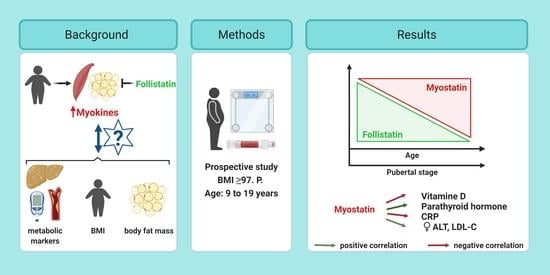
Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Analyses and Further Calculations
2.3. Statistics
2.4. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, K.; Mu, M.; Liu, K.; He, Y. Screen time and childhood overweight/obesity: A systematic review and meta-analysis. Child Care Health Dev. 2019, 45, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Himes, J.H.; Guo, Y.; Jiang, J.; Yang, L.; Lu, Q.; Ruan, H.; Shi, S. Birth Weight, Growth and Feeding Pattern in Early Infancy Predict Overweight/Obesity Status at Two Years of Age: A Birth Cohort Study of Chinese Infants. PLoS ONE 2013, 8, e64542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbeling, C.B.; Pawlak, D.B.; Ludwig, D.S. Childhood obesity: Public-health crisis, common sense cure. Lancet 2002, 360, 473–482. [Google Scholar] [CrossRef]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human Fatty Liver Disease: Old Questions and New Insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Clemente, M.G.; Mandato, C.; Poeta, M.; Vajro, P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World, J. Gastroenterol. 2016, 22, 8078–8093. [Google Scholar] [CrossRef]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jürets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum Myostatin is Upregulated in Obesity and Correlates with Insulin Re-sistance in Humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2021, 106, 181–234. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- McFarlane, C.; Langley, B.; Thomas, M.; Hennebry, A.; Plummer, E.; Nicholas, G.; McMahon, C.; Sharma, M.; Kambadur, R. Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev. Biol. 2005, 283, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Kambadur, R.; Sharma, M.; Smith, T.P.; Bass, J.J. Mutations in myostatin (GDF8) in Double-Muscled Belgian Blue and Piedmontese Cattle. Genome Res. 1997, 7, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Kim, D. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int. J. Mol. Sci. 2021, 22, 2604. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Hausman, D.B.; Johnson, M.A.; Nicklas, B.J.; Miller, M.E.; Neiberg, R.H.; Marsh, A.P.; Newman, A.B.; Blair, S.N.; et al. Change in 25-Hydroxyvitamin D and Physical Performance in Older Adults. J. Gerontol. Ser. A 2011, 66, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.; Johnson, C.; McCabe, G.; Carnell, N. Dietary protein requirements of younger and older adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef] [Green Version]
- McPherron, A.C.; Lee, S.J. Suppression of body fat accumulation in myostatin-deficient mice. J. Clin. Investig. 2002, 109, 595–601. [Google Scholar] [CrossRef]
- Wilkes, J.J.; Lloyd, D.J.; Gekakis, N. Loss-of-Function Mutation in Myostatin Reduces Tumor Necrosis Factor α Production and Protects Liver Against Obesity-Induced Insulin Resistance. Diabetes 2009, 58, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Wall, R.J.; Yang, J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem. Biophys. Res. Commun. 2005, 337, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin Inhibition in Muscle, but Not Adipose Tissue, Decreases Fat Mass and Improves Insulin Sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennequin, G.; Sirvent, P.; Whitham, M. Role of exercise-induced hepatokines in metabolic disorders. Am. J. Physiol. Metab. 2019, 317, E11–E24. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association with Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Levine, M.D.; Arslanian, S.A.; Ewing, L.J.; Houck, P.R.; Cheng, Y.; Ringham, R.M.; Sheets, C.A.; Marcus, M.D. Family-Based Treatment of Severe Pediatric Obesity: Randomized, Controlled Trial. Pediatrics 2009, 124, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating microRNAs 34a, 122, and 192 are linked to obesity-associated inflammation and metabolic disease in pediatric patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ssalamah, A.B.; Item, C.B.; de Gier, C.; Walleczek, N.; Metz, T.F.; Jakober, I.; Greber-Platzer, S.; et al. A branched-chain amino acid-based metabolic score can predict liver fat in children and adolescents with severe obesity. Pediatr. Obes. 2020, 16, e12739. [Google Scholar] [CrossRef]
- Mickey, E.; Bokor, B.R. Tanner Stages. [Updated 2021 Dec 15]. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing LLC: Orlando, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470280 (accessed on 22 January 2022).
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; Von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Ergeb. Der. Physiol. 2001, 149, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern-Matschilles, S.; Gar, C.; Wanger, L.; Haschka, S.J.; Potzel, A.L.; Hesse, N.; Then, C.; Seissler, J.; Lechner, A. Association of Serum Myostatin with Body Weight, Visceral Fat Volume, and High Sensitivity C-Reactive Protein But Not with Muscle Mass and Physical Fitness in Premenopausal Women. Exp. Clin. Endocrinol. Diabetes 2021. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, X.; Yang, X.; Zhang, D.; Zhang, X.; Du, H.; Han, Y.; Sun, L. Combination of Weight-Bearing Training and Anti-MSTN Polyclonal Antibody Improve Bone Quality In Rats. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 516–524. [Google Scholar] [CrossRef]
- Willoughby, D.S. Effects of an Alleged Myostatin-Binding Supplement and Heavy Resistance Training on Serum Myostatin, Muscle Strength and Mass, and Body Composition. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 461–472. [Google Scholar] [CrossRef]
- Kumar, S.; Hossain, J.; Inge, T.; Balagopal, P.B. Changes in Myokines in Youths With Severe Obesity Following Roux-en-Y Gastric Bypass Surgery. JAMA Surg. 2019, 154, 668–669. [Google Scholar] [CrossRef]
- Åkerfeldt, T.; Helmersson-Karlqvist, J.; Gunningberg, L.; Swenne, C.L.; Larsson, A. Postsurgical Acute Phase Reaction is Associated with Decreased Levels of Circulating Myostatin. Inflammation 2015, 38, 1727–1730. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, C.K.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell prolif-eration and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef] [Green Version]
- Ewendt, F.; Feger, M.; Föller, M. Myostatin regulates the production of fibroblast growth factor 23 (FGF23) in UMR106 osteo-blast-like cells. Pflug. Arch. 2021, 473, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ortiz, M.E.; Rodriguez, M. FGF23 as a calciotropic hormone. F1000Research 2015, 4, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; Singh, V.; Tucker, A.; Collazo, J.; Singh, R. Modulation of transforming growth factor-β/follistatin signaling and white adipose browning: Therapeutic implications for obesity related disorders. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170036. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Harasymowicz, N.S.; Wu, C.-L.; Collins, K.H.; Choi, Y.-R.; Oswald, S.J.; Guilak, F. Gene therapy for follistatin mitigates systemic metabolic inflammation and post-traumatic arthritis in high-fat diet–induced obesity. Sci. Adv. 2020, 6, eaaz7492. [Google Scholar] [CrossRef]
- El-Mottaleb, N.A.A.; Galal, H.M.; Maghraby, K.M.E.; Gadallah, A.I. Serum irisin level in myocardial infarction patients with or without heart failure. Can. J. Physiol. Pharmacol. 2019, 97, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Gonzalez-Gil, A.M.; Villarreal-Calderón, J.R.; Tamez-Rivera, O.; Rodriguez-Gutierrez, N.A.; Castillo, E.C.; Silva-Platas, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Serum Irisin Levels, Endothelial Dysfunction, and Inflam-mation in Pediatric Patients with Type 2 Diabetes Mellitus and Metabolic Syndrome. J. Diabetes Res. 2020, 2020, 1949415. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef]
| Mean (SD) or Percentages (%) | |
|---|---|
| Sex (m, %) | 68 (63%) |
| Age (years) | 13.8 ± 2.7 |
| BMI z-score | 2.8 ± 0.5 |
| Body fat mass (%) | 41.6 ± 7.1 |
| Tanner stage (male/female): | |
| 1 | 4 (13%)/27 (87%) |
| 2 | 7 (37%)/12 (63%) |
| 3 | 6 (40%)/9 (60%) |
| 4 | 9 (37%)/15 (63%) |
| 5 | 11 (73%)/4 (27%) |
| Fasting glucose (mmol/L) | 4.70 ± 0.53 |
| Insulin (pmol/L) | 194.4 ± 134 |
| C-Peptide (nmol/L) | 1.2 ±0.6 |
| HOMA-IR | 6.1 ± 5 |
| Total cholesterol (mmol/L) | 4.37 ± 0.80 |
| HDL-C (mmol/L) | 1.14 ± 0.32 |
| LDL-C (mmol/L) | 2.60 ± 0.68 |
| Triglycerides (mmol/L) | 1.45 ± 0.90 |
| Vitamin D (nmol/L) | 45.9 ± 21.3 |
| Parathyroid hormone (ng/L) | 37.6 ± 17 |
| CRP (nmol/L) | 6.7 ± 5.6 |
| IL-6 (ng/L) | 4 ± 3.6 |
| Procalcitonin (ng/L) | 200 ± 1000 |
| TNFα (g/L) | 1.1 ± 0.4 |
| ALT (U/L) | 44.9 ± 45.8 |
| Myostatin (ng/L) | Follistatin (ng/L) | Irisin (mg/L) | |
|---|---|---|---|
| Myostatin (ng/L) A | - | −0.28 ** | 0.02 |
| Follistatin (ng/L) A | −0.28 ** | - | −0.05 |
| Irisin (mg/L) A | 0.02 | −0.05 | - |
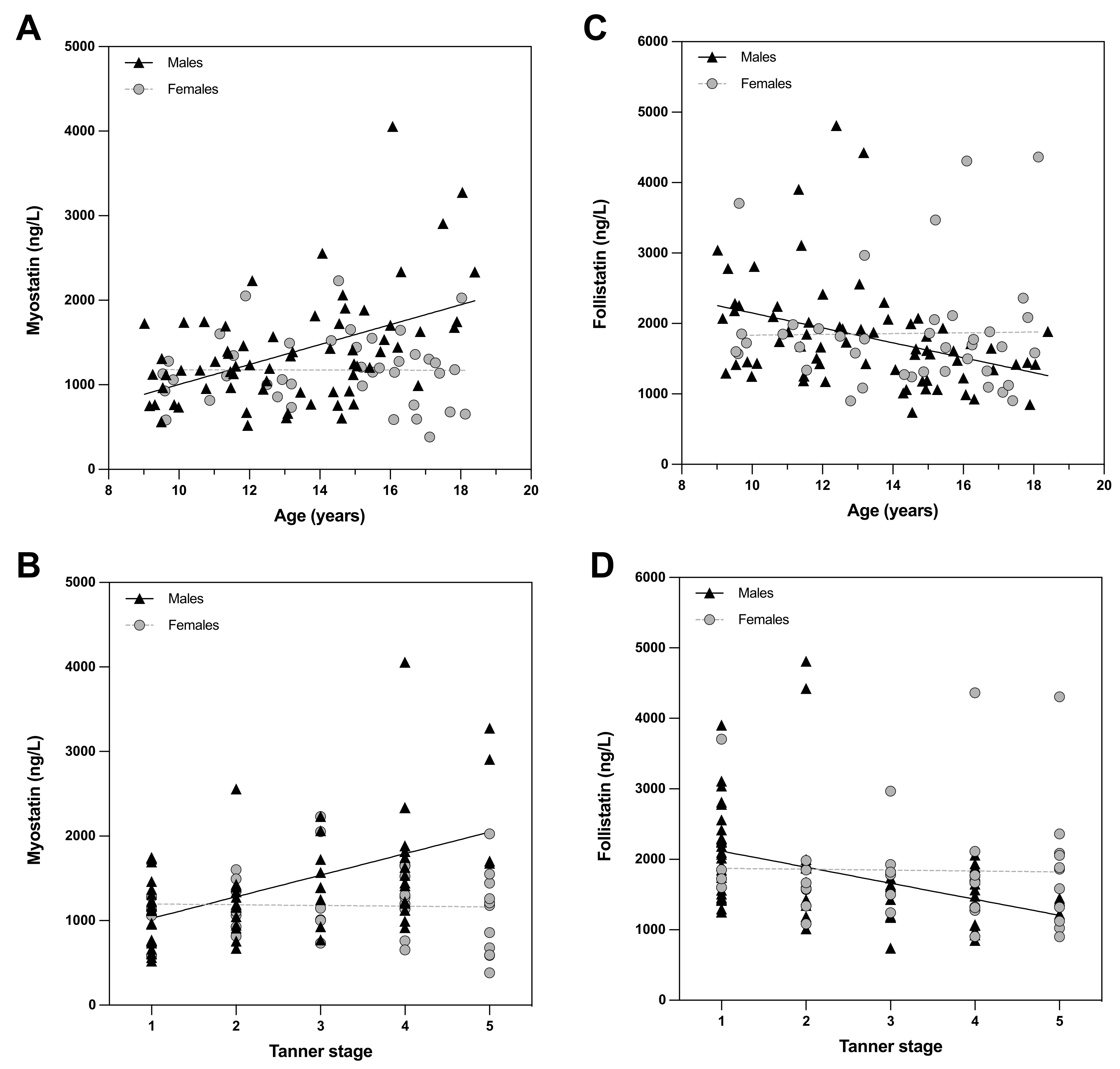
| Age (years) A | 0.24 * | −0.28 ** | 0.03 |
| Tanner stage A | 0.28 ** | −0.33 ** | 0.06 |
| BMI z-score A | 0.11 | 0.23 | −0.8 |
| Fasting glucose (mmol/L) A | −0.06 | −0.15 | 0.08 |
| Insulin (pmol/L) A | 0.26 * | −0.07 | −0.03 |
| C-Peptide (nmol/L) A | 0.17 | −0.15 | −0.01 |
| HOMA-IR A | 0.24 * | −0.11 | −0.01 |
| Cholesterol (mmol/L) A | 0.03 | 0.18 | −0.1 |
| HDL-C (mmol/L) A | −0.13 | 0.15 | 0.27 ** |
| LDL-C. (mmol/L) A | 0.11 | 0.13 | −0.26 ** |
| Triglycerides (mmol/L) A | 0.06 | 0.06 | −0.15 |
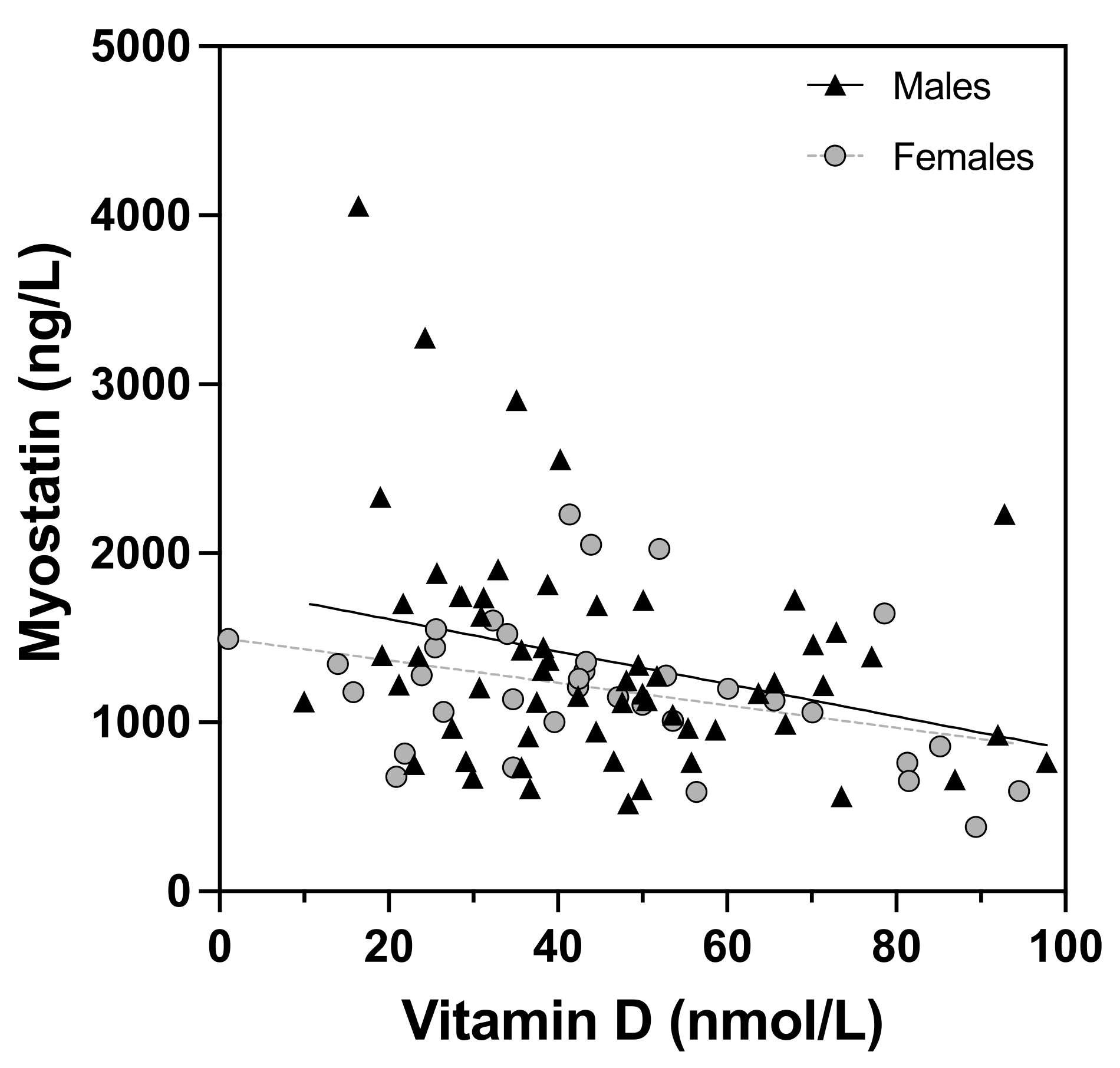
| Vitamin D (nmol/L) A | −0.31 ** | 0.11 | 0.15 |
| Parathyroid hormone (ng/L)A | 0.23 * | −0.17 | −0.20 |
| CRP (nmol/L) A | −0.24 * | 0.28 ** | −0.13 |
| IL-6 (ng/L) A | −0.34 ** | 0.29 ** | −0.21 * |
| Procalcitonin (ng/L) A | 0.04 | 0.26 ** | 0.05 |
| TNFα (ng/L) A | −0.16 | 0.17 | −0.13 |
| ALT (U/L) A | 0.27 ** | 0.07 | −0.02 |
| Myostatin (ng/L) | Follistatin (ng/L) | Irisin (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Female | Male | All | Female | Male | All | Female | Male | |
| Myostatin (ng/L) A | - | - | - | −0.23 * | −0.41 * | −0.01 | 0.02 | 0.04 | 0.14 |
| Follistatin (ng/L) A | −0.22 * | −0.41 * | −0.01 | - | - | - | 0.05 | 0.13 | −0.05 |
| Irisin (mg/L) A | 0.02 | 0.04 | 0.14 | 0.05 | 0.13 | −0.05 | - | - | - |
| BMI z-score A | −0.01 | −0.06 | 0.04 | 0.09 | 0.04 | 0.09 | −0.08 | −0.09 | −0.02 |
| Body Fat (%) A | −0.24 * | 0.04 | −0.16 | 0.14 | −0.01 | 0.08 | 0.02 | −0.05 | −0.04 |
| Fasting glucose (mmol/L) A | −0.1 | −0.07 | −0.11 | −0.15 | −0.12 | −0.19 | 0.15 | 0.37 * | −0.04 |
| Insulin (pmol/L) A | 0.01 | 0.02 | −0.09 | −0.01 | 0.12 | −0.03 | 0.01 | 0.21 | −0.11 |
| C-Peptide (nmol/L)A | −0.05 | 0.0 | −0.21 | −0.05 | −0.01 | −0.01 | 0.05 | 0.27 | −0.07 |
| HOMA-IR A | −0.04 | −0.03 | −0.11 | −0.0 | 0.15 | −0.06 | 0.05 | 0.25 | −0.13 |
| Cholesterol (mmol/L) A | 0.12 | 0.27 | 0.08 | 0.05 | 0.22 | −0.06 | −0.11 | 0.06 | −0.22 |
| HDL-C (mmol/L) A | −0.1 | −0.11 | 0.11 | 0.39 | 0.17 | 0.46 ** | 0.24 * | 0.25 | 0.22 |
| LDL-C (mmol/L) A | 0.19 | 0.37 * | 0.1 | −0.07 | 0.03 | −0.09 | −0.22 * | −0.17 | −0.25 |
| Triglycerides (mmol/L) A | −0.03 | 0.03 | −0.14 | 0.02 | 0.2 | −0.03 | −0.14 | −0.11 | −0.13 |
| Vitamin D (nmol/L) A | −0.30 ** | −0.34 * | −0.18 | 0.09 | 0.1 | −0.02 | 0.09 | 0.14 | 0.03 |
| Parathyroid hormone (ng/L)A | 0.26 * | 0.22 | 0.26 | −0.13 | −0.2 | −0.09 | −0.07 | −0.31 | 0.01 |
| CRP (nmol/L) A | −0.22 * | −0.35 * | −0.16 | 0.41 ** | 0.44 * | 0.38 ** | −0.05 | −0.21 | −0.01 |
| IL-6 (ng/L) A | −0.12 | 0.0 | −0.22 | 0.01 | −0.07 | 0.1 | −0.12 | −0.23 | −0.1 |
| Procalcitonin (ng/L) A | 0.01 | 0.04 | 0 | −0.03 | −0.11 | 0.01 | −0.05 | −0.1 | −0.03 |
| TNFα (ng/L) A | −0.05 | −0.31 | −0.14 | 0.11 | 0.25 | 0.17 | 0.0 | 0.1 | 0.04 |
| ALT (U/L) A | 0.13 | 0.38* | −0.03 | −0.05 | −0.09 | 0.01 | −0.02 | 0.32 | −0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgartner, M.; Lischka, J.; Schanzer, A.; de Gier, C.; Walleczek, N.-K.; Greber-Platzer, S.; Zeyda, M. Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity. Nutrients 2022, 14, 2133. https://doi.org/10.3390/nu14102133
Baumgartner M, Lischka J, Schanzer A, de Gier C, Walleczek N-K, Greber-Platzer S, Zeyda M. Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity. Nutrients. 2022; 14(10):2133. https://doi.org/10.3390/nu14102133
Chicago/Turabian StyleBaumgartner, Margot, Julia Lischka, Andrea Schanzer, Charlotte de Gier, Nina-Katharina Walleczek, Susanne Greber-Platzer, and Maximilian Zeyda. 2022. "Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity" Nutrients 14, no. 10: 2133. https://doi.org/10.3390/nu14102133
APA StyleBaumgartner, M., Lischka, J., Schanzer, A., de Gier, C., Walleczek, N.-K., Greber-Platzer, S., & Zeyda, M. (2022). Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity. Nutrients, 14(10), 2133. https://doi.org/10.3390/nu14102133