Abstract
Coronavirus illness (COVID-19) is an infectious pathology generated by intense severe respiratory syndrome coronavirus 2 (SARS-CoV-2). This infectious disease has emerged in 2019. The COVID-19-associated pandemic has considerably affected the way of life and the economy in the world. It is consequently crucial to find solutions allowing remedying or alleviating the effects of this infectious disease. Natural products have been in perpetual application from immemorial time given that they are attested to be efficient towards several illnesses without major side effects. Various studies have shown that plant extracts or purified molecules have a promising inhibiting impact towards coronavirus. In addition, it is substantial to understand the characteristics, susceptibility and impact of diet on patients infected with COVID-19. In this review, we recapitulate the influence of extracts or pure molecules from medicinal plants on COVID-19. We approach the possibilities of plant treatment/co-treatment and feeding applied to COVID-19. We also show coronavirus susceptibility and complications associated with nutrient deficiencies and then discuss the major food groups efficient on COVID-19 pathogenesis. Then, we covered emerging technologies using plant-based SARS-CoV-2 vaccine. We conclude by giving nutrient and plants curative therapy recommendations which are of potential interest in the COVID-19 infection and could pave the way for pharmacological treatments or co-treatments of COVID-19.
1. Introduction
In December 2019, novel evolved coronavirus called COVID-19 appeared for the first time [1]. This local epidemic has converted to a global pandemic, and more than two years later the repercussions stay unclear and dreadful [2]. At the start of 2022, the COVID-19 pandemic has been accountable for more than 100 million stated contaminations and 5 million deaths with dramatic social consequences [2].
Examination of COVID-19 at the beginning of sickness and through to the progress of the infection differs from asymptomatic to acute pneumonia with severe respiratory distress syndrome, with the most usual symptoms being cough, shortness of breath, diarrhea, fever and tiredness [3,4]. Moreover, contaminated individuals by this RNA virus presented venous thromboembolic incidents linked to endothelial damage and hypercoagulability, immoderate inflammatory status with cytokine storm, immune dysregulation, cholesterol metabolism abnormalities, oxidative stress, hypertension and new onset diabetes [5,6,7,8].
The establishment of the vaccine has been the most substantial procedure to prevent acute COVID-19 and constitutes a key function in monitoring and reducing death. However, SARS-CoV-2 exerts its adaptative capabilities by mutation of its proteins. Thus, the vaccine becomes less efficient since only a unique modification of amino acid is capable to impact the viral replication, transmission or immune control avoidance.
The most recent variant, Omicron, includes more than thirty amino acid mutations in the spike protein and displays a higher transmissibility and evade capacity from vaccines [9].
In this context, it is important to identify natural and synthetic molecules capable of enhancing the host’s natural defenses and/or to counteract viral activity.
Up to now, medicinal and aromatic plants have been exploited in various aboriginal medical strategies along with ancestral medicines for treatment of sicknesses. So, a broad range of natural products can act as a subsidiary manual to freeing the several secrets regarding human pathologies [10]. Indeed, the evaluation of potential antiviral effect of different natural sources has acquired notable attention with the appearance and re-appearance of novel viruses and in view of the advancement of technologic resources that are accessible [11].
Research on new drug discovery from natural products can permit the identification of new lead molecules, which is progressed from a screening hit to a drug candidate through structural elucidation. Plants can lead to powerful molecules against SARS-CoV-2 as they have formerly demonstrated hopeful expectations for various pivotal situations generated by deadly pathogens [12].
Herbal medicines may offer efficacious medicines with various favorable impacts towards COVID-19 and these should be evaluated. Plant therapies may allow us to inactivate the virus, block its reproduction or spread or diminish the symptoms, thus not only decreasing suffering and declining mortality, but also diminishing further spread [13].
A range of natural products have been considered, and their effectiveness towards viral infections such as COVID-19 have been established. Several isolated natural molecules and different polar or apolar plant extracts such as Artemisia annua, Agastache rugosa, Astragalus membranaceus, Cassia alata, Ecklonia cava, Gymnema sylvestre, Glycyrrhizae uralensis, Houttuynia cordata, Lindera aggregata, Lycoris radiata, Mollugo cerviana, Polygonum multiflorum, Pyrrosia lingua, Saposhnikoviae divaricate and Tinospora cordifolia have demonstrated auspicious inhibiting impact towards coronavirus [4].
Meanwhile, important quantities of antiviral substances yielded from different plant species have been explored in several studies. Scientists around the world are testing therapeutic medication from available bioactive substances and they try to discover innovative molecules from herbal medicines towards COVID-19 [12].
In parallel, several works showed that nutritional status represents a crucial function in the immune system, supporting both innate and adaptive immunity and has a role in the control of inflammation [9]. Hence, human alimentation represents an elementary function in defending against contagious diseases. Persons with nutrient deficits have diminished blood cell generation which caused distressed defense cells and augmented hazard of contaminations. Diet assistance and food quality is tremendously critical to battle against and avoid contaminations, particularly in people infected by the COVID-19 [2]. So, some investigations indicate that the outcome of COVID-19 patients is linked with their nutritional status [9]. Indeed, natural molecules such as omega 3 and 9 fatty acids and tocopherols in large quantities in the Mediterranean diet have the ability to reduce 7-ketocholesterol (7KC) toxic effects [14,15,16] and could therefore greatly reduce the complications due to COVID-19 infection associated with increased levels of 7KC in the plasma of infected patients [5,6]. It has also been reported in some countries, that hospitalized or gravely sick young individuals are at a higher risk of malnutrition, and fast valuation and care of poor nutritious health can influence medical consequences [17]. As it links to the COVID-19 pandemic, an expected 5% of these ill persons need admittance to an intensive care unit. Dietary therapy should be among the essential elements of curative regimens [3]. Thus, discrepancies in dietetic lifestyles have been assumed as acting an important role in COVID-19 geographical and mortality rate variance [7].
It is also very important to point out that at the end of acute phase of SARS-CoV-2 pandemic (long COVID), a sequence of perpetual symptoms, which last more than 12 weeks from the commencement of the infection, can occur. Cognitive dysfunction and fatigue are principal symptoms joined by sleep perturbation, deficit of concentration, depression and pain. Changes in taste and smell, headache, dizziness, coordination troubles, memory loss, anxiety and insomnia are also discovered. Indeed, long COVID also affects several healthy young people who have not been hospitalized [18,19].
Therefore, during the COVID-19 pandemic, the secure uptake of natural products such as plants, micro- and/or macronutrients can be beneficial not only to reduce the infection but also to support the immune response during COVID-19, together with their effect on long COVID [9].
Nutrition may play a substantial function in influencing the susceptibility of long COVID. Nutrients, such as vitamins (B1, B6, B9, B12, C, D and E), fatty acids, minerals (iron, zinc) and oligoelements (selenium), are recognized to perform a significant role in protecting towards neuroinflammation and oxidative stress. They have a very positive impact on cognitive functions [20].
Additionally, secondary plant metabolites such as flavonoids inhibit neuroinflammation and reduce cognitive decline. Especially, luteolin (phenolic compound) is capable of crossing the blood–brain barrier and reducing both microglial and mast cell inflammation [9].
An extraordinary number of research studies have been launched to investigate preventative options and possible anti-COVID-19 therapies using natural products either from medicinal plants or different types of foods [21].
Accordingly, this review aims to understand the prospective functions of herbal medicine and the nutrients of various food groups with their antiviral, antioxidant and anti-inflammatory activities that can contribute to enhance immunity towards viral infections attributable to SARS-CoV-2. This can help medical doctors and allows citizens to make decisions based on natural medicine and suitable dietary choices in pandemic along with post-pandemic situations.
2. Mechanism of SARS-CoV-2 Infection
With the aim of drawing up a therapeutical approach, it is necessary to understand the impact of COVID-19 on host targets. Several studies have been carried out to determine this effect [22,23]. In this review, it will help us to understand the mechanism of action of some natural products with regard to the infection of this virus. Most researches have adequately studied the mechanisms of COVID-19 entering host cells, and in specific the linkage of the spike (S) protein to its receptor, angiotensin-converting enzyme 2 (ACE2) and succeeding membrane fusion. The multistep SARS-CoV-2 entry process includes S protein synthesis and S protein structure conformational transitions necessary for association of the S protein with ACE2, engagement of the receptor-binding domain of the S protein with ACE2 receptor, proteolytic activation of the S protein by transmembrane protein serine 2 (TMPRRSS2), endocytosis and membrane fusion [24].
The spike (S) protein is made of two subunits (S1 and S2) and the partitioning and activation of the S protein are controlled by the intracellular TMPRSS2, also named furin to engender unlocked, fusion-catalyzed forms on the cell surface. This facilitates the earliest entrance of the virus. Cellular receptor ACE2 is used to enter the target cells. Notably, the heptad repeat 1 (HR1) and heptad repeat 2 (HR2) at the S2 subunit perform a few prominent functions in fusion regulating among the virus and host cell membrane. The HR1 and HR2 react to constitute a six-helix bundle, which allows the fusion of the two membranes. The organs which highly express ACE2 (cholangiocytes, small intestine and duodenum, urinary organs, testis, pancreas and heart) are vulnerable to SARS-CoV-2 infection [25] (Figure 1).
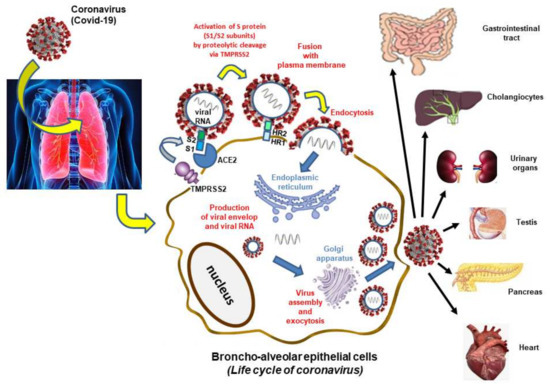
Figure 1.
Mechanism of infection by SARS-CoV-2.
The SARS-CoV-2 targets a cellular receptor in the nasal cavity, where it would multiply before disseminating in particular in the lungs. The main stages of human cell infection and virus replication take place at the plasma membrane level, in the cytoplasm (endoplasmic reticulum, Golgi apparatus) and are successively as follows: (1) Activation of the viral protein spike by furin and attachment to the ACE2 receptor by the S1 subunit which recognizes and binds to this cellular receptor. Beyond attaching the virus to the cell, the role of the spike protein is to induce fusion between the viral envelope and a cell membrane. It is a heptad repeat 1 (HR1) and heptad repeat 2 (HR2) that constitute a six-helix bundle, permitting the two membranes to fuse. This step, which corresponds to endocytosis, requires the spike protein to be cut again by a protease called TMPRSS2 (transmembrane serine protease 2). (2) Synthesis of viral messenger RNA and duplication of viral genomic RNA: when the virus has entered the cell, it releases its genomic RNA. The virus RNA polymerase synthesizes messenger RNA and copies of genomic RNA that will be used to form new virus particles. (3) Multiplication of viral particles by exploiting the cellular machinery: the virus messenger RNA uses the cellular machinery to synthesize the viral polyproteins it encodes. Once the polyprotein has been synthesized, a viral protease cuts it and allows the formation of functional viral particles. (4) The viral nucleocapsid is gathered from genomic RNA and N proteins in the cytoplasm, and thereafter bud into the lumen of endoplasmic reticulum (ER)-Golgi intermediate cavity. Virus particles are then liberated from infected cells by exocytosis. The receptor is present not only on the cells of the nose and the lungs but also in the digestive system, the heart and to a lesser extent in the kidneys and the liver. SARS-Cov-2 can therefore infect all these organs.
Both ACE2 and TMPRSS2 mRNA have been reported in different tissues. The penetration of the virus into the cells and fixation on the ACE2 receptor can generate damage including thrombosis and hypoxia immune response against the virus and cytokine storm [26].
Other receptors can manage SARS-CoV-2 infection such as dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN; genotypes CD209), liver/lymph node-specific intercellular adhesion molecule-3-grabbing integrin (L-SIGN; CLEC4M) and CD147 which explicate its elevated infectiveness [27].
TMPRSS2 inhibitors could constitute an interesting target for the prevention or treatment of COVID-19 infection [25,26]. Furthermore, different proteins or genes of SARS-Cov-2, such as the main protease, have been targeted to screen medicines; however, no small molecules have been conclusively shown to be able to treat COVID-19 patients [28]. Thus, de Oliveira et al. [29] in their in silico study indicated potential challengers from herbal compounds to inhibit the SARS-CoV-2 main protease (Mpro). They discovered that non-polar and polar groups with the occurrence of hydrogen bond acceptors have a substantial function in the herbal compounds–Mpro interactions.
3. Medicinal Plants and Their Metabolites Used in Case of COVID-19
3.1. Medicinal Plants and Their Extracts
Around 35% of the worldwide medicine market has been exchanged by medicinal products formulated by natural herbs. Hence, plants could be an additional source of molecules in the therapy towards COVID-19 [12].
Supportive therapies are used to regulate additional difficulties and organ damage generated by COVD-19. Moreover, the implementation of both modern and traditional therapies might diminish the severity of the disease and symptoms, death rate and side effects [11].
The specified mechanisms of contemporary medications under examination for COVID-19 therapy comprise the inhibition of fusion of SARS-CoV-2 with human cells, a decline in endosomal acidity, cell-membrane-generated vesicles for transport of the virus inside the host cell where the virus can replicate and obstruction of the formation of pro-inflammatory cytokines [30]. Moreover, major domains of novel medication targets are RNA-dependent RNA polymerase of the virus, cell membrane receptors, and spike proteins [4].
Plant bioactive substances can also inhibit (i) the enzymes implicated in the replication cycle of CoVs such as papain-like protease and 3CL protease, (ii) the fusion of the S protein of coronaviruses and ACE2 of the host and (iii) the associated cellular signaling pathways [12].
Natural products can probably stimulate either one or a combination of the cited impacts and the antiviral process of plant extracts depends on the structure and replication mechanism of the viruses. On the other hand, some herbs aid the enhancement of the natural antiviral immunity of the organism. In line with this, medicinal herbs can be a substitute for synthetic drugs especially since plants are the elementary origin of medical care for almost 85% of the world inhabitants and more than 40% of medications found in pharmacies originate from vegetables and microbial-based natural products [4].
Plants and natural products were exploited by populations to avoid COVID-19 since they are accessible as a first line of protection with a view to stimulate immunity and hence make the body more resistive to contaminations. They can also intervene in hygiene measures by purifying the air. A range of bioactive components are found in these herbs and many herbs are recognized for their antioxidant, anti-inflammatory and even antiviral effects [31].
COVID-19 symptoms create inflammation and hemotoxicity, which means that blood-purifying plants with anti-inflammatory, antioxidant and antiviral activities could be perceived as potential cures for COVID-19 infections [32].
Consequently, numerous research studies focused on the screening and characterization of prospective anti-inflammatory, antioxidant and anti-antiviral medicinal plants in the pandemic context. So, in this section, we will focus on plants that have these specific properties.
There are several surveys identifying the employment of diverse medicinal plants in ethnomedicine during the COVID-19 pandemic across various regions worldwide, particularly in Asian countries (China, India, Japan and Pakistan) and Africa (Algeria, Cameroon, Ethiopia, Morocco, Nigeria). However, we will only focus on those that present important results and evidence.
In fact, many researchers have pointed out the effects of the chosen medicinal plants on this virus. Most of the studies have been focused on Chinese plants, and it has been shown that traditional Chinese medicines (TCMs) decreased certain symptoms of COVID-19, such as fevers and reduced the viral load considerably [33].
For instance, Glycyrrhiza glabra (liccorice) was considered potential therapy for COVID-19 because of its prior effectiveness towards the SARS epidemic of 2003. In addition, different Chinese investigations examined chosen TCMs towards COVID-19 and stated that some of them, especially G. glabra, diminished the symptoms of this pandemic disease, notably fevers and reduced the viral load significantly [13].
Terminalia ferdinandiana Exell (commonly known as Kakadu plum) has good potential in decreasing the symptoms of COVID-19, thereby saving lives. The extract of this plant possesses exceedingly elevated antioxidant amounts such as vitamin C (~900 times (g/g) the ascorbic acid amount of blueberries) which boosts the immune system. High ascorbic acid levels enhance the immune system, thereby reducing the likeliness of infection by SARS-CoV-2 [13].
Noticeably, many research studies have demonstrated that Kakadu plum extracts inhibit the release of pro-inflammatory cytokines and promote the liberation of anti-inflammatory cytokines [13].
The Malagasy Institute for Applied Research formulated an herbal tea based on Artemisia annua, alleging prophylactic and therapeutic effects towards COVID-19 [34]. The effect of the cited herb towards SARS is recorded and has been approved in COVID-19 therapeutic practices [35].
Sambucus javanica subsp. chinensis (Lindl.) Fukuoka (Chinese elder) extract also exercised auspicious anti-human coronavirus properties. This extract significantly reduced virus yield, plaque generation and virus linking [36]. Moreover, there is preclinical indication that Sambucus nigra L. (elderberry) prevents the replication and viral linking of the human coronavirus NL63. This plant is the most efficient in preventing or fighting coronavirus infections at the early stages [37].
Rhodiola rosea L. (Golden root) has great immunoregulatory action and attenuates inflammatory harm since it regulates the differentiation of immune cells, activates inflammatory signaling pathways and secretes inflammatory factors [38]. Andrographis paniculata (Bitter weed) inhibits the augmented NOD-like receptor protein 3 (NLRP3), caspase-1 and interleukin-1β (IL-1β) particles which are strongly implicated in SARS-CoV [39].
Among the other reported species, the decoction of Qingfei Paidu, Gancaoganjiang, Sheganmahuang and of Qingfei Touxie Fuzheng exhibited promising effects [33].
Professional persons from the Zhongnan Hospital of Wuhan University comprised the usage of conventional remedies in the guidance for the treatment and prevention of COVID-19. Furthermore, to cure the infection, the specialists advise the utilization of several plant combinations depending on the pathology phase [40]. Six species are used in the TCM Yupingfeng powder, which are as follows: Astragalus mongholicus Bunge, Glycyrrhiza glabra L., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Atractylodes lancea (Thunb.) DC., Atractylodes macrocephala Koidz., Lonicera japonica Thunb. and Forsythia suspensa (Thunb.) Vahl. [41].
Likewise, numerous TCM prescriptions were formulated including the lung cleansing and detoxifying decoction (a mix of 21 plants and natural products) which is clinically assessed. This formula was efficient in 90% of the 214 enrolled COVID-19 patients and the treatment of 1262 patients, including 57 with severe symptoms, revealing that 99.28% of the patients were cured and none with mild symptoms showed severe symptoms. So, this preparation has been extensively adopted in 28 regions and has aided to the comparatively bass death proportion in between COVID-19 patients in China [42].
In India, Triyaq-e-Araba is also a formulation employed as a detoxifying and a potent antiviral agent. It includes Laurus nobilis L. berries, Bergenia ciliate (Haw.) Sternb. stem, Aristolochia indica L. roots and Commiphora myrrha (Nees) Engl [11].
In Ayurveda (an oriental holistic and preventive medicine), Tinospora cordifolia (Guduchi) and Emblica officinalis (Indian gooseberry) have immunity-boosting properties. While Phyllanthus spp., Andrographis paniculata (Creat), Glycyrrhiza glabra (Licorice), Withania somnifera (Ashwagandha) and Curcuma longa (Turmeric) have antiviral effects [43]. In metaanalysis research and in line with docking studies, it was revealed that C. longa can be employed as a preventive towards COVID-19 [32].
Tinospora cordifolia (Giloe), Ocimum tenuiflorum (Tulsi), Emblica officinalis (Indian gooseberry) and Linum usitatissimum (Linn.) (Flax seeds) have been traditionally employed as herbal remediations for various ailments since the early ages in India. They have demonstrated strong immunomodulatory, antioxidant and anti-infective activities [11].
Aqueous extract of Withania somnifera (Ashwagandha) inhibits SARS-CoV-2 from entering to cells by preventing liaisons between the viral S-protein receptor connecting domain and host ACE2 receptor [44].
AYUSH systems of medicine propagate general preventive measures aimed at preventing the spread of infection such as sustained general health by dietary modifications and herbal drugs. So, numerous formulations were developed including AYUSH kwath which is a mixture of herbs that boost immunity and are active remedies to various viral diseases, it includes Ocimum sanctum L. (Holy basil) leaves, Piper nigrum L. fruits (Black pepper), Cinnamomum verum J. Presl. stem barks (Cinnamon) and Zingiber officinale Roscoe rhizomes (Ginger) [11].
C. verum, which displays low toxicity, and rhizome of Z. officinale are employed to relieve fever and other COVID-19 symptoms in Africa [45,46]. It is worth pointing out that several other studies have demonstrated the antiviral activity of other plants which can be used against COVID-19 including Rosmarinus officinalis Linn. (Rosemary) in Africa [45,46].
Interestingly, in Morocco, based on an ethnobotanic study, 67.04% of interviewees employed medicinal plants to boost their immunity, disinfect the air or treat respiratory tract infections that may be linked to coronavirus. The most mentioned species that strengthen the immunity system were Olea europaea L. (Common olive), Vitis vinifera L. (Common grape) and Allium sativum L. (Garlic) [31]. This last one can be considered a promising plant that can act against COVID-19 since it exposed an inhibitory activity on viral replication [47]. Its extract prevents influenza A virus by inhibiting the synthesis of viral nucleoproteins and polymerase activity [48]. However, Eucalyptus globulus (Blue gum), Trigonella foenumgraecum (Fenugreek) and Aloysia triphylla (Lemon Verbena) were the species employed to alleviate some respiratory infection symptoms [31]. Additionally, Flouchi and Fikeri-Benbrahim [49] showed the efficiency of Cinchona officinalis Linn. (Quinquina), and Thymus vulgaris (Thyme) in diminishing and avoiding the hazard of contamination and in curing some COVID-19 symptoms.
Among the group of plant prevention agents during the coronavirus pandemic, we can also cite Nigella sativa L.’s (Black cumin) bioactive constituents that have been noticed as promising inhibitors of COVID-19 in molecular docking work [50].
3.2. Major Plant Metabolites
According to Bhuiyan et al. [12], plant metabolites represent a potential anti-SARS-CoV-2 effect; therefore, it is interesting to study molecules for the increased optimization and generation of medical procedures to fight COVID-19 and also subsequent pandemics that are generated by viruses. These authors suggested that about 76 natural metabolites from plant species can be successfully active towards COVID-19.
3.2.1. Polyphenols
Polyphenols (or phytophenols) are ubiquitous compounds produced by all plant species [51]. Besides their antioxidant properties, they have various physiological aspects: cell signaling, interactions with the environment (color, flavor), metal chelation, and defense against aggression either biotic (virus, bacteria, fungi infection) or abiotic (radiation, ozone, dryness) or sensor for pesticides. Several studies also support that they can stimulate immune response in cancer [52].
In animals, including humans, polyphenols have strong antioxidant effects on metabolic homeostasis. They prevent lipid, protein and nucleic acid oxidation. They also modulate the immune system by decreasing low grade inflammation. Polyphenols are good antiseptics and prevent some viral infection such as influenza virus, human immunodeficiency virus (HIV) and also SARS-CoV-2 [53].
A large variety of antiviral substances were revealed in 219 medicinal plants from 83 plant families, pre-eminent are polyphenols. Some polyphenol compounds (30-(3-methylbut-2-enyl)-30, 4-hydroxyisolonchocarpin, broussochalcone A, 4,7-trihydroxyflavane, broussochalcone B, papyriflavonol A, kazinol A, kazinol B, kazinol F, kazinol J and broussoflavan A isolated from Broussonetia papyrifera demonstrated auspicious effects towards SARS-CoV. Particularly, papyriflavonol A recorded an excellent effect against SARS-CoV (IC50 3.7, l M) [12].
Therapy with some flavonoids (hesperidin and quercetin), administered with indomethacin as a non-steroidal anti-inflammatory medicine with antiviral activities and with a low dose of aspirin as an anti-aggregating property, was suggested to be successful after 3 days from the initiation of the symptoms of SARS-CoV-2. This treatment displayed a decrease in the severances of COVID-19 and a decrease in the percentage of hospitalizations [9].
Furthermore, SARS-COV-2 contamination triggers an immune response and an oxidative response, especially to the lungs. Liu et al. [54] have shown that interleukin (IL)-6 is one of the leading factors causing death in COVID-19 patients through cytokine release. Recently, Pincemail et al. [55] reported an oxidative stress increase in COVID-19 patients hospitalized in an intensive care unit for severe pneumonia. Trujillo-Mayol et al. [56] recalled the importance of antioxidant and vitamin D intakes during the SARS-CoV-2/COVID-19 pandemic. Therefore, vulnerable populations such as the elderly and obese individuals should benefit from antioxidants and vitamins especially through the Mediterranean diet to improve their antioxidant response. Although evidence remains scarce, there is some indication that a healthy diet, along with supplemental antioxidant intake, is beneficial to COVID-19 patients.
Since the start of the COVID-19 pandemic, some hypothesis merged about a possible preventative effect of polyphenols towards SARS-COV-2 effects. Moreover, many reviews and present papers demonstrate some interesting data. So far, the prevailing hypothesis is the following: SARS-CoV-2 replication can be inhibited by polyphenols which are derived from edible plants [28].
The in vitro effects of polyphenols on COVID-19 were demonstrated by their binding on the main protease involved in the virus replication and enzyme inhibition. The authors have screened a series of plant flavan-3-ols and of pro-anthocyanidins for instance: (+)-catechin (CA), (-)-epigallocatechin (EGC), (+)-gallocatechin (GC), (-)-epiafzelechin (EAF), (+)-afzelechin (AF), (-)-epicatechin-3-O-gallate (ECG), (+)-catechin-3-O-gallate (CAG), (-)-gallatechin-3-O-gallate (GCG) and (-)-epigallocatechin-3-O-gallate (EGCG). On the other hand, they tested plant extracts rich in such polyphenols. Flavan-3-ols are present in fruits, drinks (grape extracts), parsley, strawberries, blackcurrants, blackberries, cassis, cocoa, chocolate and green tea.
The dimers of pro-anthocyanidins A1, A2, B1 and B2 are powerful antioxidants with the following properties: antiviral, antibacterial, anticancer, anti-vascular alterations and anti-aging activities.
Two approaches have been undertaken: (1) Modeling (docking) of these molecules to protease Mpro and (2) measurement of the protease Mpro activity. According to Figure 1, the main protease is involved in maturation of viral protein precursors synthesized by cell protein synthesis machinery oriented for the benefits to viral multiplication.
Six compounds bind in three of the four sites S1, S’1, S2, S4 located in the binding pocket of the peptide N3 inhibitor of Mpro. The highest affinity scores are for EAF, AF and CA, and the weakest for PA2 and PB2.
IC50 inhibition averages of measured protease activities are: 3 μM (CAG), 5 μM (ECG), 6 μM (GCG), 7 μM (EGCG) and 75 μM PB2. The other compounds do not show any inhibitory activities.
Furthermore, assays have been performed on extracts rich in CAG, ECG, GAG, EGCG and PB2 where results of IC50 are the following: green tea (3 µg/mL), grape muscadine (30 µg/mL), cocoa (153 µg/mL) and dark chocolate (256 µg/mL).
In conclusion, (1) the inhibitors’ effects are closely linked to affinity on the protease and (2) galloylation and these natural compounds are not immunizing drugs but can be preventive nutrients such cocoa, grape and green tea. These results have recently been confirmed by Bahun et al. [57].
In silico study shows that polyphenols can inhibit SARS-CoV-2 Mpro and RdRp efficiently and flavonoids exhibited powerful antiviral effects towards SARS-CoV. For example, apigenin and quercetin displayed effects towards SARS-CoV by inhibiting Mpro enzymes with an IC50 of 38.4 μM and 23.8 μM, respectively [12].
In addition, resveratrol (a polyphenol of the stilbene chemical family), under its trans-configuration, is a peculiar promising polyphenol for COVID-19 infection prevention. Indeed, resveratrol is produced in huge amounts by grape plants in response to fungi infection and accumulates in grape berries, especially in the skin. Resveratrol is present in red wine in significant amounts [58,59]. Resveratrol is a strong antioxidant [60] and shows natural anti-inflammatory properties. Its preventing properties have been verified towards the COVID-19 infection. For instance, resveratrol [61] and its analogue hopeaphenol [62], Polygonum cuspidatum extracts [63] and pterostilbene [64] inhibit SARS-CoV-2 replication. It has been proposed [65] that resveratrol may switch off interference between a link between COVID-19 and obesity via the inhibition of HIF-1α to HIF-2α (Hypoxia-inducible factor).
McLachlan [66] and Perrella et al. [67] have studied the activation of ACE2 receptor by resveratrol and other natural compounds. In an empirical investigation, it has proposed that an association of resveratrol and copper at 5.6 mg and 560 ng, respectively, orally once every 6 h has substantially reduced the death rate of very sick COVID-19 patients [68]. Kelleni [69] has proposed nanocarriers, resveratrol/pterostilbene-zinc nanoparticle administration to COVID-19 patients as adjuvant therapy. The anti-inflammatory and immunomodulatory activities of the associations were well known and can be considered as a main pharmacokinetic benefit for COVID-19.
3.2.2. Terpenoids
Terpenoids or isoprenoids constitute the major class of secondary metabolites only containing carbon, hydrogen and oxygen atoms [70,71]. Several terpenoids act as defensive constituents towards microorganisms. So, multitude terpenoids have marked pharmacological effects and are intriguing for medicine and biotechnology. Around 36,000 specific structures of this class have been mentioned [72].
In humans, terpenes are known to have anti-inflammatory, analgesic, antiviral and antibacterial activities. Regarding antiviral properties, studies have shown potential efficacy against human immunodeficiency virus 1, bronchitis virus, herpes simplex virus and West Nile virus [73,74,75,76].
Ten diterpenes, two sesquiterpenes and two triterpenes demonstrated an anti-SARS effect with IC50 of 3–10 μM. In in silico work, it has been shown that terpene Ginkgolide A can strongly inhibit the SARS CoV-2 protease enzyme [12]. In addition, Glycyrrhizin, a triterpene found in licorice roots, could inhibit SARS-CoV replication in vitro [77] and its efficacy was also obtained in patients [78]. Squalene, a natural triterpene which is a precursor of sterols and other bioactive terpenoids, was extracted from pumpkin seed oil. Then, it was assessed in the form of a nanostructure in a clinical study to treat COVID-19. It showed marked properties by reducing fever and cough during the treatment period and the treated patients did not need oxygen therapy [79].
Another study showed that Laurus nobilis L. essential oil, containing β-ocimene, 1,8-cineole, α-pinene and β-pinene, has antiviral activity against SARS-CoV [80]. An NT-VRL-1 formulation, containing about 30 terpenes with β-caryophyllene, eucalyptol and citral as main constituents, was tested on human lung fibroblast cells MRC-5 [81]. This formulation has antiviral effects which are amplified when used with cannabidiol. These effects would be most effective when the formulation is used prior to exposure to the virus, the compounds would affect the attachment and entry of the virus into cells [81]. An in silico study by Ibrahim et al. [82] focused on a protease involved in viral replication, blocking the activity of this enzyme would block viral replication and thus SARS-CoV-2. They screened in silico via their binding affinity to the protease involved in SARS-CoV-2 replication, biologically active terpene metabolites identified from a coral reef community unique to the Red Sea by comparing them to lopinavir (a known inhibitor of this protease, proposed as a treatment for COVID-19). Through molecular docking calculations, molecular dynamics (MD) simulations and molecular mechanics/generalized born surface area binding energy calculations, they identified a candidate molecule—erylosides B (226) [82]. A review written by Meeran et al. [83] evaluates the hypothesis that limonene, a cyclic monoterpene that constitutes about 98% of the essential oils in the peel, leaf and flower of many citrus fruits, including oranges, mandarins, lemons, pummelos, grapefruits and limes, could be used in the fight against COVID-19 due to its immunomodulatory, anti-inflammatory and antiviral properties. An interesting diagram summarizes the proposed possible effects of limonene and its possible involvement in SARS-CoV-2 infection.
In the following section, we will pay more attention to carotenoids and phytosterols.
Carotenoids
Carotenoids are the most abundant plant pigments responsible for the red, orange, yellow and green colors of fruits, vegetables, flowers and algae, with 700 different fat-soluble compounds identified. They can be easily extracted in ether petroleum. They are not synthesized by humans, and there are only 50 compounds in food that can be absorbed and metabolized [84]. Some carotenoids are in the majority, such as β-carotene, β-cryptoxanthin, α-carotene, lycopene, lutein and zeaxanthin, they account for 95% of the carotenoids present in the blood. This family is divided into two groups: xanthophylls (containing oxygen, hydroxyl, epoxy, carboxyl group for example): lutein, zeaxanthin and β-cryptoxanthin; and carotenes (hydrocarbons not containing oxygen): β-carotene, lycopene and α-carotene. Carotenoids are described as having antioxidant properties [85] and as being able to interact with certain cellular signaling pathways involved in the suppression of oxidative stress and inflammatory processes [86]. The review by Khalil et al. presents the chemical composition, classification, natural sources and biopharmaceutical activities of a number of carotenoids and may provide useful information for their use [87]. One team studied the antiviral activity of two marine carotenoids against SARS-CoV-2 virus entry: fucoxanthin, a polar, orange xanthophyll, found in several brown algae, and siphona xanthin, a polar xanthophyll, found in green algae and an oxidative metabolite of lutein [88]. Molecular docking studies showed that siphona xanthin could bind to the ACE2 binding region of the SARS-CoV-2 spike protein. These data were confirmed by in vitro experiments on HEK/ACE2 cells infected with a SARS-CoV-2 pseudovirus. In the review by Khalil et al. [87], the authors analyzed the existing bibliography and gathered all the existing data concerning the impact of carotenoids (β-carotene, α-carotene, β-cryptocanthin, lutein/zeaxanthin, lycopene, astaxanthin, crocins and crocetin (present in high amount in saffron) [89], phytoene and phytofluene and fucoxanthin) on inflammation, immunity and immune function, as well as on their antiviral activity. As a result of this analysis, the authors suggest that carotenoids could be used as potential drugs (with the necessary restrictions associated with certain supplementations) to combat the inflammatory storm resulting from COVID-19 infection, but also to boost the immune response (effects on several anti-inflammatory and antioxidant pathways) [87]. The authors targeted an interesting candidate for future development: astaxanthin, because of its innumerable properties and antioxidant activities against several symptoms of viral infections, such as inflammation and its acceptable safety profile. It is closely followed by lycopene, crocin and crocetin.
Phytosterols
Currently, several studies have shown that cholesterol oxidation products, called oxysterols, may have antiviral activities [90] and may be involved in the COVID-19 infection which can lead to a severe acute respiratory syndrome (SARS-CoV-2) that can be fatal especially in the elderly and obese subjects. This is the case for 27-hydroxycholesterol (27-OHC) and 7KC, for which a decrease and an increase in plasma levels are, respectively, observed in elderly subjects with mild and severe forms of the COVID-19 infection [5,6]. Meanwhile, Foo et al. [91] demonstrated in their review study that oxysterols including 7KC can be deleterious in acute viral infections. A diseased function of 7KC has been advocated with various viral pathogens because of its recognized cytotoxic activities at elevated amounts. 7KC stimulates a pro-inflammatory phenotype in macrophages and can with other oxysterols participate in immoderate inflammation. Furthermore, antiviral activities against COVID-19 have been described with synthetic oxysterols [92]. Withaferin A (WFA), a steroidal lactone with anti-inflammatory and anti-tumorigenic properties, also binds to the SARS-CoV-2 viral spike protein [93]. In addition, among 117 steroidal plant-derived pregnanes (PDPs) which were docked in the active regions of human glucocorticoid receptors (hGRs) in a comparative molecular docking analysis, 20 were selected and analyzed for their possible interactions with the human Janus kinases 1 and interleukins-6 and SARS-CoV-2 3-chymotrypsin-like protease which is a papain-like protease and RNA-dependent RNA polymerase [94]. The most efficient PDPs were bregenin, hirundigenin, anhydroholantogenin, atratogenin A, atratogenin B, glaucogenin A, glaucogenin C and glaucogenin D [95]. Altogether, these data suggest that plant sterols called phytosterols (cholesterol derivatives with either an extra methyl or ethyl group on carbon 24 or an extra double bond on the side chain [96,97]) may have an impact on the COVID-19 infection. To counteract this type of infection, traditional medicines using medicinal plants containing phytosterols are therefore a rational solution. Among these traditional medicines, Chinese medicine is an important source of substances potentially rich in phytosterols (in particular β-sitosterol) capable of acting against the viral infection and/or its consequences at the level of the immune, bronchial, epithelial and vascular endothelial cells by intervening in the MAPK, NF-κB and TLR signaling pathways on the basis of in silico analyses [98]. A molecular docking strategy targeting the Mpro protein (3CL protease from coronavirus SARS-CoV-2) involved in viral replication constitutes a reliable strategy to identify drugs with antiviral activities which could be used in infected patients [99]. In this context, phytosterols from an Ayurvedic plant, Boswellia serrata, are believed to have antiviral activities against COVID-19 [100]. From an in silico study, using the admetSAR tool along with the SwissADME and Molinspiration chemoinformatics tools, it was found that castasterone, which is a brassicasterol, could also be efficient against the SARS-CoV-2 Mpro [101]. Furthermore, due to the ability of phytosterols, such as β-sitosterol, to interact with the plasma membrane, including lipid rafts, these molecules could also oppose viral infection by inhibiting the endocytosis that promotes intracellular virus accumulation [102]. Based on the small number of studies carried out either in silico or in vitro, it appears however that phytosterols can have an effect on both the viral infection (virus entry into the cells, viral cycle) and also on the inflammatory process, which makes these molecules significant in the fight against the COVID-19 infection in infected patients.
Regularly studied medicinal plants and their active metabolites which are recommended to be used against the COVID-19 infection are summarized in Table 1.

Table 1.
Medicinal plants efficient against COVID-19 infection.
4. Major Food Groups Efficient in COVID-19 Pathogenesis
Some nutrients such as vitamins A, B6, B12, C, D, E, folates and trace elements, (zinc, iron, copper, selenium, and magnesium) are formerly recognized as immune system enhancers. Deficits in these molecules unfavorably impact the activation of the immune system in infections. Different searches advised the earliest usage of molecules including zinc, selenium and vitamin D, together with other micronutrients, to increase resistance to COVID-19 [9].
Food has evolved into a substantial element in the treatment of persons during COVID-19. A diminution in food consumption during hospitalization is immediately related to deterioration clinical consequences [2].
It has been stated that diet complementation with some nutraceuticals performs a basic function in the treatment of respiratory symptoms, since several foods and their derivatives offer an immune response to respiratory viruses. Moreover, they regulate the activity of the inflammation generated by COVID-19 [113].
Suitable nutrition is necessary for immune system cells to play their role perfectly. Hence, this allows immune cells to begin efficient reactions towards germs, solve the response quickly and avert any underlying chronic inflammation [114].
During this pathogenesis it is essential to take care of nutritional practices, taking a healthy and balanced nutritional plan with high quantities of minerals, vitamins and antioxidants. Micronutrients can boost immune function too [115].
Dietary characteristics including unavailability of nutrient-rich food, changes in dietary pattern and more prone to eat processed food, malabsorption and maldigestion of food and excessive alcohol consumption compromised the immune system which increased the risk of infection [1].
4.1. Macronutrients
In the immune system, the alimentary elements that induce malfunctioning are the deficient consumption of macronutrients and source of energy. Some macronutrients possess special functions in developing and preserving an efficient immune system in diminishing chronic inflammation [114]. Protein hydrolysates boosted barrier function and IgA generation in animal models [116]. The amino acid arginine is vital for the formation of nitric oxide by macrophages [114].
Glutamine offers a substantial energy resource for cells implicated in immune responses. Furthermore, it helps in the nucleotide synthesis, notably appropriate for speedily dividing immune cells over an immune response. In the case of infection, the content of glutamine intake by immune cells is equal or higher than that for glucose. Glutamine intervenes in the roles of neutrophils, macrophages and lymphocytes [117].
COVID-19 ill patients receiving an enteral nutrition for >7 days and getting a considerable daily level of proteins and calories per kilogram showed a bass death rate comparable with patients with a less than suitable protein provision [2].
Treatment of asymptomatic, pauci-symptomatic and mild COVID-19 patients with lactoferrin (a milk-derived 80-kDa glycoprotein) revealed faster virus negativization and faster clinical recovery in comparison with untreated patients [9].
Probiotics are live microorganisms which can provide health benefits when consumed. Probiotics can activate barrier function [114]. Outside sleep-inducing effects, milk products including yogurt could increase natural killer cell properties and decrease the hazard of respiratory infections [115]. The daily intake of probiotics was suggested to be advantageous to human health by strengthening the immune reaction by regulating the gut bacterial ecosystem and enhancing the antiviral defense. Probiotics act with macrophages to promote the formation of interleukin-12 that activates the generation of interferon-γ, a principal antiviral cytokine. Moreover, yogurt bioactive peptides have powerful angiotensin-converting enzyme-inhibitory and bradykinin-enhancing properties. Hence, they can be efficient in the fight against the COVID-19 disease and its prejudicial health outcomes. Thus, it was demonstrated that the uptake of probiotics from milk significantly decreased the prevalence of respiratory tract contamination [7]. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri was determined in experimental models by Eguchi et al. [118].
COVID-19 individuals can exhibit intestinal microbial dysbiosis described by minimal numbers of different probiotic species such as Bifidobacterium and Lactobacillus, thereby presumably needing probiotic consumption to re-establish the intestinal flora equilibrium and reduce the hazards linked to COVID-19 [119]. A dietary source of probiotics is fermented foods and numerous research studies explore the efficiency of probiotics in treating or preventing COVID-19. Chourasia et al. [120] investigated the ability of 1420 bioactive peptides characterized in soy cheese peptidome developed by Lactobacillus delbrueckii in the inhibition of the SARS-CoV-2 main protease and S1 glycoprotein. Furthermore, a computer-guided research study determined the capacity of two bioactive peptides produced by β-lactoglobulin from goat milk whey fraction in inhibiting SARS-CoV-2 and angiotensin-converting enzyme [121].
4.2. Micronutrients (Vitamins and Minerals)
Micronutrients are gaining considerable attentiveness worldwide over the COVID-19 pandemic for their capability to affect the sensitivity to infection. Immunity implies vitamins renewing the ability of cells to produce some cytokines that impact the processes of immune cells. Among the cited vitamins, such as vitamin B complexes and vitamin C (water-soluble vitamins), vitamin A, vitamin D and vitamin E, vitamin E is a fat-soluble vitamin which covers a set of eight organic molecules, four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienol) [122]. These vitamins combined with some trace elements such as iron and magnesium were employed across the world to treat individuals affected by COVID-19 [1].
4.2.1. Water-Soluble Vitamins
Vitamin B-complexes have different functions in the treatment of COVID-19. Neutrophil infiltration into the lungs could be highly prevented by vitamin B3 usage which showed anti-inflammatory properties during ventilator-caused lung injury [123]. It is found in numerous foods such as brown rice, legumes, sunflower seeds and nuts, bananas, citrus fruits, dark leafy vegetables, red meat, poultry, fish, eggs, salmon, liver and other organ meats, milk, cheese, oysters, mussels, pork, cheese, yogurt, nutritional and brewer’s yeasts and fortified cereal [1].
Vitamin B6 can help in the treatment of COVID-19 patients since they are subject to a lack of oxygen. Deficit in vitamin B6 will immediately inhibit the hemoglobin biosynthesis guiding the diminution of oxygen content in the body. In grave instances, it is among the principal causes of deaths. Diets with abundant vitamin B6 may be a substitute resolution. The foods rich in this vitamin are bread, whole grain cereals (brown rice, oatmeal), vegetables, soybean, potatoes, banana, spinach, seeds, carrot, pork, fish, poultry (chicken, turkey), eggs, milk and beef liver [1].
Vitamin C (ascorbic acid), a powerful antioxidant, neutralizes free radicals and assists in the prevention or reversal of cellular damage and it also has immunomodulatory effect [34]. Vitamin C possesses a role in the epithelial and endothelial barrier function, sustains vasodilatation and diminishes pro-inflammatory modulators [124]. Vitamin C has vital roles in the improvement of phagocytosis, chemotaxis and production of ROS, reducing necrosis and tissue injury [125]. It also plays a substantial function in the immune system, as since this vitamin practices an antioxidant effect by offering electrons, it defends cells against oxidative stress generated by infections, particularly infections impacting the lungs. Vitamin C enhanced functionality of phagocytes, proliferation of T-lymphocytes and formation of interferon, even lowering the replication of viruses. In the case of the COVID-19 pandemic, as 167 patients in the USA were provided 15 mg/day IV of vitamin C, a decline in the death rate was noticed [126].
Vitamin C was employed in 2003 during the SARS-CoV-1 emergence and as a nonspecific therapy for various respiratory tract infections [127]. Administering vitamin C to patients is an efficacious and economic scheme for the COVID-19 pandemic. The amounts of vitamin C in the serum of severely affected persons by COVID-19 found in the intensive care units were undetected or very bass [128]. Administering vitamin C shortened the patient stay in intensive care units by 8.6% and the period under mechanical ventilation by 18.2% [129,130].
Vitamin C is abundant in citrus fruits and red peppers, and the sources of vitamin C also include mangoes, broccoli, cauliflower, sweet potato, strawberries, tomatoes, papaya, beef liver, oysters, liver and eggs [1,115].
4.2.2. Fat-Soluble Vitamins: Vitamin D and Vitamin E
Evidence of a reduction in vitamin D in the serum of COVID-19 patients has been reported since the early stages of the pandemic [131].
Vitamin D diminishes the hazard of usual colds and infections including COVID-19 via three processes namely a physical barrier, cellular natural immunity, adaptive immunity and stimulates the liberation of antimicrobial peptides [127]. Pro-inflammatory cytokines and B cells were down regulated, and anti-inflammatory cytokines were up regulated by vitamin D in the adaptive immune system [132]. The occurrence of the vitamin D receptor in most immune cells proposes its crucial role in immune cell properties [133]. Vitamin D deficit in winter is linked to viral epidemics. Moreover, vitamin D preserves respiratory tract protecting tight junctions, killing enveloped viruses through the induction of cathelicidin and defensins, declining the formation of pro-inflammatory cytokines by the innate immune system and diminishing the hazard of a cytokine storm which could lead to pneumonia [115].
The incidence of vitamin D deficit in ill persons with intense COVID-19 symptoms found in intensive care units was elevated (96.82%), in comparison with patients without any manifestations (32.96%) [134]. A retrospective work performed in the Philippines on 212 persons revealed an essential relationship between the vitamin D level in the plasma of COVID-19 patients and clinical outcomes [135].
Severe COVID-19 patients displayed vitamin D deficits with lower serum levels of 25-hydroxyvitamin D and with higher levels of inflammatory markers compared to asymptomatic patients. Moreover, low levels of 25-hydroxyvitamin are related to the severity of COVID-19. Patients treated with a high dose of cholecalciferol addition exhibited higher SARS-CoV-2 negativization than those who do not have the addition. Furthermore, the initial usage of vitamin D is connected with augmented survival between COVID-19 hospitalized patients. An adequate vitamin D status diminishes the employment of intensive care and results in a diminution in death. The initial uptake of an elevated dose of vitamin D in association with hydroxychloroquine and azithromycin significantly decreases the disease severity and access to intensive care compared to treatment with hydroxychloroquine or azithromycin alone [9].
It is important to note that eating a diet rich in vitamin D may help prevent infection with COVID-19. Furthermore, because the time spent outside and therefore sun exposure is restricted, it is recommended that individuals receive more vitamin D from foods. Foods that include vitamin D are fish, seaweeds, oat, soy milk, cereal, marine fish, beef liver, cheese, egg yolk, milk, shrimp, mushrooms, cheese, fortified soy milk, fortified cereal and foods with added vitamin D (e.g., milk, yogurt) [1,115].
Vitamin E, which covers a set of eight organic molecules, four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienol) [122], can exert multiple various immunological properties and can act as antioxidant, inhibiting the activity of protein kinase C and eventually interacting with transport proteins and enzymes [136]. Vitamin E is indispensable to get rid of chronic viral infections and decrease the rate of inflammation. Thus, vitamin E must be used in a sufficient content to diminish the probability of being infected by SARS-CoV-2.
Vitamin E supplementation at 500 mg/kg may act as a treatment drug to inhibit ferroptosis in COVID-19 patients and decline ferroptosis damages to multiple organs, namely the lungs, kidneys, liver, gut, heart and nervous system. It reduces the ferric iron center in 15-lipoxygenase to inactive ferrous (Fe2+), resulting in 15-lipoxygenase inhibition and preventing lipid hydroperoxides formation [137].
Foods rich in this vitamin are vegetable oils (soybean, sunflower, corn, wheat germ, and walnut), nuts, seeds, green leafy vegetables (spinach, and broccoli), marine fish, octopus, goose meat and vitamin E fortified oil [1,115].
4.3. Trace Elements
Various trace elements (metals: zinc, iron, copper; oligoelements: magnesium, selenium) have been revealed to display a satisfying impact on reinforcing the human immune response [1].
4.3.1. Magnesium
Magnesium exerts different functions in the immune system and it is involved in both innate and acquired responses. It acts as a cofactor for participation in immunoglobulin biosynthesis and antibody generation [138]. Magnesium associated with vitamins B12 and D reduces patient demands for oxygen support and intensive care support in China [139]. Magnesium can be found in green leafy vegetables, banana, avocado, nuts, seeds, legumes, peas, spinach, oatmeal, seafood (Salmon, mackerel, tuna), shrimp, egg, milk, beef and chicken [1].
4.3.2. Iron
Iron has a key function in systemic oxygen transfer and serves as an electron donor or acceptor in several biological properties [140]. SARS-CoV-2 intrudes the heme metabolism in the body by attacking the 1-β chain of hemoglobin and hence capturing porphyrin, which results in an iron deficit. This is responsible for the evolvement of regular acute respiratory tract infections. A suitable level of iron can protect from the respiratory tract infections in gravely infected coronavirus patients. People who are iron deficient can get iron by eating these foods: pumpkin seeds, nuts, oats, brown rice, spinach, beans, potatoes, organ meats, beef, spleen, clams, egg yolk, shrimp and dark chocolate [1]. However, during an intensified inflammatory state, cytokines, such as IL-6, stimulate ferritin and hepcidin synthesis which is the key iron regulatory substance [140].
4.3.3. Zinc
Another vital trace element that is critical for the sustenance of the immune system is zinc. It prevented grave acute respiratory syndrome coronavirus RNA-dependent RNA polymerase template linking and extension in Vero-E6 cells [115]. Zinc inhibits SARS-CoV and retrovirus RNA polymerase activity [34].
Zinc and vitamin A are fundamental for a successful proliferative response for the immune system since they regularize cell division and act as a cofactor with both catalytic and structural roles in several proteins [114].
It is found that zinc can be employed to treat COVID-19 patients owing to its modulation of immune response and antiviral properties. Despite the fact that this element is most abundant in oysters, other common aliments to obtain zinc are constituted from poultry, red meat, nuts, pumpkin seeds, sesame seeds, beans, lentils, soybeans, whole grains, shellfish, dairy products, eggs, cheese, dark chocolate and cocoa powder [1,115].
4.3.4. Selenium
Selenium is essential for optimum immune functioning and it is the selenoproteins that regulate immunity. Selenium deficit may result in immune incompetence which heightens the vulnerability to infections. The evidence for the relevance of the selenium level in infectious diseases including human immunodeficiency virus infection and chronic hepatitis C virus prevalence was demonstrated [141].
There is no reliable research realized on selenium to ascertain its effect on SARS-CoV-2. However, the results of Moghaddam et al. [142] consolidate the concept of a pertinent function of selenium for COVID-19 convalescence and promote the discussion on adjuvant selenium complementation in gravely ill and selenium-deficient patients.
Selenium complementation is immuno-stimulatory, and is determined by T cell proliferation, NK cell activity, innate immune cell functions and others [141].
Almonds, pumpkin seeds, sunflower seeds, whole, wheat bread, fish, eggs pork, beef, chicken and turkey contain considerable amounts of this trace element [1].
4.4. Polyunsaturated Fatty Acids
Fatty acids are part of the constitution of the lipid double layer of biological membranes. They perform several functions (energy source, signaling molecules and precursors for the synthesis of eicosanoids) and are related to perform different functions in immune cells. Essential fatty acids such as omega-3 fatty acids regulate immune properties through its effect on inflammatory response [1]. These fatty acids must be procured from foods of marine origin (fish, shrimp and oysters) or vegetables such as walnuts, canola oil, spinach and soybeans [1,143].
For the cardiovascular apparatus, n-3 polyunsaturated fatty acids improve non-controlled inflammatory reactions, decreasing oxidative stress and attenuating coagulopathy. The anti-inflammatory effects of these fatty acids perform a critical function in mitigating the uncontrollable immune reaction in the lungs resulting from viral infections that might be beneficial in the regulation of COVID-19 [143].
So, severely diseased patients undergoing intravenous diet therapy fortified with fish oil lipid emulsion had a diminished hazard for infection by 40% and sepsis by 56% and a decrease in hospital and intensive care unit sojourn by approximately two days [144]. In the same trend, in order to cure gravely ill patients with COVID-19 by inhibiting cytokine excretion and reducing the inflammatory reaction, a parenteral complementation with fish oil emulsions, including considerable levels of eicosapentaenoic acid and docosahexaenoic acid (DHA; C22:6 n − 3) (4–6 g/d), was adopted [145].
In a pilot investigation, blood omega-3 amount from 100 COVID-19 patients was conversely linked to the risk of mortality [9].
In a survey consisting of 240 subjects with COVID-19, where the first cluster experienced standard care and the second cluster received 2 g/day of eicosapentaenoic acid capsules, the effectiveness of this fatty acid in the treatment of the sickness was demonstrated [143].
Nutrient effect results demonstrated in relevant studies are summarized in Table 2.

Table 2.
Mode of action of some nutrients tested against COVID-19.
A summary diagram of the main effects of different natural products (nutrients and secondary metabolites) is shown in Figure 2.
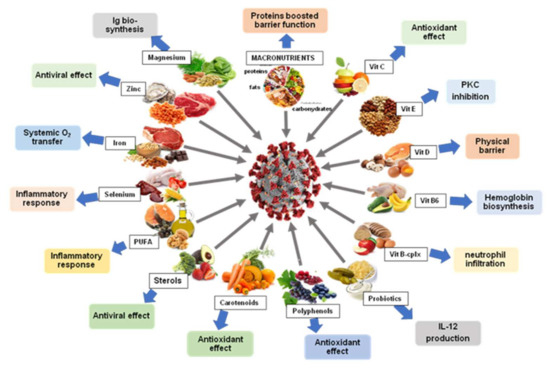
Figure 2.
Different food classes with positive effects in the treatment of the COVID-19 infection.
Nutritional recommendations and several food groups have been shown to be effective either in boosting up adaptive immunity or by acting as antivirals, anti-inflammatory and exogenous antioxidants. Among these food groups, we distinguish macro- and micronutrients (vitamins and minerals), trace elements (iron, zinc, magnesium, selenium), polyunsaturated fatty acids, different classes of secondary metabolites mainly phenolic compounds and terpenoids without forgetting other elements such as probiotics.
5. Perspectives and Emerging Technologies: Plant-Based SARS-CoV-2 Vaccines
Vaccination is currently the most efficient approach to prevent the proliferation of any viral illness [151,152]. The majority of the efficacious vaccine fabrication is performed by animal cell culture procedures. However, this takes several months to generate an important quantity of clinical grade vaccine doses. Other disadvantages are related to this method such as preservation, stabilization, recurrent contamination or infection of the cell culture systems [153].
Otherwise, the authorized vaccines were mostly founded on three common techniques of their production, (i) inactivated viral particles; (ii) mRNA formulated vaccines, and (iii) adenoviral or recombinant adenoviral vaccine [151]. Nevertheless, the usage of attenuated viruses and viral vectors in humans as vaccines may present some health hazards including the probability of mutation when using attenuated viruses and recombination by adopting viral vectors. Furthermore, the production of monoclonal antibodies towards COVID-19 cannot be a durable resolution because of prospective detrimental response [154].
Plant-based engineering, exploited in conventional and contemporary medication for several ailments such as infectious pathologies, possesses the ability to process efficacious, steady and cost-effective vaccines [155]. So, medicinal plant species may offer a resolution as an origin of natural antiviral constituents by the assemblage of secondary metabolites and also by performing as a platform to express the viral immunogenic proteins [156]. Secondary metabolites have an important function in protection due to biological properties and molecular farming is adopted for their large-scale synthesis. Moreover, metabolic technology can be employed to oppress the bioactive substance accessibility restrictions from herbal medicines and to enhance the productiveness advantages from bioprocessing and molecular farming. This consists of producing advisable recombinant proteins by whole plants or in vitro cultured plant tissues/cells. This can involve the additional refining of plant extracts, especially ones which have been employed formerly to satisfactorily inactivate SARS-CoV, as they can also have a role in inhibiting COVID-19 [154].
This is practicable to generate numerous sorts of vaccine, including protein subunit, virus-like particle (VLP), chimeric VLP and multiepitope vaccines, with the involvement of transient and stable expression. The plant transient expression process is quicker than any other procedure and offers a vaccine in 20 days after the protein amino acid sequence becomes accessible [157]. VLPs are made up of the plant lipid membrane as well as the COVID-19 spike protein, and they are comparable in dimension and form to the existent coronavirus; however, they do not contain nucleic acid so they are infective [154].
This methodology is extensively utilized for the manufacturing of recombinant proteins in various plant species including Lactuca sativa, Arabidopsis thaliana, Nicotiana tabacum and Nicotiana benthamiana which is the more suitable one [158]. The exploitation of tobacco plants for the expression of the coronavirus viral antigens can be a target for the prospect vaccinal investigation of the novel coronavirus because of the effectiveness of expression and intrinsic antiviral activities [156].
The traditional method for expression of transgenes in plants includes transgene inclusion into the nuclear genome. Presently, Agrobacterium-mediated transformation is the best popular procedure to attain this change. This bacterium possesses the capacity to transmit big stands of DNA with limited reorganization at elevated effectiveness with a bass number of insertions [152].
Vaccines produced in plants have been reeled to evoke a powerful immune reaction in humans [154]. These vaccines are perceived as third-generation vaccines [155], are secure, do not contain any consequent human pathogenic infection and are mainly high-quality clinical grade doses which can be developed within weeks. Distribution is the key to all vaccination efforts, particularly in developing countries [152].
Initial plant-based vaccines were produced for the Hepatitis B virus in 1992 [159]. Plant-based vaccines for COVID-19 can be generated in two ways: (1) By expressing the antigenic constituent of SARS-CoV-2 to provoke active immunity or (2) by expressing the antibody towards the virus to give passive safeguarding [155]. Vaccines that use individual proteins as antigens in a prime boost system alongside an adequate adjuvant, or as VLPs with various viral antigens could be valuable against COVID-19 [156].
In the USA, professionals at Kentucky Bio-Processing (KBP) cloned a portion of the genetic sequence of SARS-CoV-2, which they employed to perform a prospective Ag which was included in Nicotiana benthamiana for fabrication. The vaccine has induced a positive immune reaction in pre-clinical assays and will be entering phase 1 human clinical assays [160]. In Thailand, the aiyaPharming™ protein expression platform was used to produce a subunit-based vaccine to target COVID-19, also in N. benthamiana [158]. In South Africa, Cape Bio Pharms (CBP) is making spike S1 reagents composed of different zones of the glycoprotein associated with several fusion proteins and is trying to fabricate antibodies against these proteins [154].
Interestingly, the stage I clinical assay study of a plant-based COVID-19 vaccine has been developed by MedicagoInc, Canada. In this technique, Nicotiana benthamiana was used as plant system for the vaccine production and Agrobacterium tumefaciens as bacterial vector which is found in soils and is not toxic to plants. It has the ability to transfer its genetic material into the plant cells. This bacterium is injected with a plasmid containing the gene that codes for the spike protein of the coronavirus [161].
Potential targets included the spike, nucleocapsid, membrane, envelope, viral RNA polymerase and 3-chymotrypsin-like protease (3CLpro), that splits the virus polyprotein at 11 different positions to produce numerous non-structural proteins which are primordial for viral replication, all these proteins may be utilized to formulate eventual vaccines [154]. The main target being the S protein and its antigenic identification revealed central immunogenic proteins that can be expressed in the plants for the fabrication of a plant-based vaccine towards COVID-19. Thus, antibodies developed against the receptor-binding domain (RBD) of the S protein have been demonstrated to counteract COVID-19 [156].
However, it should be noted that various novel variants of COVID-19 are appearing worldwide, and existing vaccines are less sensitive to them. The cause can be the elevated proportions of S protein mutations, since the majority of the vaccines are focused on this protein. Therefore, new vaccines targeting other proteins, such as the N protein, can be considered a good alternative. The N protein is greatly immunogenic and highly preserved, offering lower susceptibility to mutation. Moreover, immunization by several antigens can offer satisfying preservation, suppressing the necessity for an amplifier dose. In line with this, the production of multiepitope and multivalent vaccines incorporated with plants might be effectual procedures to resolving the difficulties generated by COVID-19 mutational variants [157].
6. Conclusions
Several researchers have carried out state of the art in order to improve the understanding of the emergence of the new COVID-19 pandemic. Some of them have focused on medicinal plants and on their bioactive substances. We highlight the importance of natural products in COVID-19 pathogenesis complications and draw attention towards medicinal plants and different foods’ potential roles in mitigating the pandemic complications. Currently, there is direct evidence of a beneficial impact of numerous biologically active metabolites in COVID-19 patients. Indeed, biologically active molecules found either in medicinal plants or in diet possess the ability to regulate several of the detrimental actions caused by infection. Depending on the nature of the compounds, they are involved in different processes: they can modulate an excessive immune response, inactivate enveloped viruses, enhance macrophage phagocytic capability, improve coagulopathy, amend cell signaling and gene expression, change the model of the lipid metabolites generated under stress conditions to a more anti-inflammatory metabolite profile and activate the antioxidative property of the body.
Furthermore, numerous food ingredients determine the gut microbial composition and can consequently contribute to better regulate the immune response. A diet combined with medicinal plants with immunomodulatory, antiviral and anti-inflammatory properties can highly activate this safeguard. Diet must be abundant in vitamins and antioxidants, such as vitamins C, D, E, zinc, selenium and polyunsaturated fatty acids which have immunomodulatory activities and are substantial in protecting against the COVID-19 infection.
Additional studies, haphazard controlling assays and observational surveys are however needed to check and convert these advocated properties in the prevention and/or treatment of the actual COVID-19 infection and other possible infections. In addition, it should be noted that despite medicinal plants can have great potential against COVID-19, some of their compounds can also be toxic, teratogenic, mutagenic and carcinogenic. Hence, toxicological and pharmacological research studies are required to determine the safety and efficiency profile of the considered preparations and products. Herbal self-medication for serious diseases such as COVID-19 infection should be used with caution. However, as several foods and herbs possess immunomodulatory, anti-inflammatory and antiviral activities, and as a balanced diet and dietary intake of nutrients affect the immune system in many ways, the use of natural molecules from plants is an attractive nutritional and pharmacological approach that should not be overlooked and deserves to be explored in order to prevent and deal with pandemics, the scale of which may have significant and detrimental socioeconomic consequences.
All in all, extensive randomized, controlled investigations are required to appoint the functions of medicinal plants and of their active molecules, micro- and/or macronutrients in the various phases of COVID-19 and to assay their favorable and/or negative impacts, before the endorsement of their therapeutic usage in infected patients.
Author Contributions
Conceptualization: F.B., A.V. and G.L.; study management: G.L., F.B., A.V. and I.G.; writing—original draft: F.B., G.L., A.V. and I.G.; proofreading: G.L., F.B. and A.V.; mainly, K.M., L.B.-M., A.Z., T.G., S.E., S.M. (Stéphane Mandard), V.L., G.P., D.V.-F., O.K., A.E.M., A.G.A., S.M. (Smail Meziane), N.L., B.N., B.B.-Z., O.M.-K. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Université de Bejaia (F.B., Bejaia, Algeria), Université de Bourgogne (G.L., A.V., S.M., Dijon, France), Université de Monastir (A.Z., Monastir, Tunisia), Université Tunis El Manar (T.G., Tunis, Tunisia), Université de Sousse (A.Z., Sousse, Tunisia), Université Hassan 1er (B.N., Settat, Morocco).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Imen Ghzaiel (I.G.) received financial support from Nutrition Méditerranéenne et Santé (NMS; N.L.), Institut Européen des Antioxydants (IEA; S.M.) and was awarded the NMS prize (Romanée-Conti) in 2021. I.G. also received financial support from ABASIM (Association Bourguignonne pour les Applications des Sciences de l’Information en Médecine; Dijon, France). Mohamed Ksila (M.K.) received financial support from PHC Utique (G.L./T.G. and O.M.-K.; 2021–2022; code CMCU22G089/code Campus France 47608V). A.G.A., A.Z., F.B. and G.L. are members of the International Natural Product Sciences Taskforce (INPST: https://inpst.net/ accessed on 20 April 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, S.; Bhuiyan, F.R.; Emon, T.H.; Hasan, M. Prospects of nutritional interventions in the care of COVID-19 patients. Heliyon 2021, 7, e06285. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, E.S.; dos Santos Muniz, L.S.; Holanda, J.L.G.; Oliveira, B.D.D.; de Carvalho, M.C.F.; Leitão, A.M.M.; de Alencar Cavalcante, M.I.; de Oliveira, R.C.P.; da Silva, C.A.B.B.; Ferreira Carioca, A.A. Enteral nutritional support for patients hospitalized with COVID-19: Results from the first wave in a public hospital. Nutrition 2022, 94, 111512. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Alexander, C.; Casssady, B.A. Nutrition risk prevalence and nutrition care recommendations for hospitalized and critically-ill patients with COVID-19. Clin. Nutr. ESPEN 2021, 44, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Marcello, A.; Civra, A.; Bonotto, R.M.; Alves, L.N.; Rajasekharan, S.; Giacobone, C.; Caccia, C.; Cavalli, R.; Adami, M.; Brambilla, P.; et al. The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients. Redox. Biol. 2020, 36, 101682. [Google Scholar] [CrossRef] [PubMed]
- Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Nury, T.; Ksila, M.; Leoni, V.; Bouhaouala-Zahar, B.; Hammami, S.; Hammami, M.; Mackrill, J.J.; et al. 7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19. J. Steroid Biochem. Mol. Biol. 2021, 212, 105939. [Google Scholar] [CrossRef]
- Gouda, A.S.; Adbelruhman, F.G.; Alenezi, H.S.; Mégarbane, B. Theoretical benefits of yogurt-derived bioactive peptides and probiotics in COVID-19 patients–A narrative review and hypotheses. Saudi J. Biol. Sci. 2021, 28, 5897–5905. [Google Scholar] [CrossRef]
- Schmelter, F.; Föh, B.; Mallagaray, A.; Rahmöller, J.; Ehlers, M.; Lehrian, S.; von Kopylow, V.; Künsting, I.; Lixenfeld, A.S.; Martin, E.; et al. Metabolic and lipidomic markers differentiate COVID-19 from non-hospitalised and other intensive care patients. Front. Mol. Biosci. 2021, 1091, 737039. [Google Scholar] [CrossRef]
- Motti, M.L.; Tafuri, D.; Donini, L.; Masucci, M.T.; De Falco, V.; Mazzeo, F. The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19). Nutrients 2022, 14, 1000. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Ahmad, S.; Zahiruddin, S.; Parveen, B.; Basist, P.; Parveen, A.; Parveen, R.; Ahmad, M. Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front. Pharmacol. 2021, 2470, 578970. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. An Opinion: Herbal Medicines may Provide a Means of Controlling the COVID-19 Pandemic. Pharmacogn. Commun. 2020, 10, 140–142. [Google Scholar] [CrossRef]
- Brahmi, F.; Vejux, A.; Sghaier, R.; Zarrouk, A.; Nury, T.; Meddeb, W.; Rezig, L.; Namsi, A.; Sassi, K.; Yammine, A.; et al. Prevention of 7-ketocholesterol-induced side effects by natural compounds. Crit. Rev. Food Sci. Nutr. 2019, 59, 3179–3198. [Google Scholar] [CrossRef]
- Debbabi, M.; Nury, T.; Zarrouk, A.; Mekahli, N.; Bezine, M.; Sghaier, R.; Grégoire, S.; Martine, L.; Durand, P.; Camus, E.; et al. Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of trolox, on 7-Ketocholesterol-Induced Mitochondrial and Peroxisomal Dysfunction in Microglial BV-2 Cells. Int. J. Mol. Sci. 2016, 17, 1973. [Google Scholar] [CrossRef]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by dietary polyphenols (resveratrol, quercetin, apigenin) against 7-Ketocholesterol-Induced oxiapoptophagy in neuronal N2a cells: Potential interest for the treatment of neurodegenerative and age-related diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Kurtz, A.; Grant, K.; Marano, R.; Arrieta, A.; Feaster, W.; Steele, C.; Ehwerhemuepha, L. Long-term effects of malnutrition on severity of COVID-19. Sci. Rep. 2021, 11, 14974. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Sharanya, C.; Sabu, A.; Haridas, M. Potent phytochemicals against COVID-19 infection from phyto-materials used as antivirals in complementary medicines: A review. Future J. Pharm. Sci. 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. How the coronavirus infects cells-and why Delta is so dangerous. Nature 2021, 595, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Disoma, C.; Zheng, R.; Zhou, M.; Razzaq, A.; Liu, P.; Zhou, Y.; Dong, Z.; Du, A.; et al. SARS-CoV-2: Mechanism of infection and emerging technologies for future prospects. Rev. Med. Virol. 2021, 31, e2168. [Google Scholar] [CrossRef]
- Soldevila, B.; Puig-Domingo, M.; Marazuela, M. Basic mechanisms of SARS-CoV-2 infection. What endocrine systems could be implicated? Rev. Endocr. Metab. Disord. 2021, 23, 137–150. [Google Scholar] [CrossRef]
- Cai, G.; Cui, X.; Zhu, X.; Zhou, J. A hint on the COVID-19 risk: Population disparities in gene expression of three receptors of SARS-CoV. Preprints 2020, 2020020408. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Xie, D.-Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef]
- De Oliveira, O.V.; Cristina Andreazza Costa, M.; Marques da Costa, R.; Giordano Viegas, R.; Paluch, A.S.; Miguel Castro Ferreira, M. Traditional herbal compounds as candidates to inhibit the SARS-CoV-2 main protease: An in silico study. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef]
- Acedhars Unilag COVID-19 Response Team; Akindele, A.J.; Agunbiade, F.O.; Sofidiya, M.O.; Awodele, O.; Sowemimo, A.; Ade-Ademilua, O.; Akinleye, M.O.; Ishola, I.O.; Orabueze, I.; et al. COVID-19 Pandemic: A case for phytomedicines. Nat. Prod. Commun. 2020, 15, 5086. [Google Scholar] [CrossRef]
- Belhaj, S.; Zidane, L. Medicinal plants used to boost immunity and decrease the intensity of infection caused by SARS-COV-2 in Morocco. Ethnobot. Res. Appl. 2021, 21, 1–17. Available online: https://ethnobotanyjournal.org/era/index.php/era/article/view/2495 (accessed on 31 January 2022).
- Jamiu, A.T.; Aruwa, C.E.; Abdulakeem, I.A.; Ajao, A.A.; Sabiu, S. Phytotherapeutic Evidence Against Coronaviruses and Prospects for COVID-19. Pharmacogn. J. 2020, 12, 1252–1267. [Google Scholar] [CrossRef]
- Ren, J.-l.; Zhang, A.-H.; Wang, X.-J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Fongnzossie Fedoung, E.; Biwole, A.B.; Nyangono Biyegue, C.F.; Ngansop Tounkam, M.; Akono Ntonga, P.; Nguiamba, V.P.; Essono, D.M.; Forbi Funwi, P.; Tonga, C.; Nguenang, G.M.; et al. A review of Cameroonian medicinal plants with potentials for the management of the COVID-19 pandemic. Adv. Tradit. Med. 2021, 1–26. [Google Scholar] [CrossRef]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Lin, C.-S.; Lai, H.-C.; Lin, Y.-P.; Wang, C.-Y.; Tsai, Y.-C.; Wu, K.-C.; Huang, S.-H.; Lin, C.-W. Antiviral activity of Sambucus Formosana Nakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019, 273, 197767. [Google Scholar] [CrossRef]
- Tuiskunen Bäck, A.; Lundkvist, Å. Dengue viruses–an overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Ao, X.; Gong, X.; Liu, C.; Xu, D.; Huang, Y.; Liu, Z.; Zhao, B.; Liu, X.; et al. Meta-analysis of transcriptome datasets: An alternative method to study IL-6 regulation in coronavirus disease 2019. Comput. Struct. Biotechnol. J. 2021, 19, 767–776. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.; Shang, Y.; Liang, S.; Yang, M.; Robinson, N.; Liu, J.-P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020, 26, 243–250. [Google Scholar] [CrossRef]
- Weng, J.-K. Plant solutions for the COVID-19 pandemic and beyond: Historical reflections and future perspectives. Mol. Plant 2020, 13, 803–807. [Google Scholar] [CrossRef]
- Gangal, N.; Nagle, V.; Pawar, Y.; Dasgupta, S. Reconsidering traditional medicinal plants to combat COVID-19. AIJR Prepr. 2020, 34, 1–6. [Google Scholar]
- Balkrishna, A.; Pokhrel, S.; Singh, J.; Varshney, A. Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Drug Des. Devel Ther. 2021, 15, 1111–1133. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Vroh, B.T.A. Diversity of Plants Used in Traditional Medicine against the Main Symptoms of COVID-19 in Sub-Saharan Africa: Review of the Literature. Ethnobot. Res. Appl. 2020, 20, 1–14. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/2161 (accessed on 30 December 2021).
- Mirzaie, A.; Halaji, M.; Dehkordi, F.S.; Ranjbar, R.; Noorbazargan, H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complement. Ther. Clin. Pract. 2020, 40, 101214. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal plants to strengthen immunity during a pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar] [CrossRef]
- Flouchi, R.; Fikeri-Benbrahim, K. Prevention of COVID 19 by aromatic and medicinal plants: A systematic review. Int. J. Pharm. Sci. Res. 2020, 12, 1106–1111. [Google Scholar]
- Bouchentouf, S.; Missoum, N. Identification of Compounds from Nigella Sativa as New Potential Inhibitors of 2019 Novel Coronasvirus (COVID-19): Molecular Docking Study. ChemRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Latruffe, N. Mediterranean Diet and Wine and Health; EUD: Dijon, France, 2017; 204p. (In French) [Google Scholar]
- Ghiringhelli, F.; Rebe, C.; Hichami, A.; Delmas, D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anticancer Agents Med. Chem. 2012, 12, 852–873. [Google Scholar] [CrossRef]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, X.; Wei, X.; Li, J.; Yang, J.; Tan, H.; Zhu, J.; Wu, J. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020, 92, 595–601. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Le Goff, C.; Misset, B.; et al. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Guerra-Valle, M.; Casas-Forero, N.; Sobral, M.M.C.; Viegas, O.; Alarcón-Enos, J.; Mplvo Ferreira, I.; Pinho, O. Western dietary pattern antioxidant intakes and oxidative stress: Importance during the SARS-CoV-2/COVID-19 pandemic. Adv. Nutr. 2021, 12, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Poklar Ulrih, N. Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Latruffe, N.; Rifler, J.-P. Bioactive polyphenols from grapes and wine emphasized with resveratrol. Curr. Pharm. Des. 2013, 19, 6053–6063. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Sureda, A.; Tur, J.A.; Andreoletti, P.; Cherkaoui-Malki, M.; Latruffe, N. How efficient is resveratrol as an antioxidant of the Mediterranean diet, towards alterations during the aging process? Free Radic. Res. 2019, 53 (Suppl. S1), 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.-T.; Wu, C.-C.; Wu, S.-F.V.; Lee, M.-C.; Hu, W.-C.; Tsai, K.-W.; Yang, C.H.; Lu, C.L.; Chiu, S.K.; Lu, K.C. Resveratrol as an adjunctive therapy for excessive oxidative stress in aging covid-19 patients. Antioxidants 2021, 10, 1440. [Google Scholar] [CrossRef]
- Pasquereau, S.; Nehme, Z.; Haidar Ahmad, S.; Daouad, F.; Wallet, J.V.A.C.; Schwartz, C.; Rohr, O.; Morot-Bizot, S.; Herbein, G. Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro. Viruses 2021, 13, 354. [Google Scholar] [CrossRef]
- Tietjen, I.; Cassel, J.; Register, E.T.; Zhou, X.Y.; Messick, T.E.; Keeney, F.; Lu, L.D.; Beattie, K.D.; Rali, T.; Tebas, P.; et al. The Natural Stilbenoid (–)-Hopeaphenol Inhibits Cellular Entry of SARS-CoV-2 USA-WA1/2020, B. 1.1. 7, and B. 1.351 Variants. Antimicrob. Agents Chemother. 2021, 65, e00772-21. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Song, S.; Xiao, Z.; Chen, X.; Huang, B.; Sun, M.; Su, G.; Zhou, D.; Wang, G.; et al. Effective inhibition of coronavirus replication by Polygonum cuspidatum. Front. Biosci. 2021, 26, 789–798. [Google Scholar] [CrossRef]
- Ter Ellen, B.M.; Dinesh Kumar, N.; Bouma, E.M.; Troost, B.; van de Pol, D.P.; Van der Ende-Metselaar, H.H.; Apperloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C.; et al. Resveratrol and pterostilbene inhibit SARS-CoV-2 replication in air–liquid interface cultured human primary bronchial epithelial cells. Viruses 2021, 13, 1335. [Google Scholar] [CrossRef]
- AbdelMassih, A.; Yacoub, E.; Husseiny, R.J.; Kamel, A.; Hozaien, R.; El Shershaby, M.; Rajab, M.; Yacoub, S.; Eid, M.A.; Elahmady, M.; et al. Hypoxia-inducible factor (HIF): The link between obesity and COVID-19. Obes. Med. 2021, 22, 100317. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, C.S. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin. Hypertens. 2020, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Perrella, F.; Coppola, F.; Petrone, A.; Platella, C.; Montesarchio, D.; Stringaro, A.; Ravagnan, G.; Fuggetta, M.P.; Rega, N.; Musumeci, D. Interference of Polydatin/Resveratrol in the ACE2: Spike recognition during COVID-19 infection. A focus on their potential mechanism of action through computational and biochemical assays. Biomolecules 2021, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mittra, I.; de Souza, R.; Bhadade, R.; Madke, T.; Shankpal, P.; Joshi, M.; Qayyumi, B.; Bhattacharjee, A.; Gota, V.; Gupta, S.; et al. Resveratrol and Copper for treatment of severe COVID-19: An observational study (RESCU 002). medRxiv 2020. [Google Scholar] [CrossRef]
- Kelleni, M.T. Resveratrol-zinc nanoparticles or pterostilbene-zinc: Potential COVID-19 mono and adjuvant therapy. Biomed. Pharmacother. 2021, 139, 111626. [Google Scholar] [CrossRef]
- Abboud, R.; Charcosset, C.; Greige-Gerges, H. Interaction of triterpenoids with human serum albumin: A review. Chem. Phys. Lipids 2017, 207, 260–270. [Google Scholar] [CrossRef]
- Vattekkatte, A.; Garms, S.; Brandt, W.; Boland, W. Enhanced structural diversity in terpenoid biosynthesis: Enzymes, substrates and cofactors. Org. Biomol. Chem. 2018, 16, 348–362. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Wiley: Hoboken, NJ, USA, 2010; pp. 258–303. ISBN 9781444320503. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149. [Google Scholar]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative anti-infectious bronchitis virus (IBV) activity of (-)-pinene: Effect on nucleocapsid (N) protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef]
- Zamora, A.P.; Edmonds, J.H.; Reynolds, M.J.; Khromykh, A.A.; Ralph, S.J. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology 2016, 495, 18–32. [Google Scholar] [CrossRef]
- Bicchi, C.; Rubiolo, P.; Ballero, M.; Sanna, C.; Matteodo, M.; Esposito, F.; Zinzula, L.; Tramontano, E. HIV-1-inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med. 2009, 75, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Haiying, L.; Na, H.; Xiaoyuan, X. The curative effects of glycyrrhizin on patients with SARS. In Annual Meeting of the Society of Infectious and Parasitic Diseases; Chinese Medical Association: Wuhan, China, 2003; pp. 18–22. [Google Scholar]
- Ebrahimi, M.; Farhadian, N.; Amiri, A.R.; Hataminia, F.; Soflaei, S.S.; Karimi, M. Evaluating the efficacy of extracted squalene from seed oil in the form of microemulsion for the treatment of COVID-19: A clinical study. J. Med. Virol. 2022, 94, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z.; et al. In vitro evaluation of the activity of terpenes and cannabidiol against human coronavirus E229. Life 2021, 11, 290. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdelrahman, A.H.; Mohamed, T.A.; Atia, M.A.; Al-Hammady, M.A.; Abdeljawaad, K.A.; Elkady, E.M.; Moustafa, M.F.; Alrumaihi, F.; Allemailem, K.S.; et al. In silico mining of terpenes from red-sea invertebrates for SARS-CoV-2 main protease (Mpro) inhibitors. Molecules 2021, 26, 2082. [Google Scholar] [CrossRef]
- Meeran, M.N.; Seenipandi, A.; Javed, H.; Sharma, C.; Hashiesh, H.M.; Goyal, S.N.; Jha, N.K.; Ojha, S. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Heliyon 2021, 7, e05703. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Khalil, A.; Tazeddinova, D.; Aljoumaa, K.; Kazhmukhanbetkyzy, Z.A.; Orazov, A.; Toshev, A.D. Carotenoids: Therapeutic Strategy in the Battle against Viral Emerging Diseases, COVID-19: An Overview. Prev. Nutr. Food. Sci. 2021, 26, 241. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.-K.; Kim, I.; Warren, B.; Kim, J.; Jung, K.; Ku, B. Antiviral Activity of Two Marine Carotenoids against SARS-CoV-2 Virus Entry In Silico and In Vitro. Int. J. Mol. Sci. 2021, 22, 6481. [Google Scholar] [CrossRef] [PubMed]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Cagno, V.; Civra, A.; Poli, G. Oxysterols: An emerging class of broad spectrum antiviral effectors. Mol. Aspects Med. 2016, 49, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.X.; Bartlett, S.; Ronacher, K. Oxysterols in the Immune Response to Bacterial and Viral Infections. Cells 2022, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Wang, F.; Stappenbeck, F.; Tsuchimoto, K.; Kobayashi, C.; Saso, W.; Kataoka, M.; Yamasaki, M.; Kuramochi, K.; Muramatsu, M.; et al. Identification of anti-severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) oxysterol derivatives in vitro. Int. J. Mol. Sci. 2021, 22, 3163. [Google Scholar] [CrossRef]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 79. [Google Scholar] [CrossRef]
- Vandyck, K.; Deval, J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr. Opin. Virol. 2021, 49, 36–40. [Google Scholar] [CrossRef]
- Gyebi, G.A.; Ogunyemi, O.M.; Ibrahim, I.M.; Afolabi, S.O.; Adebayo, J.O. Dual targeting of cytokine storm and viral replication in COVID-19 by plant-derived steroidal pregnanes: An in silico perspective. Comput. Biol. Med. 2021, 134, 104406. [Google Scholar] [CrossRef]
- Kritchevsky, D. Phytosterols. Diet. Fiber Health Dis. 2021, 134, 104406. [Google Scholar] [CrossRef]
- Lizard, G. Phytosterols: To be or not to be toxic; that is the question. Br. J. Nutr. 2008, 100, 1150–1151. [Google Scholar] [CrossRef] [PubMed]
- Ping, F.; Wang, Y.; Shen, X.; Tan, C.; Zhu, L.; Xing, W.; Xu, J. Virtual Screening and Molecular Docking to Study the Mechanism of Chinese Medicines in the Treatment of Coronavirus Infection. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e934102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, J. A review of the latest research on M pro targeting SARS-COV inhibitors. RSC Med. Chem. 2021, 12, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Menon, T. Evaluation of bioactive compounds from Boswellia serrata against SARS-CoV-2. Vegetos 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hammed, M.; Adedotun, I.O.; Olajide, M.; Irabor, C.O.; Afolabi, T.I.; Gbadebo, I.O.; Rhyman, L.; Ramasami, P. Virtual screening, ADMET profiling, PASS prediction, and bioactivity studies of potential inhibitory roles of alkaloids, phytosterols, and flavonoids against COVID-19 main protease (Mpro). Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Dhuli, K.; Anpilogov, K.; Bressan, S.; Dautaj, A.; Dundar, M.; Beccari, T.; Ergoren, M.C.; Bertelli, M. Natural compounds as inhibitors of SARS-CoV-2 endocytosis: A promising approach against COVID-19. Acta Bio Med. Atenei Parmensis 2020, 91, e2020008. [Google Scholar] [CrossRef]
- Nivetha, R.; Bhuvaragavan, S.; Muthu Kumar, T.; Ramanathan, K.; Janarthanan, S. Inhibition of multiple SARS-CoV-2 proteins by an antiviral biomolecule, seselin from Aegle marmelos deciphered using molecular docking analysis. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Kumar, M.P.; Sundaram, K.M.; Ramasamy, M. Coronavirus spike (S) glycoprotein (2019-ncov) targeted siddha medicines kabasura kudineer and thonthasura kudineer–in silico evidence for corona viral drug. Asian J. Pharm. Res. Health Care 2020, 12, 20–27. [Google Scholar] [CrossRef]
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother. Res. 2021, 35, 1925–1938. [Google Scholar] [CrossRef]
- Khan, M.Y.; Kumar, V. Mechanism & inhibition kinetics of bioassay-guided fractions of Indian medicinal plants and foods as ACE inhibitors. J. Tradit. Complement. Med. 2019, 9, 73–84. [Google Scholar] [CrossRef]
- Liang, S.; Junwei, N.; Chunhua, W.; Baoying, H.; Wenling, W.; Na, Z.; Yao, D.; Huijuan, W.; Fei, Y.; Shan, C.; et al. High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J. Virol. 2022, 93, e00023-19. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.I.; Siddiqui, A.; Erum, N.; Kamran, M. Phytomedicine and the COVID-19 pandemic. In Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 693–708. [Google Scholar] [CrossRef]
- Mazraedoost, S.; Behbudi, G.; Mousavi, S.M.; Hashemi, S.A. COVID-19 treatment by plant compounds. Adv. Nano Res. Technol. 2021, 2, 23–33. [Google Scholar] [CrossRef]
- Jahan, I.; Ahmet, O. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk. J. Biol. 2020, 44, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Mishra, P.; Selvaraj, C.; Singh, S.K.; Chaube, R.; Garg, N.; Tripathi, Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)—A molecular docking study. J. Biomol. Struct. Dyn. 2022, 40, 190–203. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: A scoping review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Nutritional recommendations for CoVID-19 quarantine. Eur. J. Clin. Nutr. 2020, 74, 850–851. [Google Scholar] [CrossRef]
- Kiewiet, M.B.; Faas, M.M.; De Vos, P. Immunomodulatory protein hydrolysates and their application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of COVID-19: The Zhejiang experience. Zhejiang Da Xue Xue Bao 2020, 49, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Padhi, S.; Chiring Phukon, L.; Abedin, M.M.; Singh, S.P.; Rai, A.K. A potential peptide from soy cheese produced using Lactobacillus delbrueckii WS4 for effective inhibition of SARS-CoV-2 main protease and S1 glycoprotein. Front. Mol. Biosci. 2020, 7, 601753. [Google Scholar] [CrossRef] [PubMed]
- Çakır, B.; Okuyan, B.; Şener, G.; Tunali-Akbay, T. Investigation of beta-lactoglobulin derived bioactive peptides against SARS-CoV-2 (COVID-19): In silico analysis. Eur. J. Pharmacol. 2021, 891, 173781. [Google Scholar] [CrossRef]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef]
- Drewnowski, A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095S–1101S. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case Rep. 2020, 30, 101063. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Njiki, S.; Enow, J.; Ikome, O.; Latinwo, L.; Long, R.; Ngnepieba, P.; Alo, R.A.; Tchounwou, P.B. Pharmacological Effects of Selected Medicinal Plants and Vitamins against COVID-19. Jacobs J. Food. Nutr. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: A meta-regression analysis. J. Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019, 11, 708. [Google Scholar] [CrossRef]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25 [OH] D) levels in patients hospitalised with COVID-19 are associated with greater disease severity: Results of a local audit of practice. medRxiv 2020. [Google Scholar] [CrossRef]
- Suaini, N.H.; Zhang, Y.; Vuillermin, P.J.; Allen, K.J.; Harrison, L.C. Immune modulation by vitamin D and its relevance to food allergy. Nutrients 2015, 7, 6088–6108. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.S.; Abdel-Mottaleb, Y. In search for effective and safe drugs against SARS-CoV-2: Part III] The electronic factors of Remdesivir and the naturally extracted Aspirochlorine drugs. ChemRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Tavakol, S.; Seifalian, A.M. Vitamin E at a high dose as an anti-ferroptosis drug and not just a supplement for COVID-19 treatment. Appl. Biochem. Biotechnol. 2022, 3, 601–619. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.A.; van Lieshout, L.E.; van den Heuvel, E.G.; Matthys, C.; Péter, S.; de Groot, L.C. Conventional foods, followed by dietary supplements and fortified foods, are the key sources of vitamin D, vitamin B6, and selenium intake in Dutch participants of the NU-AGE study. Nutr. Res. 2016, 36, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Bassiouni, W.; Sosnowski, D.K.; Seubert, J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021, 219, 107703. [Google Scholar] [CrossRef]
- Pradelli, L.; Mayer, K.; Klek, S.; Omar Alsaleh, A.J.; Clark, R.A.; Rosenthal, M.D.; Axel, R.; Heller, M.D.; Maurizio Muscaritoli, M.D. ω-3 fatty-acid enriched parenteral nutrition in hospitalized patients: Systematic review with meta-analysis and trial sequential analysis. J. Parenter. Enter. Nutr. 2020, 44, 44–57. [Google Scholar] [CrossRef]
- Bistrian, B.R. Parenteral fish-oil emulsions in critically Ill COVID-19 emulsions. J. Parenter. Enter. Nutr. 2020, 44, 1168. [Google Scholar] [CrossRef]
- Baral, P.K.; Amin, M.T.; Rashid, M.; Or, M.; Hossain, M.S. Assessment of Polyunsaturated Fatty Acids on COVID-19-Associated Risk Reduction. Rev. Bras. Farmacogn. 2022, 32, 50–64. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be well: A potential role for vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef]
- Tang, C.-F.; Ding, H.; Jiao, R.-Q.; Wu, X.-X.; Kong, L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886, 173546. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of current clinical trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox. Biol. 2020, 37, 101715. [Google Scholar] [CrossRef] [PubMed]
- Bhar, A. Is It Possible to Ensure COVID19 Vaccine Supply by Using Plants? Springer: Berlin/Heidelberg, Germany, 2021; pp. 137–141. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Márquez-Escobar, V.A.; González-Ortega, O.; Nieto-Gómez, R.; Arévalo-Villalobos, J.I. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines 2020, 8, 183. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-based vaccines against viruses. Virol. J. 2014, 11, 205. [Google Scholar] [CrossRef]
- Mahmood, N.; Nasir, S.B.; Hefferon, K. Plant-based drugs and vaccines for COVID-19. Vaccines 2021, 9, 15. [Google Scholar] [CrossRef]
- Dhama, K.; Natesan, S.; Iqbal Yatoo, M.; Patel, S.K.; Tiwari, R.; Saxena, S.K.; Harapan, H. Plant-based vaccines and antibodies to combat COVID-19: Current status and prospects. Hum. Vaccines Immunother. 2020, 16, 2913–2920. [Google Scholar] [CrossRef]
- Yonesi, M.; Rezazadeh, A. Plants as a prospective source of natural anti-viral compounds and oral vaccines against COVID-19 coronavirus. Preprints 2020, 2020040321. [Google Scholar] [CrossRef]
- Maharjan, P.M.; Choe, S. Plant-based COVID-19 vaccines: Current status, design, and development strategies of candidate vaccines. Vaccines 2021, 9, 992. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Siriwattananon, K.; Malla, A.; Phoolcharoen, W. Potential for developing plant-derived candidate vaccines and biologics against emerging coronavirus infections. Pathogens 2021, 10, 1051. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, D.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.U.; Kadiresen, K.; Gan, W.C.; Ling, A.P.K. Current updates and research on plant-based vaccines for coronavirus disease 2019. Clin. Exp. Vaccine Res. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).