Y.; Yang, Z.P.; Wang, Z.H.; Jing, J.; Luo, J.Y.; Zhang, X.; Luo, C.Y.; Wang, H.; Zhao, H.P.; et al. Association between high birth weight and hypertension in children and adolescents: A cross-sectional study in China. J. Hum. Hypertens. 2017, 31, 737–743. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Ma, Y.; Wang, H.; Luo, J.; Zhang, X.; Luo, C.; Wang, H.; Zhao, H.; Pan, D.; et al. A national school-based health lifestyles interventions among Chinese children and adolescents against obesity: Rationale, design and methodology of a randomized controlled trial in China. BMC Public Health 2015, 15, 210. [Google Scholar] [CrossRef]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef]
- Association for Maternal Child Health Study; Expert Committee on Obesity Controlling for Women Children; Expert Committee on Definition of Metabolically Healthy Obesity; Screening Metabolically Unhealthy Obesity in Chinese Children. The expert consensus on definition of metabolically healthy obesity and screening metabolically healthy obesity in Chinese children. Matern. Child Health China 2019, 30, 1487–1490. [Google Scholar]
- National Health Commission of the People’s Republic of China. Screening for Overweight and Obesity among School-Age Children and Adolescents; National Health Commission of the People’s Republic of China: Beijing, China, 2018.
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Child Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Salvo, D.; Ranjit, N.; Nielsen, A.; Akhavan, N.; van den Berg, A. Characterizing Micro-scale Disparities in Childhood Obesity: Examining the Influence of Multilevel Factors on 4-Year Changes in BMI, Healthy Eating, and Physical Activity, Among a Cohort of Children Residing in Disadvantaged Urban Enclaves. Front. Public Health 2019, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Essman, M.; Popkin, B.M.; Corvalán, C.; Reyes, M.; Taillie, L.S. Sugar-Sweetened Beverage Intake among Chilean Preschoolers and Adolescents in 2016: A Cross-Sectional Analysis. Nutrients 2018, 10, 1767. [Google Scholar] [CrossRef]
- Noor, S.; Dehghan, M.; Lear, S.A.; Swaminathan, S.; Ibrahim, Q.; Rangarajan, S.; Punthakee, Z. Relationship between diet and acculturation among South Asian children living in Canada. Appetite 2020, 147, 104524. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society. Dietary Guidelines for School-Age Children; People ’s Medical Publishing House: Beijing, China, 2016. [Google Scholar]
- Fan, M.; Lyu, J.; He, P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 961–964. [Google Scholar]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Martin, R.M.; Kramer, M.S.; Patel, R.; Rifas-Shiman, S.L.; Thompson, J.; Yang, S.; Vilchuck, K.; Bogdanovich, N.; Hameza, M.; Tilling, K.; et al. Effects of Promoting Long-term, Exclusive Breastfeeding on Adolescent Adiposity, Blood Pressure, and Growth Trajectories: A Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatrics 2017, 171, e170698. [Google Scholar] [CrossRef]
- O’Tierney, P.F.; Barker, D.J.; Osmond, C.; Kajantie, E.; Eriksson, J.G. Duration of breast-feeding and adiposity in adult life. J. Nutr. 2009, 139, 422S–425S. [Google Scholar] [CrossRef]
- Phillips, D.I.; Barker, D.J.; Osmond, C. Infant feeding, fetal growth and adult thyroid function. Acta Endocrinol. 1993, 129, 134–138. [Google Scholar] [CrossRef][Green Version]
- Heimberg, M.; Olubadewo, J.O.; Wilcox, H.G. Plasma lipoproteins and regulation of hepatic metabolism of fatty acids in altered thyroid states. Endocr. Rev. 1985, 6, 590–607. [Google Scholar] [CrossRef]
- Fall, C.H.; Barker, D.J.; Osmond, C.; Winter, P.D.; Clark, P.M.; Hales, C.N. Relation of infant feeding to adult serum cholesterol concentration and death from ischaemic heart disease. BMJ 1992, 304, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; van Goudoever, J. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, D.; Chen, L.; Ma, T.; Ma, Y.; Chen, M.; Dong, B.; Dong, Y.; Ma, J.; Arnold, L. The Association between Breastfeeding Duration and Lipid Profile among Children and Adolescents. Nutrients 2021, 13, 2728. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; The European Childhood Obesity Trial Study Group; Luque, V.; Ferré, N.; Mendez-Riera, G.; Koletzko, B.; Grote, V.; Demmelmair, H.; Bluck, L.; Wright, A.; et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int. J. Obes. 2012, 36, 548–553. [Google Scholar] [CrossRef]
- Pearce, J.; Taylor, M.A.; Langley-Evans, S.C. Timing of the introduction of complementary feeding and risk of childhood obesity: A systematic review. Int. J. Obes. 2013, 37, 1295–1306. [Google Scholar] [CrossRef]
- Sobti, J.; Mathur, G.P.; Gupta, A. WHO’s proposed global strategy for infant and young child feeding: A viewpoint. J. Indian Med. Assoc. 2002, 100, 502–504. [Google Scholar]
- Forsyth, J.S. Policy and pragmatism in breast feeding. Arch. Dis. Child. 2011, 96, 909–910. [Google Scholar] [CrossRef]
- Fewtrell, M.; Wilson, D.C.; Booth, I.; Lucas, A. Six months of exclusive breast feeding: How good is the evidence? BMJ 2010, 342, c5955. [Google Scholar] [CrossRef][Green Version]
- Nieuwoudt, S.; Daniels, L.; Sayed, N. Food and Nutrition Security of Infants and Young Children: Breastfeeding and Complementary Feeding; Children’s Institute, University of Cape Town: Cape Town, South Africa, 2021. [Google Scholar]
- OECD Directorate for Employment LaSASPD. OECD Family Database. CO1.5 Breastfeeding Rates. 2009. Available online: www.oecd.org/dataoecd/30/56/43136964.pdf (accessed on 13 March 2022).
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- ESPGHAN Committee on Nutrition; Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; et al. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef]
- Grummer-Strawn, L.M.; Rollins, N. Summarising the health effects of breastfeeding. Acta Paediatr. 2015, 104, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, H.; Cesar, V.; World Health Organization. Long-Term Effects of Breastfeeding: A Systematic Review; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).