Vitamin D and Pancreatitis: A Narrative Review of Current Evidence
Abstract
:1. Introduction
2. Search Strategy
3. Vitamin D Metabolism and Its Biological Actions in Basic Studies
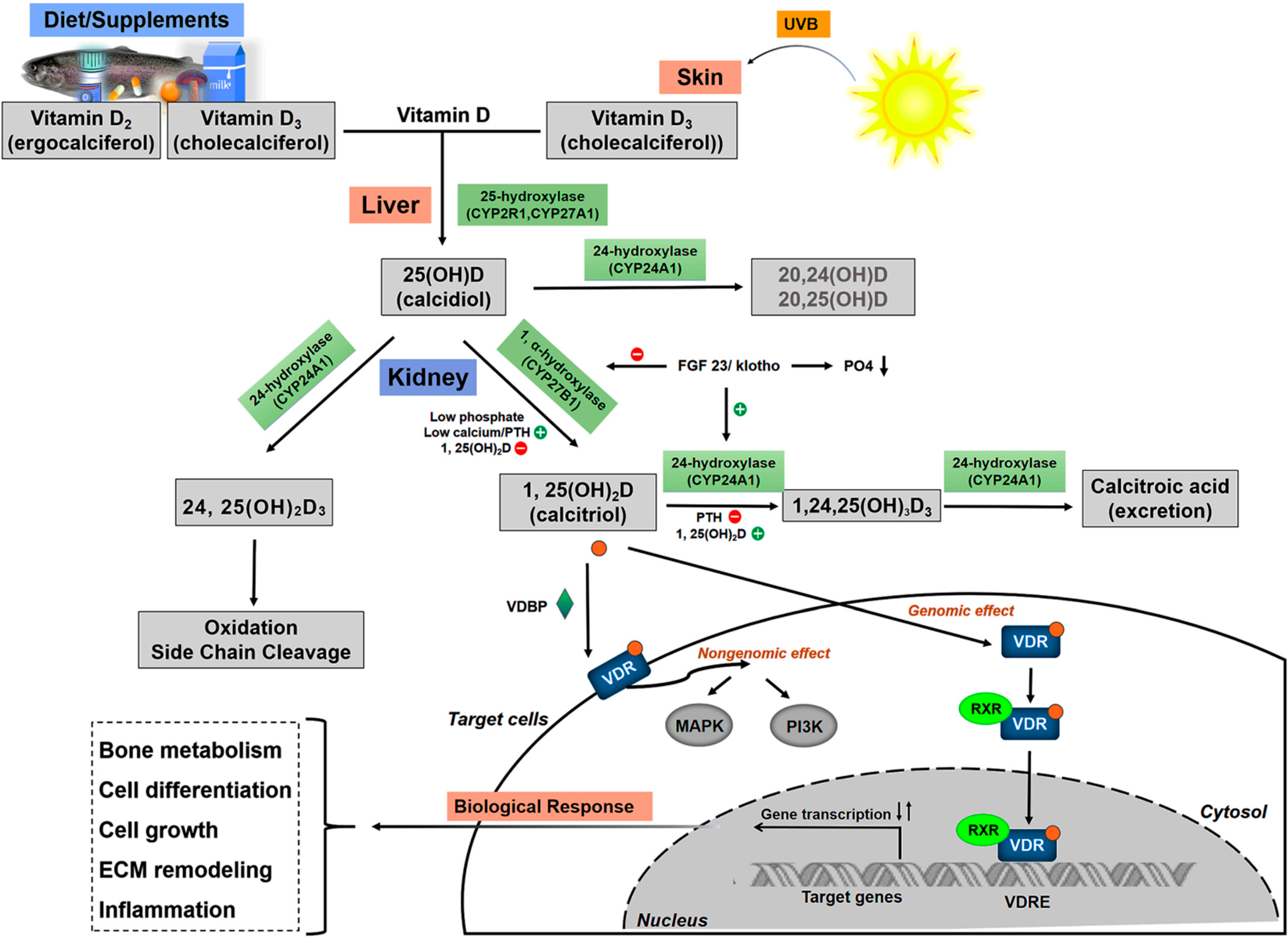
3.1. Vitamin D Metabolism
3.2. Biological Action of Vitamin D in Pancreatic Cells
4. Vitamin D and Pancreatitis in Clinical Studies
4.1. Vitamin D and Acute Pancreatitis
4.1.1. Vitamin D Status in Patients with AP
4.1.2. Imbalance of Vitamin D Metabolism as a Risk Factor for AP
Vitamin D Deficiency and Hypercalcemia-Mediated AP
Vitamin D3 Poisoning-Induced Pancreatitis
4.1.3. Vitamin D Disorders Affect the Severity of AP
Vitamin D Levels Affect the Severity of AP
Gene Polymorphisms
4.2. Vitamin D and Chronic Pancreatitis
4.2.1. The Prevalence of Vitamin D Deficiency/Insufficiency in Patients with CP
4.2.2. Vitamin D Deficiency/Insufficiency Associated with the Severity of Exocrine Function
4.2.3. CP-Related Osteopathy
4.2.4. CP-Related Diabetes
5. Vitamin D Supplementation and Its Analogs’ Potential in Pancreatitis
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Christakos, S.; Hewison, M.; Gardner, D.G.; Wagner, C.L.; Sergeev, I.N.; Rutten, E.; Pittas, A.G.; Boland, R.; Ferrucci, L.; Bikle, D.D. Vitamin D: Beyond bone. Ann. N. Y. Acad. Sci. 2013, 1287, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Society, E. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Sahota, O. Understanding vitamin D deficiency. Age Ageing 2014, 43, 589–591. [Google Scholar] [CrossRef] [Green Version]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Mann, S.T.; Stracke, H.; Lange, U.; Klor, H.U.; Teichmann, J. Vitamin D3 in patients with various grades of chronic pancreatitis, according to morphological and functional criteria of the pancreas. Dig. Dis. Sci. 2003, 48, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kim, J.W.; Lee, K.J. Vitamin D deficiency predicts severe acute pancreatitis. United Eur. Gastroenterol. J. 2019, 7, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef] [PubMed]
- Frulloni, L.; Falconi, M.; Gabbrielli, A.; Gaia, E.; Graziani, R.; Pezzilli, R.; Uomo, G.; Andriulli, A.; Balzano, G.; Benini, L.; et al. Italian consensus guidelines for chronic pancreatitis. Dig. Liver Dis. 2010, 42 (Suppl. 6), S381–S406. [Google Scholar] [CrossRef]
- Martínez, J.; Abad-González, A.; Aparicio, J.R.; Aparisi, L.; Boadas, J.; Boix, E.; de Las Heras, G.; Domínguez-Muñoz, E.; Farré, A.; Fernández-Cruz, L.; et al. The Spanish Pancreatic Club recommendations for the diagnosis and treatment of chronic pancreatitis: Part 1 (diagnosis). Pancreatology 2013, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Munoz, J.E.; Drewes, A.M.; Lindkvist, B.; Ewald, N.; Czakó, L.; Rosendahl, J.; Löhr, J.M. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology 2018, 18, 847–854. [Google Scholar] [CrossRef]
- Arvanitakis, M.; Ockenga, J.; Bezmarevic, M.; Gianotti, L.; Krznarić, Ž.; Lobo, D.; Löser, C.; Madl, C.; Meier, R.; Phillips, M.; et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin. Nutr. 2020, 39, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Arcot, J.; Cunningham, J.; Greenfield, H.; Hsu, J.; Padula, D.; Strobel, N.; Fraser, D.R. New data for vitamin D in Australian foods of animal origin: Impact on estimates of national adult vitamin D intakes in 1995 and 2011–13. Asia Pac. J. Clin. Nutr. 2015, 24, 464–471. [Google Scholar]
- Liu, J.; Greenfield, H.; Strobel, N.; Fraser, D.R. The influence of latitude on the concentration of vitamin D3 and 25-hydroxy-vitamin D3 in Australian red meat. Food Chem. 2013, 140, 432–435. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [PubMed] [Green Version]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Rafi, L.; Mitschele, T.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003, 164, 239–246. [Google Scholar] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Wallbaum, P.; Rohde, S.; Ehlers, L.; Lange, F.; Hohn, A.; Bergner, C.; Schwarzenböck, S.M.; Krause, B.J.; Jaster, R. Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J. Gastroenterol. 2018, 24, 170–178. [Google Scholar] [CrossRef]
- Bläuer, M.; Sand, J.; Laukkarinen, J. Physiological and clinically attainable concentrations of 1,25-dihydroxyvitamin D3 suppress proliferation and extracellular matrix protein expression in mouse pancreatic stellate cells. Pancreatology 2015, 15, 366–371. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.S.; Wang, C.; Han, X.L.; Du, J.J.; Li, Y.Y.; Zhang, C. Design, synthesis and biological evaluation of non-secosteriodal vitamin D receptor ligand bearing double side chain for the treatment of chronic pancreatitis. Eur. J. Med. Chem. 2018, 146, 541–553. [Google Scholar] [CrossRef]
- Clark, S.A.; Stumpf, W.E.; Sar, M.; DeLuca, H.F.; Tanaka, Y. Target cells for 1,25 dihydroxyvitamin D3 in the pancreas. Cell Tissue Res. 1980, 209, 515–520. [Google Scholar] [CrossRef]
- Hummel, D.; Aggarwal, A.; Borka, K.; Bajna, E.; Kállay, E.; Horváth, H.C. The vitamin D system is deregulated in pancreatic diseases. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Shang, F.; Zhu, Y.; Sun, Y.; Sudi, R.S. Modulation of VDR and Cell Cycle-Related Proteins by Vitamin D in Normal Pancreatic Cells and Poorly Differentiated Metastatic Pancreatic Cancer Cells. Nutr. Cancer 2019, 71, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.; Ma, M.T.; Leung, K.K.; Leung, P.S. Vitamin D and vitamin A receptor expression and the proliferative effects of ligand activation of these receptors on the development of pancreatic progenitor cells derived from human fetal pancreas. Stem Cell Rev. Rep. 2011, 7, 53–63. [Google Scholar] [CrossRef]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Kumar, R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am. J. Physiol. 1994, 267, E356–E360. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.A.; Sommer, S.; Sussman, C.R.; Grande, J.P.; Kumar, R. Expression and regulation of the vitamin D receptor in the zebrafish, Danio rerio. J. Bone Miner. Res. 2008, 23, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhou, Z.G.; You, S.; Huang, G.; Lin, J.; Yang, L.; Li, X.; Zhou, W.D.; Chao, C. Modulation of monocyte hyperresponsiveness to TLR ligands by 1,25-dihydroxy-vitamin D3 from LADA and T2DM. Diabetes Res. Clin. Pract. 2009, 83, 208–214. [Google Scholar] [CrossRef]
- Dai, Z.H.; Tan, B.; Yang, H.; Wang, O.; Qian, J.M.; Lv, H. 1,25-hydroxyvitamin D relieves colitis in rats via down-regulation of toll-like receptor 9 expression. Croat. Med. J. 2015, 56, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Bang, U.C.; Novovic, S.; Andersen, A.M.; Fenger, M.; Hansen, M.B.; Jensen, J.E. Variations in serum 25-hydroxyvitamin D during acute pancreatitis: An exploratory longitudinal study. Endocr. Res. 2011, 36, 135–141. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [Green Version]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Scientific Advisory Committee on Nutrition. Vitamin D and Health. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 16 March 2022).
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [Green Version]
- Arabi, A.; El Rassi, R.; El-Hajj Fuleihan, G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat. Rev. Endocrinol. 2010, 6, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Sarafin, K.; Durazo-Arvizu, R.; Tian, L.; Phinney, K.W.; Tai, S.; Camara, J.E.; Merkel, J.; Green, E.; Sempos, C.T.; Brooks, S.P. Standardizing 25-hydroxyvitamin D values from the Canadian Health Measures Survey. Am. J. Clin. Nutr. 2015, 102, 1044–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiangpeng, L.; Zengli, Z.; Honghong, Z.; Hanmin, Z.; Jianlie, Z.; Qiren, H.; Zhixue, W.; Liang, W.; Zhonghou, L. Application Guideline for Vitamin D and Bone Health in Adult Chinese (2014 Standard Edition). Chin. J. Osteoporos. 2014, 20, 1011–1030. [Google Scholar]
- Cates, M.C.; Singh, S.M.; Peick, A.L.; Harvey, M.H.; Reber, H.A. Acute hypercalcemia, pancreatic duct permeability, and pancreatitis in cats. Surgery 1988, 104, 137–141. [Google Scholar]
- Kim, D.I.; Kim, H.; Son, P.; Kang, J.H.; Kang, B.T.; Yang, M.P. Serum 25-hydroxyvitamin D concentrations in dogs with suspected acute pancreatitis. J. Vet. Med. Sci. 2017, 79, 1366–1373. [Google Scholar] [CrossRef] [Green Version]
- Abou Saleh, M.; Alkhayyat, M.; Mansoor, E.; Khoudari, G.; Simons-Linares, C.R.; Vargo, J.; Chahal, P.; Stevens, T. The Risk of Vitamin D Deficiency, Osteoporosis, and Fractures in Acute Pancreatitis. Pancreas 2020, 49, 629–633. [Google Scholar] [CrossRef] [PubMed]
- De Waele, B.; Vierendeels, T.; Willems, G. Vitamin status in patients with acute pancreatitis. Clin. Nutr. 1992, 11, 83–86. [Google Scholar] [CrossRef]
- Bang, U.C.; Brandt, L.; Benfield, T.; Jensen, J.-E.B. Changes in 1,25-Dihydroxyvitamin D and 25-Hydroxyvitamin D Are Associated with Maturation of Regulatory T Lymphocytes in Patients with Chronic Pancreatitis: A Randomized Controlled Trial. Pancreas 2012, 41, 1213–1218. [Google Scholar] [CrossRef]
- Leerhoy, B.; Shabanzadeh, D.M.; Nordholm-Carstensen, A.; Novovic, S.; Hansen, M.B.; Jorgensen, L.N. Pancreatic function following post-endoscopic retrograde cholangiopancreatography pancreatitis: A controlled cohort study with long-term follow-up. United Eur. Gastroenterol. J. 2018, 6, 586–594. [Google Scholar] [CrossRef]
- Tešić-Rajković, S.; Radovanović-Dinić, B.; Mitić, B.; Dinić-Radović, V.; Jovanović, M. Hyperparathyroidism as a cause of recurrent acute pancreatitis: A case report. Vojnosanit. Pregl. 2016, 73, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Saif, A. Primary hyperparathyroidism presenting with acute pancreatitis and asymptomatic bone involvement. Clin. Cases Miner. Bone Metab. 2015, 12, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.T.; Fehmi, S.M. Hypercalcemic Pancreatitis Triggered by Pregnancy with a CYP24A1 Mutation. Pancreas 2016, 45, e31–e32. [Google Scholar] [CrossRef]
- Woods, G.N.; Saitman, A.; Gao, H.; Clarke, N.J.; Fitzgerald, R.L.; Chi, N.W. A Young Woman with Recurrent Gestational Hypercalcemia and Acute Pancreatitis Caused by CYP24A1 Deficiency. J. Bone Miner. Res. 2016, 31, 1841–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waele, B.D.; Smitz, J.; Willems, G. Recurrent pancreatitis secondary to hypercalcemia following vitamin D poisoning. Pancreas 1989, 4, 378–380. [Google Scholar] [CrossRef]
- Feyles, F.; Mussa, A.; Peiretti, V.; Tessaris, D.; Santanera, A.; Corrias, A.; de Sanctis, L.; Calvo, L. Iatrogenic acute pancreatitis due to hypercalcemia in a child with pseudohypoparathyroidism. J. Pediatr. Endocrinol. Metab. 2014, 27, 149–152. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, S.K.; Mithal, A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin. Endocrinol. 2015, 83, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, C.; Winograd, R.; Koch, A.; Abuzahra, F.; Trautwein, C.; Wasmuth, H.E. Acute necrotic pancreatitis induced by severe hypercalcaemia due to tacalcitol ointment. Br. J. Dermatol. 2007, 156, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.; Aziz, M.; Chandran, J.; Souferi, B.; Berry, R.; Elias, S.; Tabibian, J.H. Vitamin D-Induced Acute Pancreatitis. 2021. Available online: https://journals.lww.com/americantherapeutics/Citation/9000/Vitamin_D_Induced_Acute_Pancreatitis.98086.aspx (accessed on 25 March 2022).
- El-Mahdy, R.I.; Ramadan, H.K.; Mohammed, H.; Ahmed, E.H.; Mokhtar, A.A.; Hosni, A. Impact of the etiology and Vitamin D receptor TaqI rs731236 gene polymorphism on the severity of acute pancreatitis. J. Hepatobiliary Pancreat. Sci. 2020, 27, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Cieslinska, A.; Kostyra, E.; Fiedorowicz, E.; Snarska, J.; Kordulewska, N.; Kiper, K.; Savelkoul, H.F.J. Single Nucleotide Polymorphisms in the Vitamin D Receptor Gene (VDR) May Have an Impact on Acute Pancreatitis (AP) Development: A Prospective Study in Populations of AP Patients and Alcohol-Abuse Controls. Int. J. Mol. Sci. 2018, 19, 1919. [Google Scholar] [CrossRef] [Green Version]
- Skipworth, J.R.; Nijmeijer, R.M.; van Santvoort, H.C.; Besselink, M.G.; Schulz, H.U.; Kivimaki, M.; Kumari, M.; Cooper, J.A.; Acharya, J.; Shankar, A.; et al. The effect of renin angiotensin system genetic variants in acute pancreatitis. Ann. Surg. 2015, 261, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, H.; Wall, B.M.; Cooke, C.R. Osteomalacia and secondary hyperparathyroidism after kidney transplantation: Relationship to vitamin D deficiency. Am. J. Med. Sci. 2007, 333, 58–62. [Google Scholar] [CrossRef]
- Martinez-Moneo, E.; Stigliano, S.; Hedstrom, A.; Kaczka, A.; Malvik, M.; Waldthaler, A.; Maisonneuve, P.; Simon, P.; Capurso, G. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology 2016, 16, 988–994. [Google Scholar] [CrossRef]
- Hoogenboom, S.A.; Lekkerkerker, S.J.; Fockens, P.; Boermeester, M.A.; van Hooft, J.E. Systematic review and meta-analysis on the prevalence of vitamin D deficiency in patients with chronic pancreatitis. Pancreatology 2016, 16, 800–806. [Google Scholar] [CrossRef]
- Olesen, S.S.; Poulsen, J.L.; Vestergaard, P.; Drewes, A.M. Vitamin-D deficiency in patients with chronic pancreatitis—Prevalence and pitfalls. Pancreatology 2017, 17, 22–23. [Google Scholar] [CrossRef]
- Tang, X.Y.; Ru, N.; Li, Q.; Qian, Y.Y.; Sun, H.; Zhu, J.H.; He, L.; Wang, Y.C.; Hu, L.H.; Li, Z.S.; et al. Prevalence and Risk Factors for Osteopathy in Chronic Pancreatitis. Dig. Dis. Sci. 2021, 66, 4008–4016. [Google Scholar] [CrossRef]
- Joker-Jensen, H.; Mathiasen, A.S.; Kohler, M.; Rasmussen, H.H.; Drewes, A.M.; Olesen, S.S. Micronutrient deficits in patients with chronic pancreatitis: Prevalence, risk factors and pitfalls. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Serena, S.; Waldthaler, A.; Martinez-Moneo, E.; Lionetto, L.; Robinson, S.; Malvik, M.; Hedstrom, A.; Kaczka, A.; Scholdei, M.; Haas, S.; et al. Vitamins D and K as Factors Associated with Osteopathy in Chronic Pancreatitis: A Prospective Multicentre Study (P-BONE Study). Clin. Transl. Gastroenterol. 2018, 9, e197. [Google Scholar]
- Min, M.; Patel, B.; Han, S.; Bocelli, L.; Kheder, J.; Vaze, A.; Wassef, W. Exocrine Pancreatic Insufficiency and Malnutrition in Chronic Pancreatitis: Identification, Treatment, and Consequences. Pancreas 2018, 47, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.H.; Sood, A.K.; Manrai, M. Occult metabolic bone disease in chronic pancreatitis. Niger. J. Clin. Pract. 2017, 20, 1122–1126. [Google Scholar] [CrossRef]
- Pezzilli, R.; Melzi d’Eril, G.V.; Barassi, A. Markers of Bone Metabolism in Patients with Chronic Pancreatitis and Pancreatic Ductal Adenocarcinoma. Medicine 2015, 94, e1754. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.; Cahen, D.L.; Koch, A.D.; Braat, H.; Poley, J.W.; Kuipers, E.J.; Bruno, M.J. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology 2013, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, S.; Richter, E.; Klapdor, R. Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Res. 2012, 32, 1991–1998. [Google Scholar]
- Dujsikova, H.; Dite, P.; Tomandl, J.; Sevcikova, A.; Precechtelova, M. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology 2008, 8, 583–586. [Google Scholar] [CrossRef]
- Duggan, S.N.; Purcell, C.; Kilbane, M.; O’Keane, M.; McKenna, M.; Gaffney, P.; Ridgway, P.F.; Boran, G.; Conlon, K.C. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: A case-matched study. Am. J. Gastroenterol. 2015, 110, 336–345. [Google Scholar] [CrossRef]
- Duggan, S.N.; Smyth, N.D.; O’Sullivan, M.; Feehan, S.; Ridgway, P.F.; Conlon, K.C. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr. Clin. Pract. 2014, 29, 348–354. [Google Scholar] [CrossRef]
- Prabhakaran, A.; Bhasin, D.K.; Rana, S.S.; Bhadada, S.K.; Bhansali, A.; Rao, C.; Gupta, R.; Khandelwal, N. Bone mineral metabolism and bone mineral density in alcohol related and idiopathic chronic pancreatitis. Trop. Gastroenterol. 2014, 35, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Duggan, S.N.; O’Sullivan, M.; Hamilton, S.; Feehan, S.M.; Ridgway, P.F.; Conlon, K.C. Patients with Chronic Pancreatitis Are at Increased Risk for Osteoporosis. Pancreas 2012, 41, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Reddy, S.V.; Bhatia, V.; Choudhuri, G.; Singh, R.K.; Singh, N.; Bhatia, E. High prevalence of low bone mineral density in patients with tropical calcific pancreatitis. Pancreas 2011, 40, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, K.; Chacko, A.; Thomas, N.; Selvakumar, R.; George, B.; Paul, T.V.; Seshadri, M.S. Predictors of osteodystrophy in patients with chronic nonalcoholic pancreatitis with or without diabetes. Endocr. Pract. 2011, 17, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.V.; Ramesh, V.; Bhatia, E. Double blind randomized control study of intramuscular vitamin D3 supplementation in tropical calcific pancreatitis. Calcif. Tissue Int. 2013, 93, 48–54. [Google Scholar] [CrossRef]
- Frøkjær, J.B.; Olesen, S.S.; Drewes, A.M. Fibrosis, atrophy, and ductal pathology in chronic pancreatitis are associated with pancreatic function but independent of symptoms. Pancreas 2013, 42, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, J.; Riemann, J.F.; Lange, U. Prevalence of exocrine pancreatic insufficiency in women with obesity syndrome: Assessment by pancreatic fecal elastase 1. ISRN Gastroenterol. 2011, 2011, 951686. [Google Scholar] [CrossRef] [Green Version]
- Teichmann, J.; Mann, S.T.; Stracke, H.; Lange, U.; Hardt, P.D.; Klör, H.U.; Bretzel, R.G. Alterations of vitamin D3 metabolism in young women with various grades of chronic pancreatitis. Eur. J. Med. Res. 2007, 12, 347–350. [Google Scholar] [PubMed]
- Duggan, S.N.; Smyth, N.D.; Murphy, A.; Macnaughton, D.; O’Keefe, S.J.; Conlon, K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Mann, S.T.; Stracke, H.; Lange, U.; Klör, H.U.; Teichmann, J. Alterations of bone mineral density and bone metabolism in patients with various grades of chronic pancreatitis. Metabolism 2003, 52, 579–585. [Google Scholar] [CrossRef]
- Haaber, A.B.; Rosenfalck, A.M.; Hansen, B.; Hilsted, J.; Larsen, S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int. J. Pancreatol. 2000, 27, 21–27. [Google Scholar] [CrossRef]
- Duggan, S.N. Negotiating the complexities of exocrine and endocrine dysfunction in chronic pancreatitis. Proc. Nutr. Soc. 2017, 76, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.R.; Bolland, M.J. Calcium and/or Vitamin D Supplementation for the Prevention of Fragility Fractures: Who Needs It? Nutrients 2020, 12, 1011. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, V.W.; Pandol, S.J.; Porcel, J.; Wei, P.C.; Wilkens, L.R.; Le Marchand, L.; Pike, M.C.; Monroe, K.R. Dietary Factors Reduce Risk of Acute Pancreatitis in a Large Multiethnic Cohort. Clin. Gastroenterol. Hepatol. 2017, 15, 257–265.e3. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Sharma, R.; Rana, S.S.; Chaudhary, V.; Bhasin, D.K. 537 Impact of Vitamin D Supplementation on the Course, Severity, Complications and Outcome of Patients of Acute Pancreatitis with Vitamin D Deficiency. Gastroenterology 2014, 146, S-95. [Google Scholar] [CrossRef]
- Ryzen, E.; Singer, F.R. Hypercalcemia in leprosy. Arch. Intern. Med. 1985, 145, 1305–1306. [Google Scholar] [CrossRef]
- Ryzen, E.; Rea, T.H.; Singer, F.R. Hypercalcemia and abnormal 1,25-dihydroxyvitamin D concentrations in leprosy. Am. J. Med. 1988, 84, 325–329. [Google Scholar] [CrossRef]
- Bar-Shavit, Z.; Teitelbaum, S.L.; Reitsma, P.; Hall, A.; Pegg, L.E.; Trial, J.; Kahn, A.J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1983, 80, 5907–5911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderschueren, D.; Pye, S.R.; O’Neill, T.W.; Lee, D.M.; Jans, I.; Billen, J.; Gielen, E.; Laurent, M.; Claessens, F.; Adams, J.E.; et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: The European Male Aging Study (EMAS). J. Clin. Endocrinol. Metab. 2013, 98, 995–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, U.C.; Matzen, P.; Benfield, T.; Beck Jensen, J.E. Oral cholecalciferol versus ultraviolet radiation B: Effect on vitamin D metabolites in patients with chronic pancreatitis and fat malabsorption—A randomized clinical trial. Pancreatology 2011, 11, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Gärtner, S.; Doller, J.; Tran, Q.T.; Frost, F.; Bannert, K.; Jaster, R.; Berlin, P.; Valentini, L.; Meyer, F.; et al. Nutritional management of chronic pancreatitis: A systematic review and meta-analysis of randomized controlled trials. J. Gastroenterol. Hepatol. 2021, 36, 588–600. [Google Scholar] [CrossRef]
- Zou, W.; Ru, N.; Wu, H.; Hu, L.; Ren, X.; Jin, G.; Wang, Z.; Du, Y.; Cao, Y.; Zhang, L.; et al. Guidelines for the diagnosis and treatment of chronic pancreatitis in China (2018 edition). Hepatobiliary Pancreat. Dis. Int. 2019, 18, 103–109. [Google Scholar] [CrossRef]
- Gardner, T.; Adler, D.; Forsmark, C.; Sauer, B.; Taylor, J.; Whitcomb, D. ACG Clinical Guideline: Chronic Pancreatitis. Am. J. Gastroenterol. 2020, 115, 322–339. [Google Scholar] [CrossRef]
- Srivoleti, P.; Yang, A.L.; Jin, D.X.; Banks, P.A.; McNabb-Baltar, J. Does Provider Type Affect Bone Health Surveillance in Chronic Pancreatitis? Dig. Dis. Sci. 2021, 66, 2235–2239. [Google Scholar] [CrossRef]
- Lawrencia, C.; Charrier, A.; Huang, G.; Brigstock, D.R. Ethanol-mediated expression of connective tissue growth factor (CCN2) in mouse pancreatic stellate cells. Growth Factors 2009, 27, 91–99. [Google Scholar] [CrossRef]
| Pancreatic Cells | VDR Expression | Vitamin D Induced Targets Expression | Biological Actions |
|---|---|---|---|
| Pancreatic stellate cells [26,27,28] | High | IL-6, Collagen I, α-SMA and fibronectin↓ | Inhibitory effects against proliferation and fibrosis in vitro or in chronic pancreatitis models |
| Islets cells [29,30,31] | Low | VDR, CYP24A1, CaSR↑ | 1,25 Dihydroxyvitamin D3 has a direct and genomic action on β-cell functions including insulin secretion; in CP patients, the highest CYP24A1 levels were found in the endocrine cells. |
| Pancreatic acinar cells [31] | Absent or low basal level | VDR, CYP24A1, CaSR↑ | CYP24A1 is increased both during inflammation (as in chronic pancreatitis) and during malignant transformation (as in pancreatic ductal adenocarcinoma) |
| Pancreatic ductal cell [32] | Low | Increased VD-induced VDR, CDKN1A, CDK1 expression↑, high-dose VD downregulated VDR expression | Promoting the cell cycle of normal ductal cells |
| Pancreatic progenitor cells [33] | VDR expressing in the nucleus, cytoplasm, and plasma membrane | VD-induced VDR expression↑ | Promote cell viability and proliferation. |
| Author, Year | Study Design | Country | AP Patients (n) | Etiology of AP (%) | Vitamin D Deficiency (n, %) | Osteoporosis (n, %) |
|---|---|---|---|---|---|---|
| Abou Saleh et al., 2020 [53] | Retrospective cohort study | USA | 196,080 | NA | Deficiency (17.7) | 17,120 (8.7) |
| Bang et al., 2011 [55] | Prospective cohort study | England | 73 | Gallstones (52), Alcohol consumption (30), Idiopathic (11), Alcohol and gallstone (3), Other (4) | severe deficiency <13 nmol/L (23) deficiency 13–25 nmol/L (20) insufficiency 26–50 nmol/L (40) | NA |
| Huh et al., 2019 [12] | Prospective cohort study | Korea | 242 | Gallstones (52.5), Alcohol consumption (36), Hypertriglyceridemia (5), Idiopathic (6.6) | Deficiency < 10 ng/mL (56.2) Insufficiency 10–20 ng/mL (28.5) | NA |
| Leerhøy et al., 2018 [56] | Prospective cohort study | Denmark | 29 | Post-ERCP (100) | Insufficiency < 50 nmol/L (34.5) | NA |
| Study | Patients | Sample Size | Age, Years * | Etiology (%) | PEI (%) | PERT (%) | EI (%) | Osteopathy (%) | Serum 25(OH)D Deficiency |
|---|---|---|---|---|---|---|---|---|---|
| Observational Studies (Cross-Sectional Studies) | |||||||||
| Olese et al., 2017, Denmark [72] | CP | 147 | NA | NA | NA | NA | NA | NA | 42% (<50 nmol/L) |
| Tang et al., 2021, China [73] | CP | 104 | 46.1 (14.4) | Idiopathic, 68.3 Tropical alcoholic 31.7 | 27.9 | 49.0 | 26.9 | Osteopenia, 30.8; Osteoporosis, 5.8 | 73% (<20 ng/mL) |
| Joker-Jensen et al., 2020, England [74] | CP | 115 | 57.9 (13.0) | Alcoholic, 50 Tropical, NA Idiopathic, NA | 60.8 | 35.6 | 37.4 | NA | 22% (<25 nmol/L) |
| Stigliano et al., 2018, European (multicenter) [75] | CP | 211 | 60 | Alcoholic 43.60 Idiopathic 18.95 Hereditary 4.26 Obstructive 5.68 Other 27.48 | 56.42 | 54.97 | 37 | Osteopenia 42.18; Osteoporosis 21.80 | 56.37% (<20 ng/mL) |
| Min et al., 2018, USA [76] | CP | 91 | 48.6 (10.4) | Toxic/metabolic 59.3 Idiopathic 18.7 Genetic 14.3 Autoimmune 5.8 Obstructive 2.2 | 84.6 | NA | NA | Osteopenia 46.7; Osteoporosis 22.2 | 62.50% |
| Kumar et al., 2017, India [77] | CP | 102 | 40.8 (12.6) | Alcoholic 67 Tropical 35 | NA | NA | NA | Osteomalacia and low bone mass 36 | 67.6% (<30 ng/mL) |
| Pezzilli et al., 2015, Italy [78] | CP | 30 | 57.0 (13.1) | NA | 56.7 | NA | 23.3 | NA | 86.6% (<20 ng/mL) |
| Sikkens et al., 2013, Holland (Prospective) [79] | CP | 40 | 52 (11) | Alcoholic 50 Idiopathic 43 Other 7 | 70 | 48 | 45 | Osteopenia 45; Osteoporosis 10 | 53% (<38 pmol/L) |
| Klapdor et al., 2012, Germany (Prospective) [80] | CP | 37 | NA | NA | NA | 100 | NA | NA | 86.5% (<30 ng/mL), 37.8% (<10 ng/mL) |
| Dujsikova et al., 2008, Czech Republic [81] | CP | 73 | 46 (13) | Alcoholic 11 Idiopathic 89 | NA | NA | NA | Osteopathy 39; Osteopenia 26; Osteoporosis 5; Osteomalacia 8 | 86.3% (<75 nmol/L) |
| Prospective Case—Control Study | |||||||||
| Duggan et al., 2015, Ireland [82] | CP | 29 | 44.3 (12.3) | Alcoholic 62.1 Idiopathic 27.6 Other 10.3 | NA | NA | NA | Osteoporosis 31; Osteopenia 44.8 | 48.3% (<30 nmol/L) |
| Controls | 29 | 45.8 (9.8) | NA | NA | NA | NA | Osteoporosis 6.9; Osteopenia 51.7 | 20.7% (<30 nmol/L) | |
| Duggan et al., 2014, Ireland [83] | CP | 62 | 47.9 (12.5) | Alcoholic 38.7 | 34.8 | NA | NA | NA | 58% (<20 ng/mL) |
| Controls | 66 | 47.7 (11) | NA | NA | NA | NA | NA | 61.7% | |
| Prabhakaran, et al., 2014, India [84] | CP | 103 | 38.6 (20.6) | Alcoholic 70 Idiopathic 29.1 Post-traumatic 0.9 | 20.4 | NA | 37.8 | Osteoporosis 30.1; Osteopenia 39.8 | 19.4% (<10 ng/mL) |
| Controls | 40 | 36.7 (20.7) | NA | NA | NA | NA | NA | 38.59 ± 26 ng/mL * | |
| Duggan et al., 2012, Ireland [85] | CP | 62 | 47.9 (12.5) | Alcoholic 38.7 Other 61.3 | NA | NA | NA | Osteoporosis 34; Osteopenia 39.6 | 47.5 ± 21.6 mmol/L * |
| Controls | 66 | 47.74 (11) | NA | NA | NA | NA | Osteoporosis 10.2; Osteopenia 33.9 | 46.4 ± 20.4 mmol/L * | |
| Joshi et al., 2011, India [86] | CP | 72 | 31.1 (10.3) | Tropical calcific pancreatitis | 46 | 46 | 72 | The BMD Z-scores at the lumbar spine −1.0 ± 1.0 total hip −1.2 ± 1.2 | 86% (<50 nmol/L) |
| Controls | 100 | 32.6 (9.6) | NA | NA | NA | NA | NA | 85% | |
| Sudeep et al., 2011, India [87] | CP | 31 | 35.8 (9.0) | Tropical fibro calculous pancreatitis 65 Idiopathic 35 | 69 | 0 | 68 | Osteoporosis 29 | 52% (<20 ng/mL) |
| Controls | 35 | 38.6 (5.2) | NA | NA | NA | NA | Osteoporosis 9 | 24% | |
| Mann et al., 2003, Germany [11] | CP | 42 | 52.6 (13.5) | NA | 78.5 | NA | NA | DEXA Ward’s trangle (WARD) 92.2% ± 5.2% | 26.7 ± 9.7 nmol/L * |
| Controls | 20 | 48.9 (6.4) | NA | NA | NA | NA | DEXA WARD 97.1% ± 3.1% | 69.5 ± 13.5 nmol/L * | |
| Double Blinded, Randomized Controlled Trial | |||||||||
| Reddy et al., 2013, India [88] | CP | 40 | 33 (9) | Tropical Calcific (idiopathic) | NA | 52.5 | 92.5 | NA | 40% (25–50 nmol/L) 72% (<25 nmol/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Hu, C.; Chen, C.-J.; Han, Y.-P.; Lin, Z.-Q.; Deng, L.-H.; Xia, Q. Vitamin D and Pancreatitis: A Narrative Review of Current Evidence. Nutrients 2022, 14, 2113. https://doi.org/10.3390/nu14102113
Cai F, Hu C, Chen C-J, Han Y-P, Lin Z-Q, Deng L-H, Xia Q. Vitamin D and Pancreatitis: A Narrative Review of Current Evidence. Nutrients. 2022; 14(10):2113. https://doi.org/10.3390/nu14102113
Chicago/Turabian StyleCai, Fei, Cheng Hu, Chan-Juan Chen, Yuan-Ping Han, Zi-Qi Lin, Li-Hui Deng, and Qing Xia. 2022. "Vitamin D and Pancreatitis: A Narrative Review of Current Evidence" Nutrients 14, no. 10: 2113. https://doi.org/10.3390/nu14102113
APA StyleCai, F., Hu, C., Chen, C.-J., Han, Y.-P., Lin, Z.-Q., Deng, L.-H., & Xia, Q. (2022). Vitamin D and Pancreatitis: A Narrative Review of Current Evidence. Nutrients, 14(10), 2113. https://doi.org/10.3390/nu14102113







