Beyond the BMI: Validity and Practicality of Postpartum Body Composition Assessment Methods during Lactation: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Identifying the Research Question
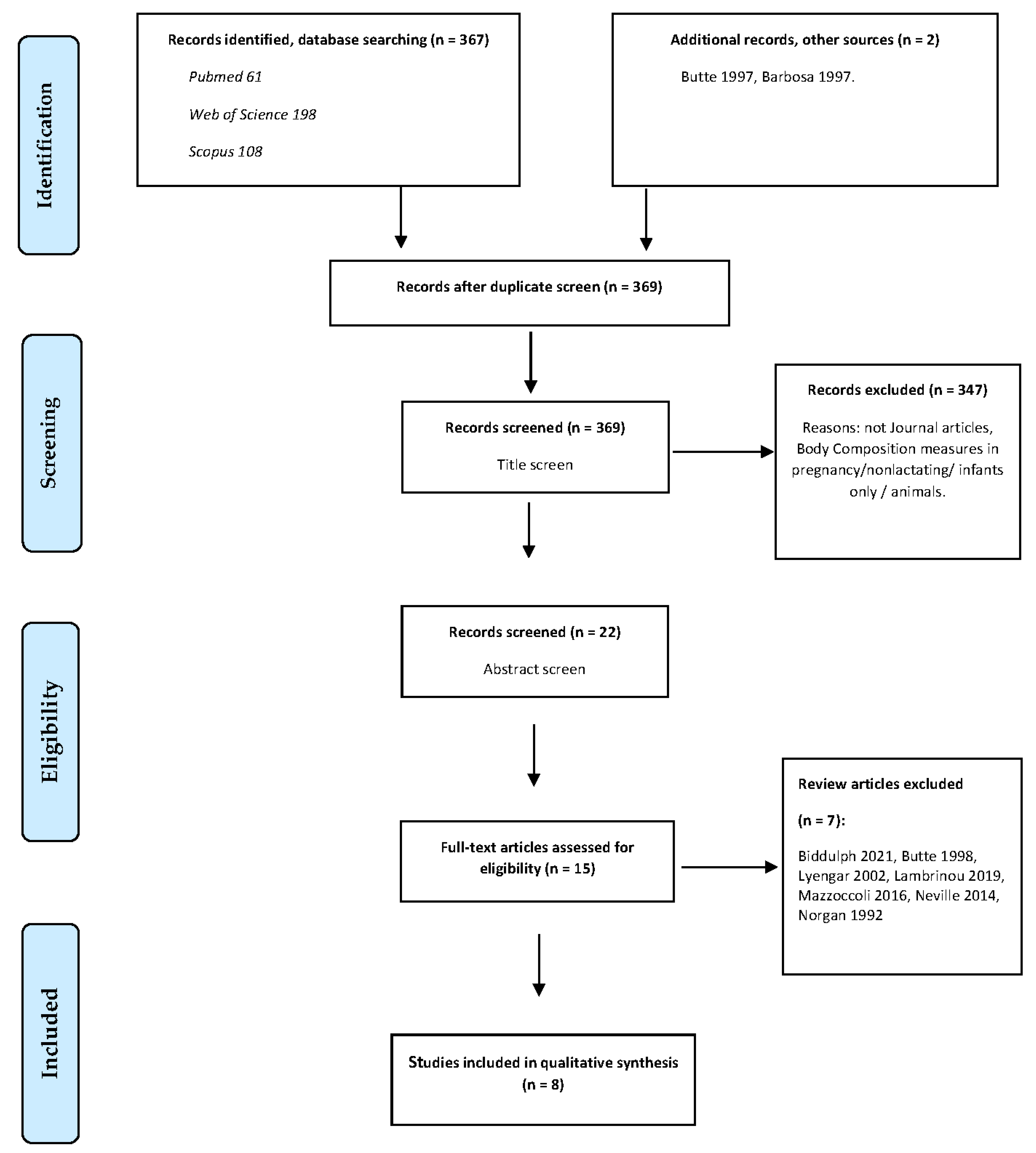
2.3. Search Strategy and Eligibility Criteria
3. Results
3.1. Synthesis
3.2. Analysis of Methodologies
3.2.1. Study Design and Sample Characteristics
3.2.2. Anthropometric Assessment Methods
3.2.3. Two-Compartment Models and Techniques
Isotope Dilution (Hydrometry)
Bioimpedance Analysis (BIA)
Dual-Energy X-ray Absorptiometry (DEXA/DXA)
Densitometry
Other Techniques
3.2.4. Multicompartment Models of Body Composition
3.2.5. Consideration of Confounders
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gila-Díaz, A.; Díaz-Rullo Alcántara, N.; Carrillo, G.H.; Singh, P.; Arribas, S.M.; Ramiro-Cortijo, D. Multidimensional Approach to Assess Nutrition and Lifestyle in Breastfeeding Women during the First Month of Lactation. Nutrients 2021, 13, 1766. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M. Body Composition Changes during Lactation Are Highly Variable among Women. J. Nutr. 1998, 128, 381S–385S. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F. Pregnancy and lactation: Physiological adjustments, nutritional requirements and the role of dietary supplements. J. Nutr. 2003, 133, 1997S–2002S. [Google Scholar] [CrossRef] [PubMed]
- Medoua, G.N.; Nana, E.S.; Essa’a, V.J.; Ntsama, P.M.; Matchawe, C.; Rikong, H.A.; Oyono, J.L.E. Body composition of Cameroonian lactating women determined by anthropometry, bioelectrical impedance, and deuterium dilution. Nutrition 2011, 27, 414–419. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Rich-Edwards, J.W. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef]
- Widen, E.M.; Gallagher, D. Body composition changes in pregnancy: Measurement, predictors and outcomes. Eur. J. Clin. Nutr. 2014, 68, 643–652. [Google Scholar] [CrossRef]
- Gunderson, E.P. Childbearing and obesity in women: Weight before, during, and after pregnancy. Obstet. Gynecol. Clin. N. Am. 2009, 36, 317–332. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Abrams, B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol. Rev. 1999, 21, 261–275. [Google Scholar] [CrossRef]
- Makama, M.; Skouteris, H.; Moran, L.J.; Lim, S. Reducing Postpartum Weight Retention: A Review of the Implementation Challenges of Postpartum Lifestyle Interventions. J. Clin. Med. 2021, 10, 1891. [Google Scholar] [CrossRef]
- Hatsu, I.E.; McDougald, D.M.; Anderson, A.K. Effect of infant feeding on maternal body composition. Int. Breastfeed. J. 2008, 3, 18. [Google Scholar] [CrossRef]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Olędzka, G.; Szostak-Węgierek, D.; Weker, H.; Wesołowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Prescott, S.L. Developmental Origins of Health and Disease: The Role of Human Milk in Preventing Disease in the 21st Century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Fosbøl, M.; Zerahn, B. Contemporary methods of body composition measurement. Clin. Physiol. Funct. Imaging 2015, 35, 81–97. [Google Scholar] [CrossRef]
- Wells, J.C.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child 2006, 91, 612–617. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M.; Ellis, K.J.; Wong, W.W.; Smith, E.O. Changes in fat-free mass and fat mass in postpartum women: A comparison of body composition models. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 874–880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopkinson, J.M.; Butte, N.F.; Ellis, K.J.; Wong, W.W.; Puyau, M.R.; Smith, E.O. Body fat estimation in late pregnancy and early postpartum: Comparison of two-, three-, and four-component models. Am. J. Clin. Nutr. 1997, 65, 432–438. [Google Scholar] [CrossRef]
- Wong, W.W.; Butte, N.F.; Smith, E.O.; Garza, C.; Klein, P.D. Body composition of lactating women determined by anthropometry and deuterium dilution. Br. J. Nutr. 1989, 61, 25–33. [Google Scholar] [CrossRef]
- Papathakis, P.C.; Rollins, N.C.; Brown, K.H.; Bennish, M.L.; Van Loan, M.D. Comparison of isotope dilution with bioimpedance spectroscopy and anthropometry for assessment of body composition in asymptomatic HIV-infected and HIV-uninfected breastfeeding mothers. Am. J. Clin. Nutr. 2005, 82, 538–546. [Google Scholar] [CrossRef][Green Version]
- Ellegård, L.; Bertz, F.; Winkvist, A.; Bosaeus, I.; Brekke, H.K. Body composition in overweight and obese women postpartum: Bioimpedance methods validated by dual energy X-ray absorptiometry and doubly labeled water. Eur. J. Clin. Nutr. 2016, 70, 1181–1188. [Google Scholar] [CrossRef]
- Lof, M.; Forsum, E. Evaluation of bioimpedance spectroscopy for measurements of body water distribution in healthy women before, during, and after pregnancy. J. Appl. Physiol. 2004, 96, 967–973. [Google Scholar] [CrossRef]
- Sohlström, A.; Forsum, E. Changes in total body fat during the human reproductive cycle as assessed by magnetic resonance imaging, body water dilution, and skinfold thickness: A comparison of methods. Am. J. Clin. Nutr. 1997, 66, 1315–1322. [Google Scholar] [CrossRef]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Lunn, P.G. Breast-feeding patterns, maternal milk output and lactational infecundity. J. Biosoc. Sci. 1992, 24, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J. Human Body Composition: In Vivo Methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Marlatt, K.L.; Altazan, A.D.; Redman, L.M. Advances in assessing body composition during pregnancy. Eur. J. Clin. Nutr. 2018, 72, 645–656. [Google Scholar] [CrossRef]
- Alex, A.; Bhandary, E.; McGuire, K.P. Anatomy and Physiology of the Breast during Pregnancy and Lactation. In Diseases of the Breast during Pregnancy and Lactation; Alipour, S., Omranipour, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–7. [Google Scholar] [CrossRef]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Wang, J.; Gallagher, D.; Thornton, J.C.; Yu, W.; Horlick, M.; Pi-Sunyer, F.X. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. Am. J. Clin. Nutr. 2006, 83, 809–816. [Google Scholar] [CrossRef]
- Wells, J.C.; Fuller, N.J.; Dewit, O.; Fewtrell, M.S.; Elia, M.; Cole, T.J. Four-component model of body composition in children: Density and hydration of fat-free mass and comparison with simpler models. Am. J. Clin. Nutr. 1999, 69, 904–912. [Google Scholar] [CrossRef]
- Mueller, W. Human Body Composition: Growth, Aging, Nutrition and Activity. By Gilbert B. Forbes. New York: Springer-Verlag New York Inc. 1987. vii + 350 pp., figures, tables, index. $66.00 (cloth). Am. J. Phys. Anthropol. 1988, 76, 559. [Google Scholar] [CrossRef]
- Withers, R.T.; LaForgia, J.; Pillans, R.K.; Shipp, N.J.; Chatterton, B.E.; Schultz, C.G.; Leaney, F. Comparisons of two-, three-, and four-compartment models of body composition analysis in men and women. J. Appl. Physiol. 1998, 85, 238–245. [Google Scholar] [CrossRef]
- Withers, R.T.; Laforgia, J.; Heymsfield, S.B. Critical appraisal of the estimation of body composition via two-, three-, and four-compartment models. Am. J. Hum. Biol. 1999, 11, 175–185. [Google Scholar] [CrossRef]
- Papathakis, P.C.; Loan, M.D.V.; Rollins, N.C.; Chantry, C.J.; Bennish, M.L.; Brown, K.H. Body Composition Changes During Lactation in HIV-Infected and HIV-Uninfected South African Women. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 43, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Widen, E.M.; Collins, S.M.; Khan, H.; Biribawa, C.; Acidri, D.; Achoko, W.; Achola, H.; Ghosh, S.; Griffiths, J.K.; Young, S.L. Food insecurity, but not HIV-infection status, is associated with adverse changes in body composition during lactation in Ugandan women of mixed HIV status. Am. J. Clin. Nutr. 2017, 105, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.W.; Chan, G.M.; Moyer-Mileur, L. Postpartum body composition changes in lactating and non-lactating primiparas. Nutrition 1999, 15, 481–484. [Google Scholar] [CrossRef]
- Glickman, S.G.; Marn, C.S.; Supiano, M.A.; Dengel, D.R. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J. Appl. Physiol. 2004, 97, 509–514. [Google Scholar] [CrossRef]
- Haarbo, J.; Gotfredsen, A.; Hassager, C.; Christiansen, C. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin. Physiol. 1991, 11, 331–341. [Google Scholar] [CrossRef]
- LaForgia, J.; Dollman, J.; Dale, M.J.; Withers, R.T.; Hill, A.M. Validation of DXA body composition estimates in obese men and women. Obesity 2009, 17, 821–826. [Google Scholar] [CrossRef]
- Tremblay, E.; Thérasse, E.; Thomassin-Naggara, I.; Trop, I. Quality Initiatives: Guidelines for Use of Medical Imaging during Pregnancy and Lactation. Radiographics 2012, 32, 897–911. [Google Scholar] [CrossRef]
- Rose, G.L.; Farley, M.J.; Ward, L.C.; Slater, G.J.; Skinner, T.L.; Keating, S.E.; Schaumberg, M.A. Accuracy of body composition measurement techniques across the age-span. Appl. Physiol. Nutr. Metab. 2022, 47, 482–494. [Google Scholar] [CrossRef]
- Xiao, J.; Purcell, S.A.; Prado, C.M.; Gonzalez, M.C. Fat mass to fat-free mass ratio reference values from NHANES III using bioelectrical impedance analysis. Clin. Nutr. 2018, 37, 2284–2287. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Tie, W.; Lai, C.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in human milk and body composition of term infants during the first 12 months of lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of Infant and Maternal Factors on Energy and Macronutrient Composition of Human Milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef] [PubMed]
| Reference | Body Composition (BC) Assessment Methods (Maternal) |
|---|---|
| Butte, N.F., et al. (1997) [23] | Two-component (TBW, UWW, SF, TBK, DXA/TOBEC), three-component (TBW and UWW -Fuller 3, Siri 3), four-component models (TBW, UWW, and BMC-Fuller 4). TBW by deuterium oxide dilution. |
| Ellegård, L., et al. (2016) [27] | BW, height, BIS, MFBIA, DXA, and TBW via DLW. |
| Hopkinson, J.M., et al. (1997) [24] | TBW, TBK, body density, BMC by deuterium dilution, whole-body potassium counting, hydrodensitometry, and DXA (postpartum only). |
| Lof, M., et al. (2004) [28] | BIS (supine), reference isotope, and bromide dilution (only 2H2O was given at the postpartum measurement). |
| Medoua, G.N., et al. (2011) [4] | Anthropometry (triceps, biceps, subscapular, suprailiac sites), BIA (supine), reference method: deuterium oxide dilution. |
| Papathakis, P.C., et al. (2005) [26] | TBW using BIS and 2H2O to measure FFM and FM. Anthropometric measurements: BW, height, BMI, MUAC and four skinfold thicknesses (triceps, biceps, subscapular, and suprailiac). |
| Sohlström, A., et al. (1997) [29] | TBW by isotope dilution, MRI (30 transaxial images over the complete body except the head, hands, and feet), biceps, triceps, subscapular, and suprailiac SKF thicknesses. |
| Wong, W.W., et al. (1989) [25] | Anthropometry (triceps, biceps, and subscapular SKF) and deuterium dilution (HM, urine, saliva, breath). |
| Reference | Study Objective(s) | Study Population | Study Design | 2C Body Composition (BC) Assessment Methods (Maternal) | 3C Body Composition (BC) Assessment Methods (Maternal) | 4C or Organ/Tissue Models for Body Composition (BC) Assessment Methods (Maternal) | Timing of BC Measurements | Method Comparison | Comparative Validity, Reliability, and/or Appropriateness | Relevant Findings | Strengths | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Butte, N.F., et al. (1997) [23] | To compare postpartum changes in BC by two-, three-, and four-compartment models and test for an effect of pregnancy or lactation on hydration, density, or potassium content of FFM. | N = 35 healthy postpartum women, lactating and nonlactating women (29 Caucasian, 2 African American, 4 Hispanic), USA. | FFM and FM estimated by nine BC models at three time points (3, 6 and 12 months postpartum) were compared using repeated measures ANOVA, as were changes in FFM and FM at two time intervals (3–6 months and 6–12 months postpartum). | TBW, UWW, SF, TBK, DXA/TOBEC. | TBW and UWW –Fuller 3, Siri 3. | TBW, UWW and BMC –Fuller 4; TBW by deuterium dilution. | 3, 6 and 12 months postpartum. | Noted systematic differences among BC models in FFM and FM measures, but not in terms of longitudinal changes in FFM and FM. | No effect of pregnancy/lactation on the postpartum composition of FFM by 3 months, so two-component models are acceptable for use in postpartum women. | Pregnancy-induced changes in the hydration, density and potassium content of FFM were not evident by 3 months postpartum. Changes in FFM and FM did not differ significantly between models. The rank order from the highest to lowest estimate of FFM was TOBEC, TBW, Fuller 3, Siri 3, Fuller 4, UWW, SF, TBK, and DXA. | Numerous models and methods used: systematic differences noted between the models. | Not all lactating: breastfeeding prevalence was 27/35, 22/35, and 9/35 at 3, 6 and 12 months, respectively. |
| Ellegård, L., et al. (2016) [27] | To validate BIS and MFBIA with the reference methods DXA and DLW, and to assess BC in overweight/obese women postpartum. | n = 70 postpartum women, Caucasian, 35 overweight and 35 obese, Sweden. | Repeated measurements of BC (FM, FFM, skeletal muscle mass, and TBW) using reference methods and simple methodology (both cross-sectional and longitudinal). | BW, height, BIS, MFBIA, and TBW via DLW (isotope dilution of both D2O and H2O). | DXA | N/A | 3 (baseline), 6 (12-week intervention) and 15 months postpartum (1-year follow-up). | Both BIS and, even more so, MFBIA underestimated FM and overestimated FFM and TBW. | Most accurate and precise TBW compared with DLW was obtained using 73% of FFM as assessed by DXA. | BIS underestimates FM, but accurately estimates muscle mass and changes in BC; MFBIA underestimates FM and overestimates TBW. Impedance devices underestimate FM in overweight and obese women. | Used accepted reference methods. Three repeated measures. | Systematic bias introduced via body positioning (impedance increases in supine over time). Bioimpedance devices used are too imprecise for individual evaluation. |
| Hopkinson, J.M., et al. (1997) [24] | To evaluate 2C and 3C models in the early-postpartum period. | n = 56 healthy women (38 lactating, 18 nonlactating), aged 19–35 years, USA. | Used a four-component model as a criterion for evaluating two- and three-component models. | TBW, hydrodensitometry. | DXA (postpartum only). | TBW, body density, BMC by deuterium dilution, whole-body potassium count (TBK). | Twice, at 36 ± 1 week gestation and 15 ± 2 days postpartum. | FM by TBK may differ by up to 6 kg from 4C model at 2 weeks postpartum. Use of standard 2C models to estimate FM results in significant error both at 2 weeks postpartum. | 3C model compared favorably with 4C Fuller et al.’s model for estimation of mean and individual FM and change in FM. | At 2 wk. postpartum, FFM hydration and density had not returned to nonpregnant values, and differed between lactating (higher) and nonlactating women (p < 0.05). Standard TBW and body density estimates of FM differed from 4C estimates at both time points (latter was higher, p < 0.005). | Used a four-component model as a criterion for evaluating two- and three-component models. | Black women have around 7–8% more bone mineral and 4% more non-osseous mineral than white women, but this sample did not provide sufficient power to address the influence of ethnicity on intermethod differences. |
| Lof, M., et al. (2004) [28] | To evaluate BIS measurements of body water distribution in healthy women before, during, and after pregnancy. | n = 21 healthy women with healthy pregnancies and deliveries, lactating, Sweden. | Methodological study comparing BIS measures of ECW, ICW, and TBW, with reference methods, over the reproductive cycle. | BIS (supine), reference isotope and bromide dilution (only 2H2O was given at the postpartum measurement). | N/A | N/A | Before pregnancy, 14, and 32 weeks gestation, 2 weeks postpartum. | Average estimates of ICW by BIS were in good agreement with the corresponding reference data (not individual). | BIS (2C) may be useful for estimating average ICW, as well as changes in ICW, in groups of women during reproduction. | Postpartum average ICW, ECW, and TBW, estimated by BIS, were in agreement with reference data. | Longitudinal measures with reference methods. | Wrist–ankle measurements of resistance in BIS, assumes that the body consist of five cylinders. |
| Medoua, G.N., et al. (2011) [4] | To compare BC estimates using deuterium dilution, MF-BIA, and SKF techniques in lactating women. | n = 44 lactating women, aged 19 to 42 years, BMI 26.94 ± 3.61 kg/m2, Africa. | Comparison of the results of BC from the deuterium dilution technique with more convenient MFBIA and skinfold thickness methods. | Anthropometry (triceps, biceps, subscapular, suprailiac sites), BIA (supine), reference method: deuterium oxide dilution. | N/A | N/A | 2.63 ± 1.31 months postpartum. | Inappropriate to use anthropometry or BIA equations to predict body composition in a population different from the population in which these equations were developed. | BC was affected (p < 0.05) by the technique used to measure it. Main factor implicated in the lack of agreement between BIA-predicted equations is lactation. | Anthropometric and BIA-based predictive equations overestimated BF by 2.7 to 11.7 kg; and underestimated TBW and FFM. Higher biases when using Black-specific equations. | Bland and Altman tests to determine bias and limits of agreement between values predicted by equations and measured by deuterium oxide dilution. | Small sample, no control group of healthy non-lactating women, use of the hydration factor 0.73. |
| Papathakis, P.C., et al. (2005) [26] | To determine the validity of BIS and anthropometric measurements to measure BC compared to the stable isotope dilution method. To describe the BC of HIV-infected lactating women. | n = 20 HIV-infected and 48 HIV-uninfected lactating women, 15–40 (median 24) years old, rural/low SES, African (Zulu), South Africa. | Compared the ability of BIS and anthropometry to determine TBW with isotope dilution (2H2O) to determine FM and FFM in HIV+ and HIV- breastfeeding women. | TBW using BIS and 2H2O to measure FFM and FM. Anthropometric measurements: BW, ht, BMI, MUAC, and four skinfold thicknesses (triceps, biceps, subscapular, and suprailiac). | N/A | N/A | Once, at 10 weeks postpartum. | Measurements determined by BIS correlated with 2H2O. BMI, MUAC, and skinfold-thickness measurements correlated strongly with FM measured by 2H2O; FFM only in HIV- mothers. | BIS comparable to reference 2H2O method. BMI and MUAC are useful in predicting FM, but are not valid measures of FFM in HIV+ mothers. | TBW by BIS was 5–6% greater than 2H2O method but FM or FFM did not differ significantly by method. Difference deemed acceptable by the authors. | Used the stable isotope deuterium oxide (2H2O) is a reference technique for measuring TBW. | Only assessed HIV-infected women without severe immune suppression. |
| Sohlström, A., et al. (1997) [29] | To compare changes in total BF assessed by MRI, body water dilution, and skinfold thickness in postpartum women. | n = 16 healthy postpartum women, lactating, Sweden. | Changes in total BF during the human reproductive cycle as assessed by MRI, body water dilution, and SKF thickness: a comparison of methods. | TBW by isotope dilution; biceps, triceps, subscapular, and suprailiac SKF thicknesses. | N/A | MRI (30 transaxial images over the complete body except the head, hands, and feet). | Before pregnancy and at 5–10 days and 2, 6, and 12 months postpartum. | Estimates of changes in BF by isotope dilution may be unreliable and invalid, as the degree of hydration of FFM may change over the course of the postpartum period in women. | Risk for bias when changes in TBF during reproduction are estimated by SKF-thickness technique and isotope dilution. | Changes in the degree of hydration of FFM may occur after delivery. SKF method tended to overestimate fat retention compared with MRI, and underestimate the amount of mobilized fat. | Results of BF from the MRI technique are more valid than changes estimated with use of body water or SKF techniques. | SKF technique: during both pregnancy and lactation, a redistribution of subcutaneous adipose tissue may occur. |
| Wong, W.W., et al. (1989) [25] | To compare estimations of BF, FFM, and TBW of lactating women by anthropometric and deuterium dilution methods. | n = 10 lactating women, aged 28.4 ± 4.2 years, USA. | Comparison of anthropometric equations and deuterium dilution method in calculating postpartum BC. | Anthropometry (triceps, biceps and subscapular SKF) and deuterium dilution (HM, urine, saliva, breath). | N/A | N/A | Once, at 3.4 ± 1.3 months postpartum. | Deuterium dilution method involves certain assumptions and errors, but is more direct and precise. | Difficulty in obtaining accurate and reproducible skinfold thickness measurements in the suprailiac regions of post-partum women, so excluded. | No significant differences in mean BF, FFM, or TBW between anthropometry and deuterium dilution; however, wide 95% CI, so not applicable to individuals. | Technical aspects of the deuterium dilution method also investigated. | Deuterium dilution overestimates TBW to the degree that deuterium exchanges with non-aqueous hydrogens. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddulph, C.; Holmes, M.; Kuballa, A.; Carter, R.J.; Maher, J. Beyond the BMI: Validity and Practicality of Postpartum Body Composition Assessment Methods during Lactation: A Scoping Review. Nutrients 2022, 14, 2197. https://doi.org/10.3390/nu14112197
Biddulph C, Holmes M, Kuballa A, Carter RJ, Maher J. Beyond the BMI: Validity and Practicality of Postpartum Body Composition Assessment Methods during Lactation: A Scoping Review. Nutrients. 2022; 14(11):2197. https://doi.org/10.3390/nu14112197
Chicago/Turabian StyleBiddulph, Caren, Mark Holmes, Anna Kuballa, Roger J. Carter, and Judith Maher. 2022. "Beyond the BMI: Validity and Practicality of Postpartum Body Composition Assessment Methods during Lactation: A Scoping Review" Nutrients 14, no. 11: 2197. https://doi.org/10.3390/nu14112197
APA StyleBiddulph, C., Holmes, M., Kuballa, A., Carter, R. J., & Maher, J. (2022). Beyond the BMI: Validity and Practicality of Postpartum Body Composition Assessment Methods during Lactation: A Scoping Review. Nutrients, 14(11), 2197. https://doi.org/10.3390/nu14112197






