Bioenergetic Balance of Continuous Venovenous Hemofiltration, a Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
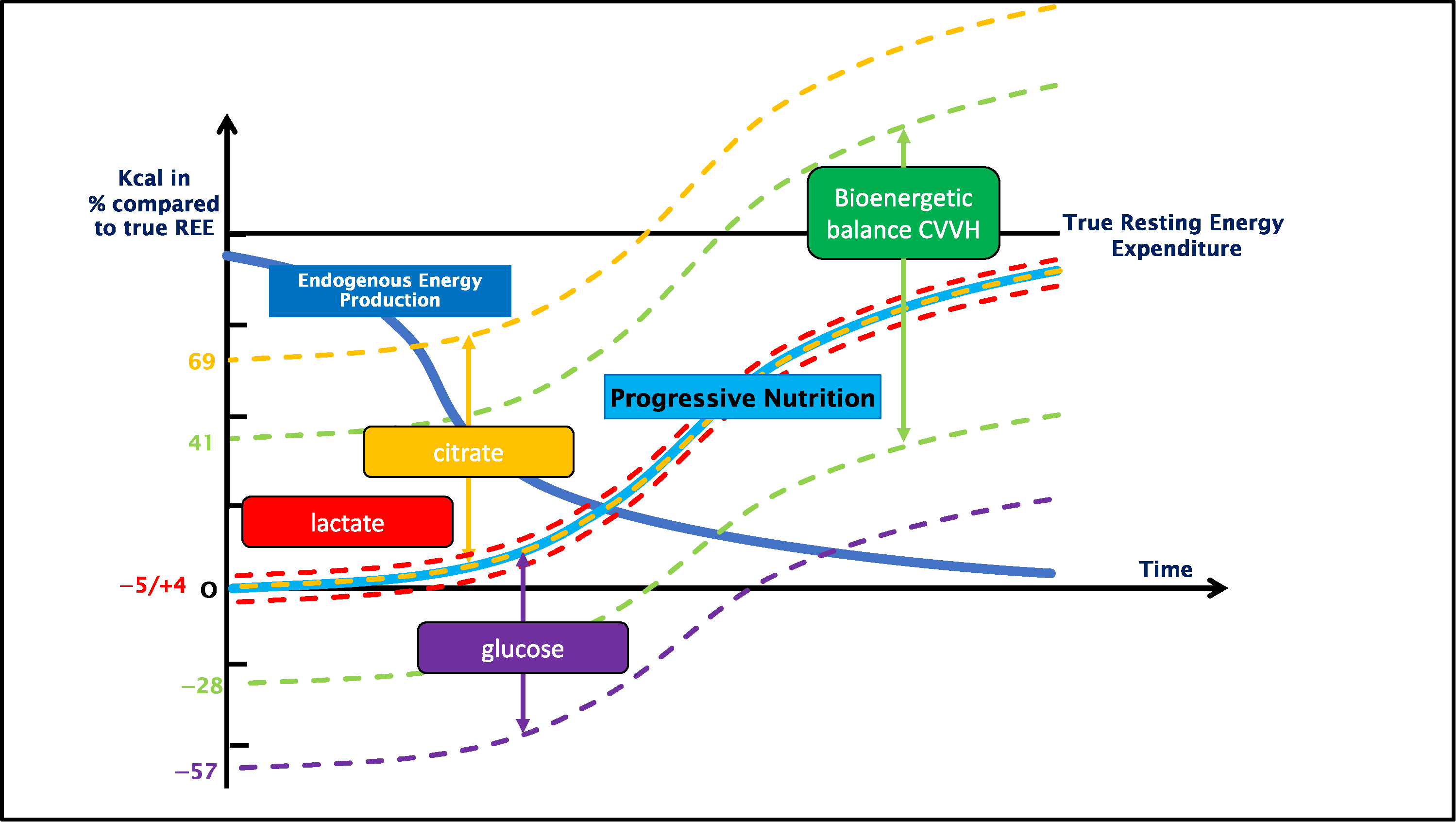
3.1. Bioenergetic Balance
3.2. Non-Intentional Calories
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Amount of Subjects (ntotal) | 9 |
|---|---|
| Mean age | 70 ± 12 years |
| Gender | |
| 6 |
| 3 |
| Mean weight | 89 ± 28 kg |
| Mean BMI | 30.3 ± 6.7 kg/m2 |
| Mean APACHE II score | 30 ± 12 |
| Ventilation (n) | |
| 4 |
| 5 |
| 30-day mortality (n/ntotal) | 7/9 |
References
- Duan, J.Y.; Zheng, W.H.; Zhou, H.; Xu, Y.; Huang, H.B. Energy delivery guided by indirect calorimetry in critically ill patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Pertzov, B.; Bar-Yoseph, H.; Menndel, Y.; Bendavid, I.; Kagan, I.; Glass, Y.D.; Singer, P. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef] [Green Version]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.M.; Aldawood, A.S.; Al-Dorzi, H.M.; Tamim, H.M.; Haddad, S.H.; Jones, G.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; Sadat, M.; et al. Permissive Underfeeding or Standard Enteral Feeding in High- and Low-Nutritional-Risk Critically Ill Adults. Post Hoc Analysis of the PermiT Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 652–662. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef]
- Case, J.; Khan, S.; Khalid, R.; Khan, A. Epidemiology of acute kidney injury in the intensive care unit. Crit. Care Res. Pract. 2013, 2013, 479730. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.G.; Bellomo, R.; Bagshaw, S.M.; Glassford, N.J.; Lo, S.; Jun, M.; Cass, A.; Gallagher, M. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: A systematic review and meta-analysis. Intensive Care Med. 2013, 39, 987–997. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Balik, M.; Zakharchenko, M.; Leden, P.; Otahal, M.; Hruby, J.; Polak, F.; Rusinova, K.; Stach, Z.; Tokarik, M.; Vavrova, J.; et al. Bioenergetic gain of citrate anticoagulated continuous hemodiafiltration—A comparison between 2 citrate modalities and unfractionated heparin. J. Crit. Care 2013, 28, 87–95. [Google Scholar] [CrossRef] [PubMed]
- New, A.M.; Nystrom, E.M.; Frazee, E.; Dillon, J.J.; Kashani, K.B.; Miles, J.M. Continuous renal replacement therapy: A potential source of calories in the critically ill. Am. J. Clin. Nutr. 2017, 105, 1559–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jonckheer, J.; Spapen, H.; Debain, A.; Demol, J.; Diltoer, M.; Costa, O.; Lanckmans, K.; Oshima, T.; Honoré, P.M.; Malbrain, M.; et al. CO2 and O2 removal during continuous veno-venous hemofiltration: A pilot study. BMC Nephrol. 2019, 20, 222. [Google Scholar] [CrossRef] [Green Version]
- Jonckheer, J.; Demol, J.; Lanckmans, K.; Malbrain, M.; Spapen, H.; De Waele, E. MECCIAS trial: Metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin. Nutr. 2020, 39, 3797–3803. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Mariano, F.; Morselli, M.; Bergamo, D.; Hollo, Z.; Scella, S.; Maio, M.; Tetta, C.; Dellavalle, A.; Stella, M.; Triolo, G. Blood and ultrafiltrate dosage of citrate as a useful and routine tool during continuous venovenous haemodiafiltration in septic shock patients. Nephrol. Dial Transpl. 2011, 26, 3882–3888. [Google Scholar] [CrossRef] [Green Version]
- Chadha, V.; Garg, U.; Warady, B.A.; Alon, U.S. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr. Nephrol. 2002, 17, 819–824. [Google Scholar] [CrossRef]
- De Waele, E.; Opsomer, T.; Honoré, P.M.; Diltoer, M.; Mattens, S.; Huyghens, L.; Spapen, H. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard? Minerva Anestesiol. 2015, 81, 272–282. [Google Scholar]
- Zusman, O.; Kagan, I.; Bendavid, I.; Theilla, M.; Cohen, J.; Singer, P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin. Nutr. 2019, 38, 1206–1210. [Google Scholar] [CrossRef]
- Stevenson, J.M.; Heung, M.; Vilay, A.M.; Eyler, R.F.; Patel, C.; Mueller, B.A. In vitro glucose kinetics during continuous renal replacement therapy: Implications for caloric balance in critically ill patients. Int. J. Artif. Organs. 2013, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Mizock, B.A. Alterations in carbohydrate metabolism during stress: A review of the literature. Am. J. Med. 1995, 98, 75–84. [Google Scholar] [CrossRef]
- De Waele, E.; Spapen, H.; Honore, P.M.; Mattens, S.; Rose, T.; Huyghens, L. Bedside calculation of energy expenditure does not guarantee adequate caloric prescription in long-term mechanically ventilated critically ill patients: A quality control study. Sci. World J. 2012, 2012, 909564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonghe, B.; Appere-De-Vechi, C.; Fournier, M.; Tran, B.; Merrer, J.; Melchior, J.C.; Outin, H. A prospective survey of nutritional support practices in intensive care unit patients: What is prescribed? What is delivered? Crit. Care Med. 2001, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Stapel, S.N.; de Boer, R.J.; Thoral, P.J.; Vervloet, M.G.; Girbes, A.R.J.; Oudemans-van Straaten, H.M. Amino Acid Loss during Continuous Venovenous Hemofiltration in Critically Ill Patients. Blood Purif. 2019, 48, 321–329. [Google Scholar] [CrossRef] [PubMed]
| Prismocitrate ® 18/0 | Prismocal B22 ® | Biphozyl ® | NaCl 0.9% | |
|---|---|---|---|---|
| Na (mmol/L) | 140 | 140 | 140 | 154 |
| K (mmol/L) | 0 | 4 | 4 | 0 |
| Cl (mmol/L) | 86 | 120.5 | 122 | 154 |
| Mg (mmol/L | 0 | 0 | 0.75 | 0 |
| P (mmol/L) | 0 | 0 | 1–2 | 0 |
| HCO3 (mmol/L) | 0 | 22 | 22 | 0 |
| Citrate (mmol/L) | 18 | 0 | 0 | 0 |
| Glucose (mmol/L) | 0 | 6.1 | 0 | 0 |
| Lactate (mmol/L) | 0 | 3 | 0 | 0 |
| Low Dose CVVH with Citrate | High Dose CVVH with Citrate | Low Dose CVVH without Citrate | |
|---|---|---|---|
| n = | 8 | 4 | 7 |
| Blood flow (mL/min) | 150 ± 0 | 150 ± 0 | 150 ± 0 |
| Predilution flow (mL/h) | 1756 ± 264 | 1700 ± 147 | 1721 ± 296 |
| Postdilution flow | 506 ± 431 | 2300 ± 1036 | 464 ± 490 |
| Postdilution fluid (n) | |||
| 1 | 0 | 1 |
| 3 | 2 | 3 |
| 4 | 2 | 3 |
| Effluent flow (mL/h) | 2363 ± 476 | 4075 ± 974 | 2279 ± 551 |
| Low Dose CVVH with Citrate | High Dose CVVH with Citrate | Low Dose CVVH without Citrate | ||
|---|---|---|---|---|
| Absolute bioenergetic balance (kcal/day) | Mean | 498 ± 110 | 262 ± 222 | −189 ± 77 |
| Range | 339 to 681 | 56 to 565 | −298 to −92 | |
| Relative bioenergetic balance (%) | Mean | 26 ± 9 | 17 ± 11 | −13 ± 8 |
| Range | 14 to 42 | 7 to 32 | −28 to −5 |
| Low Dose CVVH with Citrate | High Dose CVVH with Citrate | Low Dose CVVH without Citrate | p-Value | ||
|---|---|---|---|---|---|
| Gain due to dialysis fluid of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 759 ± 114 | 734 ± 64 | 0 | <0.001 |
| Glucose (g/24 h) | 6 ± 14 | 38 ± 45 | 7 ± 15 | 0.083 | |
| Lactate (mmol/24 h) | 16 ± 38 | 104 ± 124 | 19 ± 40 | 0.083 | |
| Loss in effluent of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 168 ± 47 | 281 ± 73 | 0 | <0.001 |
| Glucose (g/24 h) | 64 ± 28 | 107 ± 40 | 57 ± 22 | 0.032 | |
| Lactate (mmol/24 h) | 64 ± 34 | 127 ± 74 | 60 ± 38 | 0.070 | |
| Total balance of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 591 ± 81 | 453 ± 60 | 0 | <0.001 |
| Glucose (g/24 h) | −59 ± 24 | −69 ± 53 | −50 ± 20 | 0.607 | |
| Lactate (mmol/24 h) | −48 ± 16 | −22 ± 84 | −42 ± 14 | 0.567 | |
| Absolute caloric balance (kcal/day) | Citrate | 736 ± 101 | 564 ± 75 | 0 | <0.001 |
| Glucose | −222 ± 90 | −262 ± 202 | −187 ± 74 | 0.584 | |
| Lactate | −16 ± 5 | −7 ± 27 | 3 ± 15 | 0.032 | |
| Relative caloric balance compared to the true Resting Energy Expenditure (REE) (%) | Citrate | 40 ± 14% (26 to 69%) | 44 ± 16% (34 to 69%) | 0% | <0.001 |
| Glucose | −12 ± 7% (−25 to −5%) | −24 ± 24% (−57 to 0%) | −13 ± 8% (−28 to −5%) | 0.300 | |
| Lactate | −1 ± 1% (−2 to 0%) | −1 ± 3% (−5 to 1%) | 0 ± 1% (−1 to 3%) | 0.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonckheer, J.; Van Hoorn, A.; Oshima, T.; De Waele, E. Bioenergetic Balance of Continuous Venovenous Hemofiltration, a Retrospective Analysis. Nutrients 2022, 14, 2112. https://doi.org/10.3390/nu14102112
Jonckheer J, Van Hoorn A, Oshima T, De Waele E. Bioenergetic Balance of Continuous Venovenous Hemofiltration, a Retrospective Analysis. Nutrients. 2022; 14(10):2112. https://doi.org/10.3390/nu14102112
Chicago/Turabian StyleJonckheer, Joop, Alex Van Hoorn, Taku Oshima, and Elisabeth De Waele. 2022. "Bioenergetic Balance of Continuous Venovenous Hemofiltration, a Retrospective Analysis" Nutrients 14, no. 10: 2112. https://doi.org/10.3390/nu14102112
APA StyleJonckheer, J., Van Hoorn, A., Oshima, T., & De Waele, E. (2022). Bioenergetic Balance of Continuous Venovenous Hemofiltration, a Retrospective Analysis. Nutrients, 14(10), 2112. https://doi.org/10.3390/nu14102112






