A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action
Abstract
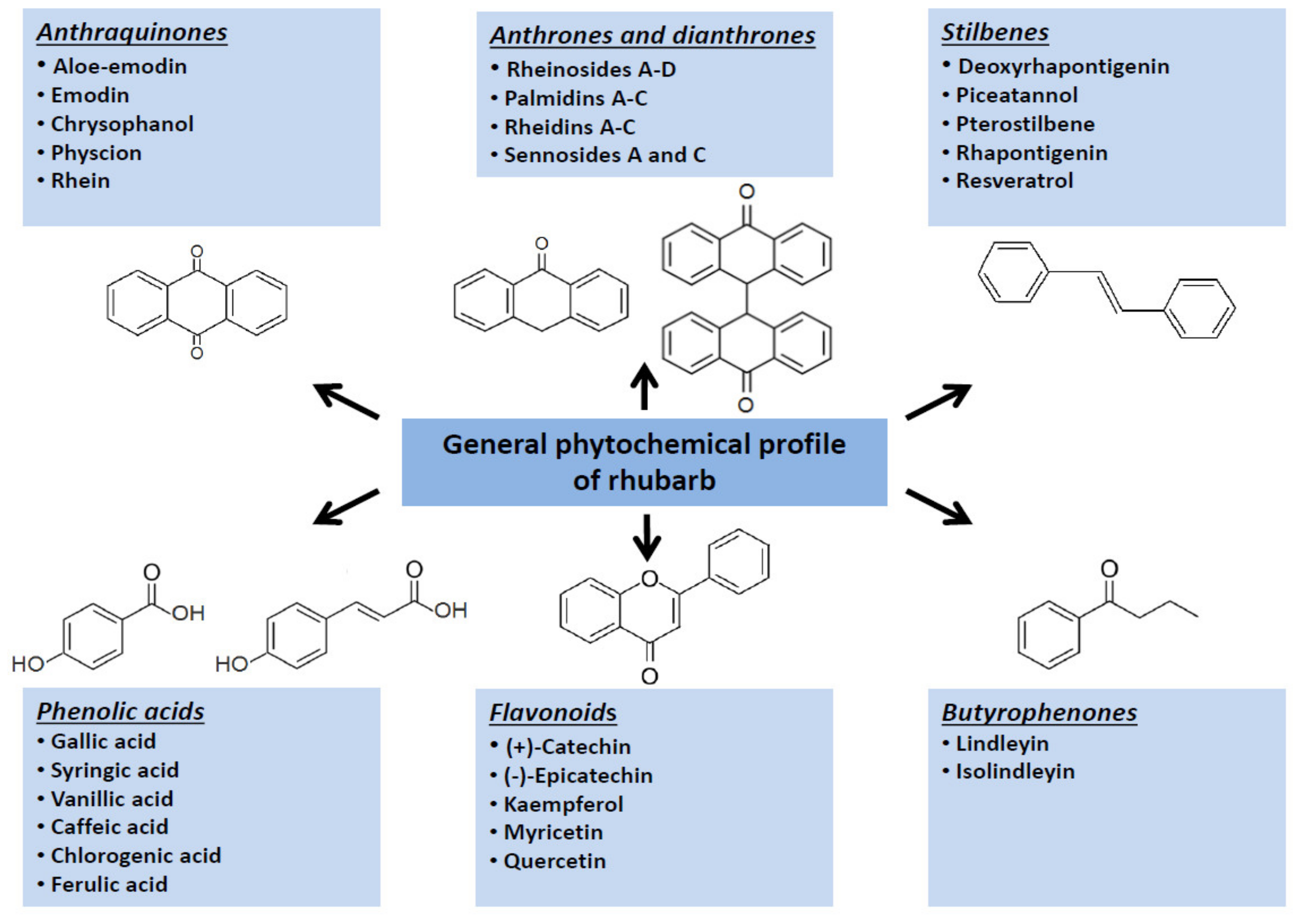
1. Introduction
2. Rhubarbs as Ethnomedicinal Plants
3. Rhubarb-Based Preparations in a Contemporary Pharmaceutical and Food Market
4. Hypolipidemic Effects of Rhubarb: Not Only the Fibre
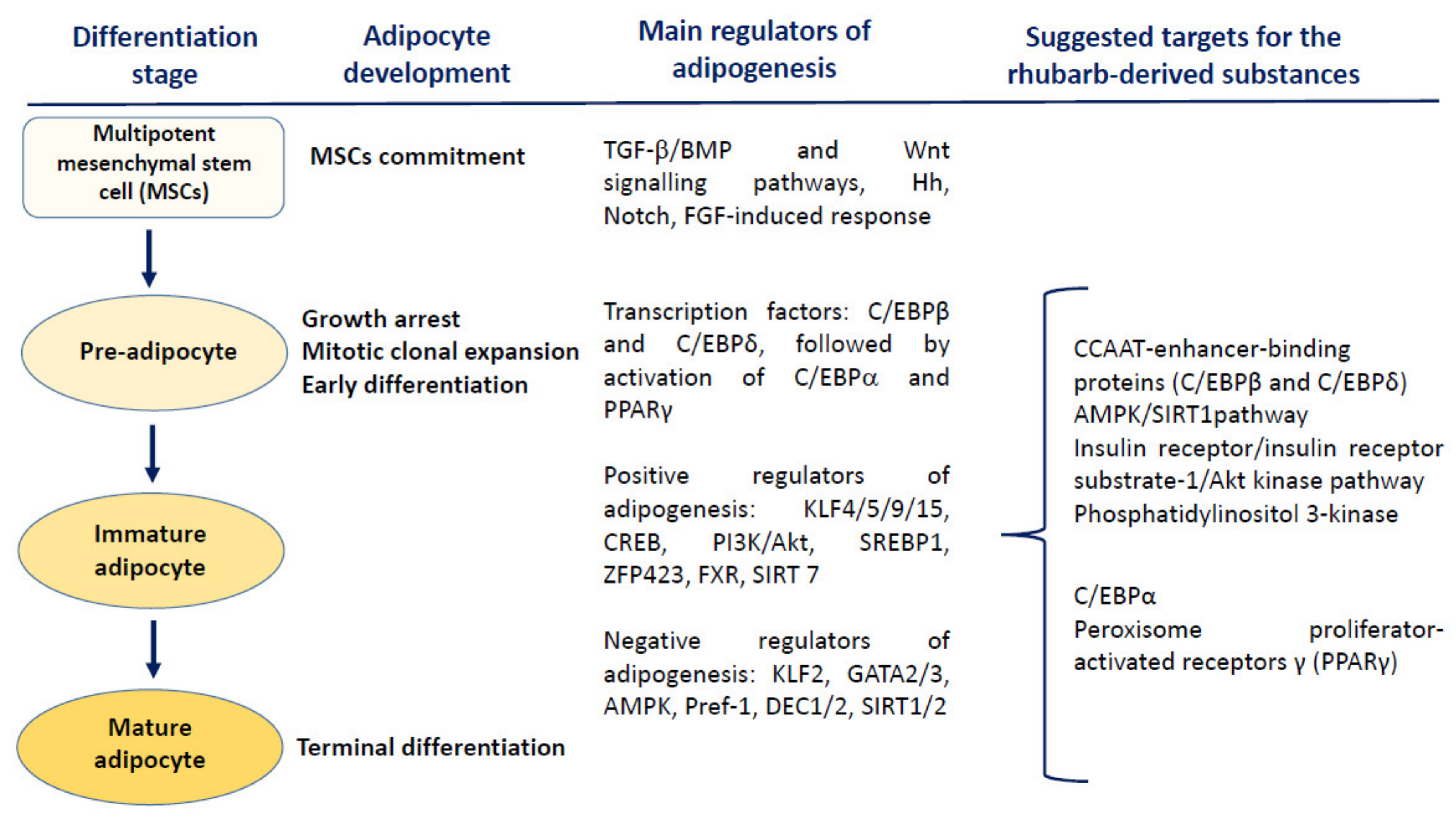
5. Molecular Targets for Rhubarb-Derived Substances
5.1. Inhibitory Effects on Key Enzymes Related to Lipid Absorption and Metabolism
5.2. Modulation of the Adipose Tissue Physiology
5.3. Metabolism and Glucose Level Regulation
5.4. Anti-Obesity Action of Rhubarb Extracts in Animal Studies
5.5. Anti-Obesity Action of Rhubarb Extracts in Clinical Trials
5.6. Laxative Effects of Rhubarb-Based Extracts and Preparations
6. Concluding Remarks
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 February 2022).
- Williams, D.M.; Nawaz, A.; Evans, M. Drug therapy in obesity: A review of current and emerging treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Mahdavi, A.; Abbasi, E.; Iranshahy, M.; Sathyapalan, T.; Sahebkar, A. The effects of phytochemicals and herbal bio-active compounds on tumour necrosis factor-α in overweight and obese individuals: A clinical review. Inflammopharmacology 2022, 30, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Hasani-Ranjbar, S.; Shahbal, N.; Qorbani, M.; Aletaha, A.; Haghi-Aminjan, H.; Soltani, A.; Khatami, F.; Nikfar, S.; Hassani, S.; et al. Effect of the herbal medicines in obesity and metabolic syndrome: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2020, 34, 526–554. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Pu, Z.-J.; Tang, Y.-P.; Shen, J.; Chen, Y.-Y.; Kang, A.; Zhou, G.-S.; Duan, J.-A. Advances in bioactive constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T. Ethnobotanical uses, phytochemistry and pharmacology of different Rheum species (Polygonaceae): A review. Adv. Exp. Med. Biol. 2021, 1308, 309–352. [Google Scholar]
- Kolodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum—A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2021, 20, 589–607. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Y.; Li, A.; Sun, A.; Yu, L.; Liu, L. Separation and purification of six components from the roots of Rheum officinaleBaill. by supercritical fluid chromatography. J. Liq. Chrom. Relat. Tech. 2017, 40, 156–164. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, T.; Chaturvedi, P. Rheum emodi Wall ex. meissn (Indian Rhubarb): Highly endangered medicinal herb. J. Med. Plants Stud. 2017, 5, 13–16. [Google Scholar]
- Sargin, S.A. Plants used against obesity in Turkish folk medicine: A review. J. Ethnopharmacol. 2021, 270, 113841. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Wang, Y. Meta analysis of umbilical area applying with rhubarb in treatment of constipation. Chin. Nurs. Res. 2016, 30, 4020–4024. [Google Scholar]
- Chang, J.-L.; Montalto, M.B.; Heger, P.W.; Thiemann, E.; Rettenberger, R.; Wacker, J. Rheum rhaponticum extract (ERr 731): Postmarketing data on safety surveillance and consumer complaints. Integr. Med. 2016, 15, 34–39. [Google Scholar]
- Park, S.; Kim, Y.N.; Kwak, H.J.; Jeong, E.J.; Kim, S.H. Estrogenic activity of constituents from the rhizomes of Rheum undulatumLinné. Bioorg. Med. Chem. Lett. 2018, 28, 552–557. [Google Scholar] [CrossRef]
- AsokanShibu, M.; Kuo, W.W.; Kuo, C.H.; Day, C.H.; Shen, C.Y.; Chung, L.C.; Lai, C.H.; Pan, L.F.; Vijaya Padma, V.; Huang, C.Y. Potential phytoestrogen alternatives exert cardio-protective mechanisms via estrogen receptors. Biomedicine 2017, 7, 11. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Bunzel, M.; Seiler, A.; Steinhart, H. Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J. Agric. Food Chem. 2005, 53, 9553–9559. [Google Scholar] [CrossRef]
- Goel, V.; Cheema, S.K.; Agellon, L.B.; Ooraikul, B.; Basu, T.K. Dietary rhubarb (Rheum rhaponticum) stalk fibre stimulates cholesterol 7a-hydroxylase gene expression and bile acid excretion in cholesterol-fed C57BL/6J mice. Br. J. Nutr. 1999, 81, 65–71. [Google Scholar] [CrossRef]
- Rana, V.; Bachheti, R.K.; Chand, T.; Barman, A. Dietary fibre and human health. Int. J. Food Saf. Nutr. Public Health 2011, 4, 101–118. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Basu, T.K.; Ooraikul, B.; Garg, M. The lipid lowering effects of rhubarb stalk fibre: A new source of dietary fibre. Nutr. Res. 1993, 13, 1017–1024. [Google Scholar] [CrossRef]
- Goel, V.; Ooraikul, B.; Basu, T.K. Cholesterol lowering effects of rhubarb fibre in hypercholesterolemic men. J. Am. Coll. Nutr. 1997, 16, 600–604. [Google Scholar]
- Goel, V.; Cheema, S.K.; Agellon, L.B.; Ooraikul, B.; McBurney, M.I.; Basu, T.K. In vitro binding of bile salt to rhubarb stalk powder. Nutr. Res. 1998, 18, 893–903. [Google Scholar] [CrossRef]
- Dongowski, G. Interactions between dietary fibre-rich preparations and glycoconjugated bile acids in vitro. Food Chem. 2007, 104, 390–397. [Google Scholar] [CrossRef]
- Cheema, S.K.; Goel, V.; Basu, T.K.; Agellon, L.B. Dietary rhubarb (Rheum rhaponticum) stalk fibre does not lower plasma cholesterol levels in diabetic rats. Br. J. Nutr. 2003, 89, 201–216. [Google Scholar] [CrossRef]
- Fernandez, M.L. Distinct mechanisms of plasma LDL lowering by dietary fibre in the guinea pig: Specific effects of pectin, guar gum and psyllium. J. Lipid Res. 1995, 36, 2394–2404. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Lin, E.C.; Trejo, A.; McNamara, D.J. Prickly pear (Opuntia sp.) pectin reverses low density lipoprotein receptor suppression induced by a hypercholesterolemic diet in guinea pigs. J. Nutr. 1992, 122, 2330–2340. [Google Scholar] [CrossRef]
- Shen, H.; He, L.; Price, R.L.; Fernandez, M.L. Dietary soluble fiber lowers plasma LDL cholesterol concentrations by altering lipoprotein metabolism in female guinea pigs. J. Nutr. 1998, 128, 1434–1441. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, E.; Ma, L.; Zhai, P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012, 11, 56. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Lee, S.-A.; Choi, M.-S. Antiobesity and vasoprotective effects of resveratrol in ApoE-deficient mice. J. Med. Food. 2014, 17, 310–316. [Google Scholar] [CrossRef]
- Rašković, A.; Ćućuz, V.; Torović, L.; Tomas, A.; Gojković-Bukarica, L.; Ćebović, T.; Milijašević, B.; Stilinović, N.; CvejićHogervorst, J. Resveratrol supplementation improves metabolic control in rats with induced hyperlipidemia and type 2 diabetes. Saudi Pharm. J. 2019, 27, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.P.; Kim, J.K.; Lim, Y.H. Antihyperlipidemic effects of rhapontin and rhapontigenin from rheum undulatum in rats fed a high-cholesterol diet. Planta Med. 2014, 80, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, M.; Lu, Y.; Wang, L.; Wu, C.; Duan, H. Rhaponticin from rhubarb rhizomes alleviates liver steatosis and improves blood glucose and lipid profiles in KK/Ay diabetic mice. Planta Med. 2009, 75, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Andrade, J.M.; Frade, A.C.; Guimarães, J.B.; Freitas, K.M.; Lopes, M.T.; Guimarães, A.L.; de Paula, A.M.; Coimbra, C.C.; Santos, S.H. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, J.; Wang, Z.; Zhang, G.; Yin, J.; Wang, X.; Wang, S.; Yu, Z. Resveratrol reduces the inflammatory response in adipose tissue and improves adipose insulin signaling in high-fat diet-fed mice. PeerJ 2018, 6, e5173. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van deWeijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 18191827. [Google Scholar] [CrossRef]
- Van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arter. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef]
- Knop, F.K.; Konings, E.; Timmers, S.; Schrauwen, P.; Holst, J.J.; Blaak, E.E. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabet. Med. 2013, 30, 1214–1218. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30-days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No beneficial effects of resveratrol on the metabolic syndrome: A randomized placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef]
- Anton, S.D.; Embry, C.; Marsiske, M.; Lu, X.; Doss, H.; Leeuwenburgh, C.; Manini, T.M. Safety and metabolic outcomes of resveratrol supplementation in older adults: Results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014, 57, 181–187. [Google Scholar] [CrossRef]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef]
- Van der Made, S.M.; Plat, J.; Mensink, R.P. Trans-resveratrol supplementation and endothelial function during the fasting and postprandial phase: A randomized placebo-controlled trial in overweight and slightly obese participants. Nutrients 2017, 9, 596. [Google Scholar] [CrossRef]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.L.; Breslow, J.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2018, 4, 122–135. [Google Scholar]
- De Ligt, M.; Bergman, M.; Fuentes, R.M.; Essers, H.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; Schrauwen, P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: A randomized trial. Am. J. Clin. Nutr. 2020, 12, 1029–1038. [Google Scholar] [CrossRef]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, L.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef]
- Most, J.; Warnke, I.; van Boekschoten, M.; Jocken, J.W.; de Groot, P.; Friedel, A.; Bendik, I.; Goossens, G.H.; Blaak, E.E. The effects of polyphenol supplementation on adipose tissue morphology and gene expression in overweight and obese humans. Adipocyte 2018, 7, 190–196. [Google Scholar] [CrossRef]
- Arzola-Paniagua, M.A.; García-Salgado López, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef]
- Su, Z.-L.; Hang, P.-Z.; Hu, J.; Zheng, Y.-Y.; Sun, H.-Q.; Guo, J.; Liu, K.-Y.; Du, Z.-M. Aloe-emodin exerts cholesterol-lowering effects by inhibiting proprotein convertase subtilisin/kexin type 9 in hyperlipidemic rats. Acta Pharmacol. Sin. 2020, 41, 1085–1092. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, S.J.; Kwon, E.Y.; Choi, M.S. Physcion reduces lipid accumulation and prevents the obesity in mice. Nutr. Metab. 2019, 16, 31. [Google Scholar] [CrossRef]
- Wang, J.; Ji, J.; Song, Z.; Zhang, W.; He, X.; Li, F.; Zhang, C.; Guo, C.; Wang, C.; Yuan, C. Hypocholesterolemic effect of emodin by simultaneous determination of in vitro and in vivo bile salts binding. Fitoterapia 2016, 110, 116–122. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Shang, F.; Ning, Y.; Huang, Z.; He, R.; Sun, J.; Dong, S. Emodin improves glucose and lipid metabolism disorders in obese mice via activating brown adipose tissue and inducing browning of white adipose tissue. Front. Endocrinol. 2021, 12, 618037. [Google Scholar] [CrossRef]
- Czernichow, S.; Batty, G.D. Withdrawal of sibutramine for weight loss: Where does this leave clinicians? Obes. Facts 2010, 3, 155–156. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Qi, X. Review of the clinical effect of orlistat. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Xiamen, China, 15–17 December 2018. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, A.G.; de la Rosa, L.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kasabri, V.; Al-Hallaq, E.K.; Bustanji, Y.K.; Abdul-Razzak, K.K.; Abaza, I.F.; Afifi, F.U. Antiobesity and antihyperglycaemic effects of Adiantumcapillus-veneris extracts: In-vitro and in-vivo evaluations. Pharm. Biol. 2017, 55, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of antilipase components of Artemisia argyi leaves based on spectrum-effect relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef]
- Vijayaraj, P.; Nakagawa, H.; Yamaki, K. Cyanidin and cyanidin-3-glucoside derived from Vignaunguiculata act as noncompetitive inhibitors of pancreatic lipase. J. Food Biochem. 2019, 43, e12774. [Google Scholar] [CrossRef]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, T.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, E.-W.; Eom, S.-H.; Kim, T.H. Pancreatic lipase inhibitory stilbenoids from the roots of Vitisvinifera. Int. J. Food Sci. Nutr. 2014, 65, 97–100. [Google Scholar] [CrossRef]
- Habtemariam, S. Antihyperlipidemic components of Cassia auriculata aerial parts: Identification through in vitro studies. Phytother. Res. 2013, 27, 152–155. [Google Scholar] [CrossRef]
- Batubara, L.; Kuspradini, H.; Muddathir, A.M.; Mitsunaga, T. Intsiapalembanica wood extracts and its isolated compounds as Propionibacterium acnes lipase inhibitor. J. Wood Sci. 2014, 60, 169–174. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Wang, Y.; Cheng, Y. Hollow fiber based affinity selection combined with high performance liquid chromatography-mass spectroscopy for rapid screening lipase inhibitors from lotus leaf. Anal. Chim. Acta 2013, 785, 75–81. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Cai, S.; Wang, O.; Ji, B. Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obes. Res. Clin. Pract. 2016, 10, 327–339. [Google Scholar] [CrossRef]
- Zheng, C.-D.; Duan, Y.-Q.; Gao, J.-M.; Ruan, Z.-G. Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J. Chin. Med. Assoc. 2020, 73, 319–324. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Shahouzebi, B.; Sharifi-Far, F. Inhibitory effect of some plant extracts on pancreatic lipase. Int. J. Pharmacol. 2010, 6, 18–24. [Google Scholar] [CrossRef]
- Abe, I.; Seki, T.; Noguchi, H.; Kashiwada, Y. Galloyl esters from rhubarb are potent inhibitors of squalene epoxidase, a key enzyme in cholesterol biosynthesis. Planta Med. 2000, 66, 753–756. [Google Scholar] [CrossRef]
- Shi, D.H.; Xu, C.; Guo, B.X.; Wang, X.T.; Chen, Y.X.; Tan, R.X. Inhibition of soluble epoxide hydrolase by extracts derived from inflammation-treating Chinese medicinal herbs. Phytother. Res. 2008, 22, 1264–1268. [Google Scholar] [CrossRef]
- Li, W.; Kim, J.H.; Zhou, W.; Shim, S.H.; Ma, J.Y.; Kim, Y.H. Soluble epoxide hydrolase inhibitory activity of phenolic components from the rhizomes and roots of Gentiana scabra. Biosci. Biotechnol. Biochem. 2015, 79, 907–911. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lee, S.; Jang, H.J.; Su, X.D.; Wang, H.S.; Kim, Y.H.; Yang, S.Y. Inhibition potential of phenolic constituents from the aerial parts of Tetrastigmahemsleyanum against soluble epoxide hydrolase and nitric oxide synthase. J. Enzyme Inhib. Med. Chem. 2019, 34, 753–760. [Google Scholar] [CrossRef]
- Han, Y.K.; Lee, J.S.; Yang, S.Y.; Lee, K.Y.; Kim, Y.H. In Vitro and in silico studies of soluble epoxide hydrolase inhibitors from the roots of lycopuslucidus. Plants 2021, 10, 356. [Google Scholar] [CrossRef]
- Jo, A.R.; Kim, J.H.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. Soluble epoxide hydrolase inhibitory components from Rheum undulatum and in silico approach. J. Enzyme Inhib. Med. Chem. 2016, 31, 70–78. [Google Scholar] [CrossRef]
- Kitamura, S.; Morisseau, C.; Inceoglu, B.; Kamita, S.G.; De Nicola, G.R.; Nyegue, M.; Hammock, B.D. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandrabrazzeanabaillon: Synthesis, quantification, and measurement of biological activities in vitro and in vivo. PLoS ONE 2015, 10, e0117438. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int. J. Endocrinol. 2016, 2016, 1216783. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, S.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Miao, J.; He, X.; Hu, J.; Cai, W. Emodin inhibits NF-κB signaling pathway to protect obese asthmatic rats from pathological damage via Visfatin. Tissue Cell 2022, 74, 101713. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Mongioì, L.M.; La Vignera, S.; Cannarella, R.; Cimino, L.; Compagnone, M.; Condorelli, R.A.; Calogero, A.E. The role of resveratrol administration in human obesity. Int. J. Mol. Sci. 2021, 22, 4362. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Seo, S.G.; Heo, Y.S.; Yue, S.; Cheng, J.-X.; Lee, K.W.; Kim, K.-H. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef]
- Yamamoto, T.; Li, Y.; Hanafusa, Y.; Yeh, Y.-S.; Maruki-Uchida, H.; Kawakami, S.; Sai, M.; Goto, T.; Ito, T.; Kawada, T. Piceatannol exhibits anti-inflammatory effects on macrophages interacting with adipocytes. Food Sci. Nutr. 2017, 5, 76–85. [Google Scholar] [CrossRef]
- Park, I.S.; Han, Y.; Jo, H.; Lee, K.W.; Song, Y.S. Piceatannol is superior to resveratrol at suppressing adipogenesis in human visceral adipose-derived stem cells. Plants 2021, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.L.; Tao, R.Y.; Niu, Y.J.; Chen, X.G.; Tian, J.Y.; Ye, F. Rhein, an inhibitor of adipocyte differentiation and adipogenesis. J. Asian Nat. Prod. Res. 2011, 13, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, S.; Hu, N.; Gu, M.; Chu, C.; Li, Y.; Lu, X.; Huang, C. Rhein reduces fat weight in db/db mouse and prevents diet-induced obesity in C57Bl/6 mouse through the inhibition of PPARγ signaling. PPAR Res. 2012, 2012, 374936. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhu, X.; Zhang, Y.; Cui, G.; Peng, L.; Lu, X.; Zang, Y.Q. Rhein protects against obesity and related metabolic disorders through liver X receptor-mediated uncoupling protein 1 upregulation in brown adipose tissue. Int. J. Biol. Sci. 2012, 8, 1375–1384. [Google Scholar] [CrossRef]
- Fang, J.Y.; Huang, T.H.; Chen, W.J.; Aljuffali, I.A.; Hsu, C.Y. Rhubarb hydroxyanthraquinones act as antiobesity agents to inhibit adipogenesis and enhance lipolysis. Biomed. Pharmacother. 2022, 146, 112497. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Xia, R.; Wei, L.; Zhang, C.; Zhang, J.; Zhao, L.; Wu, H.; Kang, L.; Yang, S. Danthron ameliorates obesity and MAFLD through activating the interplay between PPARα/RXRα heterodimer and adiponectin receptor 2. Biomed. Pharmacother. 2021, 137, 111344. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, H.; Wang, L.; Zhao, X.; He, L. Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharmazie 2018, 73, 228–233. [Google Scholar]
- Wang, Y.-J.; Huang, S.-L.; Feng, Y.; Ning, M.-M.; Leng, Y. Emodin, an 11β-hydroxysteroid dehydrogenase type 1 inhibitor, regulates adipocyte function in vitro and exerts anti-diabetic effect in ob/ob mice. Acta Pharmacol. Sin. 2012, 33, 1195–1203. [Google Scholar] [CrossRef]
- Reimer, R.A.; McBurney, M.I. Dietary fiber modulates intestinal pro-glucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 1996, 137, 3948–3956. [Google Scholar] [CrossRef]
- Reimer, R.A.; Thomson, A.B.; Rajotte, R.V.; Basu, T.K.; Ooraikul, B.; McBurney, M.I. A physiological level of rhubarb fiber increases proglucagon gene expression and modulates intestinal glucose uptake in rats. J. Nutr. 1997, 127, 1923–1928. [Google Scholar] [CrossRef][Green Version]
- Shojaei Shad, F.; Haghighi, M.J. Study of the effect of the essential oil (extract) of rhubarb stem (shoot) on glycosylated hemoglobin and fasting blood glucose levels in patients with type II diabetes. Biomedicine 2018, 8, 24. [Google Scholar] [CrossRef]
- Jiang, C.S.; Liang, L.F.; Guo, Y.W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012, 33, 1217–1245. [Google Scholar] [CrossRef]
- Saidu, Y.; Muhammad, S.A.; Abbas, A.Y.; Onu, A.; Tsado, I.M.; Muhammad, L. In vitro screening for protein tyrosine phosphatase 1B and dipeptidyl peptidase IV inhibitors from selected Nigerian medicinal plants. J. Intercult. Ethnopharmacol. 2016, 6, 154–157. [Google Scholar]
- Lee, W.; Kim, S.N.; Yoon, G. Screening of medicinal herbs for inhibitory activity against protein tyrosine phosphatase B. Korean J. Pharmacogn. 2010, 41, 227–231. [Google Scholar]
- Yue, H.; Jiang, S.; Wang, L.; Banma, C.; Zhou, G.; Shao, Y.; Tao, Y.; Zhao, X. Hypoglycemic ingredients identification of Rheum tanguticum Maxim. ex Balf. by UHPLC-triple-TOF-MS/MS and interrelationships between ingredients content and glycosidase inhibitory activities. Ind. Crops Prod. 2022, 178, 114595. [Google Scholar] [CrossRef]
- Choi, S.B.; Ko, B.S.; Park, S.K.; Jang, J.S.; Park, S. Insulin sensitizing and a-glucoamylase inhibitory action of sennosides, rheins and rhaponticin in RheiRhizoma. Life Sci. 2006, 78, 934–942. [Google Scholar] [CrossRef]
- Choi, S.Z.; Lee, S.O.; Jang, K.U.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch. Pharm. Res. 2005, 28, 1027–1030. [Google Scholar] [CrossRef]
- Hosseini, A.; Mollazadeh, H.; Amiri, M.S.; Sadeghnia, H.R.; Ghorbani, A. Effects of a standardized extract of Rheum turkestanicumJanischew root on diabetic changes in the kidney, liver and heart of streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017, 86, 605–611. [Google Scholar] [CrossRef]
- Bahnasy, R.M. Rhubarb (Rheum emodi) improves some immunity markers and blood glucose in rats exposed to glucocorticoids. Home Econ. J. 2020, 36, 111–128. [Google Scholar] [CrossRef]
- Cheng, F.R.; Cui, H.X.; Fang, J.L.; Yuan, K.; Guo, Y. Ameliorative effect and mechanism of the purified anthraquinone-glycoside preparation from Rheum Palmatum L. on type 2 diabetes mellitus. Molecules 2019, 24, 1454. [Google Scholar] [CrossRef]
- Hadjzadeh, M.A.; Rajaei, Z.; Khodaei, E.; Malek, M.; Ghanbari, H. Rheum turkestanicum rhizomes possess anti-hypertriglyceridemic, but not hypoglycemic or hepatoprotective effect in experimental diabetes. Avicenna J. Phytomed. 2017, 7, 1–9. [Google Scholar]
- Cui, H.-X.; Zhang, L.-S.; Luo, Y.; Yuan, K.; Huang, Z.-Y.; Guo, Y. A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. Front. Microbiol. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Lee, W.; Yoon, G.; Hwang, Y.R.; Kim, Y.K.; Kim, S.N. Anti-obesity and hypolipidemic effects of Rheum undulatum in high-fat diet-fed C57BL/6 mice through protein tyrosine phosphatase 1B inhibition. BMB Rep. 2012, 45, 141–146. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.; Zeng, X.; Ou, Z.; Xue, M.; Gao, D.; Liu, S.; Li, S.; Yang, S. Rheum palmatum L. attenuates high fat diet-induced hepatosteatosis by activating AMP-activated protein kinase. Am. J. Chin. Med. 2016, 44, 551–564. [Google Scholar] [CrossRef]
- Moon, M.K.; Kang, D.G.; Lee, A.S.; Yeom, K.B.; Kim, J.S.; Lee, H.S. Anti-atherogenic effects of the aqueous extract of rhubarb in rats fed an atherogenic diet. Am. J. Chin. Med. 2008, 36, 555–568. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, F.; Liu, Y.; Zhang, C.; Yu, H.; Zhang, Y.; Zhao, Y. Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. Phytother. Res. 2008, 22, 935–942. [Google Scholar] [CrossRef]
- Shokri, H.; Farokhi, F.; Heydari, R.; Manaffar, R. Renoprotective effect of hydroalcoholic extract of Rheum ribes root in diabetic female rats. Avicenna J. Phytomed. 2014, 4, 392–401. [Google Scholar]
- Hadjzadeh, M.A.R.; Parsaee, H.; Sadeghian, A. Cholesterol lowering effects of Rheum ribs in hypercholesterolemic rabbits. Med. J. Islamic Repub. Iran 2004, 18, 277–280. [Google Scholar]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; van Hul, M.; Cani, P.D. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansiamuciniphila in mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Liu, Y.-F. Treatment with rhubarb improves brachial artery endothelial function in patients with atherosclerosis: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Chin. Med. 2007, 35, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Shojaei-Shad, F.; Jahantigh-Haghighi, M.; Mansouri, A.; Jahantigh-Haghighi, M. The effect of Rhubarb stem extract on blood pressure and weight of type 2 diabetic patients. Med. Sci. 2019, 23, 159–162. [Google Scholar]
- Roerig, J.L.; Steffen, K.J.; Mitchell, J.E.; Zunker, C. Laxative abuse. Epidemiology, diagnosis and management. Drugs 2010, 70, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Zhou, Q.-X.; Zhang, D.; Jiang, X.-H. Investigation the distributions and pharmacokinetics of five rhubarb anthraquinones in rabbits and rats. J. Anal. Pharm. Res. 2016, 3, 00048. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubiacordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.S.; Chen, F.; Liu, X.H.; Xu, H.; Zhou, Y.Z. Progress in research of chemical constituents and pharmacological actions of Rhubarb. Chin. J. New Drugs 2011, 20, 1534–1548. [Google Scholar]
- Rehman, H.; Begum, W.; Anjum, F.; Tabasum, H. Rheum emodi (Rhubarb): A fascinating herb. J. Pharmacogn. Phytochem. 2014, 3, 89–94. [Google Scholar]
- Li, F.; Wang, S.-C.; Wang, X.; Ren, Q.-Y.; Wang, W.; Shang, G.-W.; Zhang, L.; Zhang, S.-H. Novel exploration of cathartic pharmacology induced by rhubarb. ZhongguoZhong Yao ZaZhi 2008, 33, 481–484. [Google Scholar]
- Wei, H.; Chang, J.; Liu, P.; Li, Z.; Miao, G.; Liu, X.; Liu, C.; Zhang, X. Design and evaluation of rhubarb total free anthraquinones oral colon-specific drug delivery granules to improve the purgative effect. Braz. J. Pharm. Sci. 2019, 55, e17110. [Google Scholar] [CrossRef]
- Liu, P.; Wei, H.; Chang, J.; Miao, G.; Liu, X.; Li, Z.; Liu, L.; Zhang, X.; Liu, C. Oral colon-specific drug delivery system reduces the nephrotoxicity of rhubarb anthraquinones when they produce purgative efficacy. Exp. Ther. Med. 2017, 14, 3589–3601. [Google Scholar] [CrossRef]
- Xie, W.; Xing, D.; Zhao, Y.; Su, H.; Meng, Z.; Chen, Y.; Du, L. A new tactic to treat postprandial hyperlipidemia in diabetic rats with gastroparesis by improving gastrointestinal transit. Eur. J. Pharmacol. 2005, 510, 113–120. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Chien, T.-Y.; Chen, J.-R.; Lin, I.-H.; Wang, C.-C. Hypolipidemic effects of three purgative decoctions. Evid.-Based Complement. Altern. Med. 2011, 2011, 249254. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.H.; Cha, J. Antiobesity effects of the combined plant extracts varying the combination ratio of phyllostachyspubescens leaf extract and scutellariabaicalensis root extract. Evid.-Based Complement. Altern. Med. 2016, 2016, 9735276. [Google Scholar] [CrossRef]

| The Examined Substances | Type of Study | Experimental Model, Doses and Concentrations | Main Effects of the Rhubarb Fibre and Stilbenes Administration | References |
|---|---|---|---|---|
| ANIMAL STUDIES | ||||
| R. rhaponticum stalk-derived preparation containing 74% of dietary fibre/dry mass (incl. 66% insoluble and 8% soluble) | animal | mice fed with cholesterol-enriched diet with 5% of rhubarb stalk fibre, for 4 weeks | ↓ the acyl CoA: cholesterol acyltransferase (ACAT) activity; no effects on the cholesterol-rich diet enhancement of the β-hydroxyβ-methyl coenzyme A reductase (HMGR) activity | [22] |
| R. rhaponticum stalk fibre | animal | cholesterol-fed C57BL/6J mice receiving the fibre-rich diet (50 g/kg b.w.) for 4 weeks | ↓ plasma cholesterol (−13%); ↓ the hepatic concentrations of total cholesterol (by 34%) and cholesteryl esters (by 34%); ↓ acyl CoA: cholesterol acyltransferase activity; ↓ the faecal bile acid loss; ↓the gallbladder bile acid pool | [19] |
| R. rhaponticum stalk fibre | animal | the diabetes-prone and the streptozotocin-induced diabetic rats receiving the fibre-rich diet (50 g/kg b.w.) for 2 weeks | No effect on the plasma cholesterol and triacylglycerol levels in diabetic rats | [26] |
| HUMAN STUDIES | ||||
| the rhubarb-stalk-derived preparation containing 74% dietary fibre/dry mass (incl. 66% insoluble and 8% soluble) | human | Ten hypercholesterolemic men (BMI of 27.9 ± 3.8 kg/m2); 27 g of rhubarb fibre/day, for 4 weeks | ↓ serum total cholesterol (−8%) and LDL cholesterol (−9%); no changes in HDL cholesterol level; a return of the cholesterol-lowering effect to baseline after the fibre supplementation withdrawal for one month | [23] |
| The Examined Substances | Experimental Model, Doses and Concentrations | Main Effects of the Stilbene Administration | References |
|---|---|---|---|
| Rhaponticin and rhapontigenin isolated from R. rhabarbarum roots | rats fed a high-cholesterol diet, followed by oral rhapontin or rhapontigenin treatment (1, 2.5 and 5 mg/kg b.w. (body weight)/day) | ↓ the serum lipid level; ↑ HDL cholesterol; improvement in the degenerating fatty liver structure; the aspartate aminotransferase (AST) and the alanine aminotransferase (ALT) levels comparable to the control group | [33] |
| Rhaponticin from R. rhabarbarum roots | KK/Ay type 2 diabetic mice treated with rhaponticin (125 mg/kg b.w., 4 weeks) | ↓ the plasma triglyceride, LDL, cholesterol, non-esterified free fatty acids; ↓ lactate dehydrogenase, creatine kinase, AST and ALT activities | [34] |
| Resveratrol | high-fat diet (HFD)-fed C57BL/6 J mice, a daily dose of 200 mg/kg b.w. of resveratrol, for 8 weeks | anti-hypercholesterolemic effects: improvement in serum lipid parameters, ↓ hepatic cholesterol, ↓ body weight, ↑ bile acid pool size, ↑ liver CYP7A1 mRNA expression and CYP7A1 enzyme activity | [30] |
| apoE-deficient mice fed an atherogenic diet containing 0.02% resveratrol (w/w), for 12 weeks | ↓ the plasma total cholesterol, LDL cholesterol, non-high-density-lipoprotein cholesterol, apoB/apoA1 ratio, hepatic cholesterol and triglyceride; ↑ the plasma HDL cholesterol | [31] | |
| mice fed standard diet plus resveratrol (4 g/kg of food to provide a 400-mpk dose), for 8 weeks | ↑ brown adipose tissue thermogenesis; ↑ mRNA of thermogenesis-related genes, incl. uncoupling protein 1 (UCP1), sirtuin 1 (SIRT1), phosphatase and tensin homolog (PTEN) and bone morphogenetic protein 7 (BMP-7) expression; ↓ fat accumulation in adipose tissue; ↓ total cholesterol and glucose levels in plasma | [35] | |
| C57BL/6 mice fed a high-fat diet with a low dose of resveratrol, i.e., 200 mg/kg b.w./day (HFD-RES/L) or with a high dose of resveratrol, i.e., 400 mg/kg b.w./day (HFD-RES/H) | ↓ insulin resistance; ↑ expressions of pAkt, glucose transporter type 4 (GLUT4) and insulin receptor substrate 1 (IRS-1) in white adipose tissue (WAT); ↓ proinflammatory cytokine levels in serum; ↓ macrophage infiltration and C-C chemokine receptor type 2 (CCR2) chemokine expression in white adipose tissue (WAT) | [36] | |
| rats with hyperlipidemia; a daily dose of 20 mg/kg b.w., of resveratrol, for 30 days | ↓ LDL and triglyceride levels; ↑ HDL levels in animals; | [32] |
| Number of Participants (n), Resveratrol Doses and Study Duration | Participant Diagnosis | Main Effects of Resveratrol Supplementation in the Context of an Anti-Obesity Action | References |
|---|---|---|---|
| n = 11; 150 mg/day, for 30 days; a randomized, placebo-controlled, double-blind and cross-over study | obesity | Calorie-restriction-like effects; reduction in the sleeping and resting metabolic rate; ↓ intrahepatic lipid content, circulating glucose and triglycerides; ↓ inflammation markers; ↓ the systolic blood pressure; improvement in the HOMA index | [37] |
| n = 24; 500 mg, 3 times/day, for 4 weeks; a randomized, placebo-controlled study | obesity (BMI > 30 kg/m2) | No effect on the total cholesterol, HDL, LDL, plasma triglyceride and blood pressure; no changes in the resting metabolic rate and lipid oxidation | [38] |
| n = 28; 75 mg/day, for 6 weeks; a randomized, placebo-controlled, double-blind, cross-over study | obesity (BMI of 33.3 ± 0.6 kg/m2) | No effects on blood pressure; the flow-mediated dilatation (FMD) increased by 23% | [39] |
| n = 45; 150 mg/day, for 4 weeks; a randomized, placebo-controlled, cross-over study | overweight or obesity (BMI of 25–35 kg/m2) | No effects on metabolic risk markers related to cardiovascular health | [40] |
| n = 50; 500 mg/day, for 12 weeks; a randomized, placebo-controlled, double-blind study | overweight (BMI of 28.35 ± 3.49 and 28.75 ± 3.50 kg/m2, for the intervention and placebo group, respectively); non-alcoholic fatty liver disease | Reduction in BMI, waist circumference, HDL cholesterol and apo A1 both in intervention and placebo group; no differences in the above parameters between these groups; ↓ alanine transferase (ALT) and hepatic steatosis, compared to placebo | [41] |
| n = 8; 1000 mg once a day for a week, then 2000 mg/day for the next week; a randomized, placebo-controlled study | overweight or obesity (BMI of 27.0–40.0 kg/m2), mild hypertriglyceridemia | No effects on insulin sensitivity and blood plasma triglyceride level; ↓ apoB-48 and apoB-100 production rate | [42] |
| n = 10; 150 mg/a day, for 30 days; a randomized, placebo-controlled, double-blind, cross-over study | obesity (BMI of 32 ± 1 kg/m2) | Suppression of postprandial glucagon responses; no changes in fasting glucagon levels | [43] |
| n = 32; 300 mg/day or 1000 mg/day for 90 days; a randomized, placebo controlled, double-blind study | overweight or obesity (BMI of 25.0–34.9 kg/m2) | ↓ glucose levels compared to placebo; no changes in blood pressure, body weight and waist circumference | [47] |
| n = 11; 150 mg/day for 30 days; a randomized, placebo-controlled, double-blind, cross-over study | obesity (BMI of 28–36 kg/m2) | ↓ adipocyte size; changes in the adipose tissue morphology: reduction in the proportion of large and very large adipocytes; increase in small adipocytes; enhanced adipogenesis | [44] |
| n = 23; 200 mg/day for 26 weeks, a placebo-controlled study | overweight (BMI of 25–30 kg/m2), healthy older adults (50–80 years) | ↓ body fat and leptin increase compared to placebo; no significant changes in body weight, BMI or blood pressure compared to placebo | [45] |
| n = 161; 100 mg of resveratrol or 120 mg orlistat + 100 mg resveratrol (O-R group), 3 times a day, for 6 months; the participants consumed 500 k calories fewer than the usual diet; a randomized, placebo-controlled study | obesity (BMI of 30.0–39.9 kg/m2) | No significant changes in the group treated with resveratrol solely; ↓ BMI, waist circumference and fat mass in the orlistat-treated and O-R groups; the most effective one was the O-R combination | [55] |
| n = 74; 150 mg or 1000 mg/day, for 16 weeks; a randomized, placebo-controlled study | obesity (BMI of 33.8 ± 0.44 kg/m2) | No effect on blood pressure, body composition, lipid deposition in the liver or striated muscle; no beneficial effect on glucose and lipid metabolism; 1000 mg dose increased the total cholesterol and LDL compared to placebo group | [46] |
| n = 45; 75 mg twice a day, for 4 weeks; a randomized, placebo-controlled study | overweight or slight obesity (BMI of 28.3 ± 3.2 kg/m2) | No changes in plasma biomarkers of endothelial function or inflammation (both in the fasting state and postprandial phase); no changes in serum triglyceride and insulin level | [49] |
| n = 38; a combination of 282 or 80 mg/day of the epigallocatechin-3-gallate and resveratrol (EGCG + RES), for 12 weeks; a randomized, placebo-controlled study | overweight or obesity (BMI of 29.7 ± 0.5 kg/m2) | ↓ visceral adipose tissue mass; no effect on insulin-stimulated glucose disposal, endogenous glucose production or lipolysis; | [53] |
| n = 25; 282 or 80 mg/day of the EGCG + RES, for 12 weeks; a randomized, placebo-controlled study | overweight or obesity (BMI of 29.7 ± 1.1 kg/m2) | No changes in adipocyte size or surface area in abdominal subcutaneous adipose tissue; EGCG + RES downregulated pathways contributing to adipogenesis, cell cycle and apoptosis in the abdominal subcutaneous adipose tissue | [54] |
| n = 112; 75 mg, twice a day, for 12 weeks; a randomized, placebo-controlled study | overweight or obesity (BMI ≥ 27 kg/m2), insulin resistance | No effects on cardiometabolic risk parameters and liver fat content | [48] |
| n = 28; 1000 mg, twice a day, for 30 days; a randomized, placebo-controlled study | obesity (BMI of 30–40 kg/m2), metabolic syndrome | No changes in insulin resistance; no changes in adipose tissue metabolism | [50] |
| n = 41; 150 mg/day, for 6 months; a randomized, placebo-controlled study | overweight or obesity (BMI of 27–35 kg/m2) | No effects on intrahepatic lipid level, energy metabolism, blood pressure, physical performance, quality of life and sleep | [51] |
| n = 25; 250 mg/day, with physical training and diet, for 3 months; a randomized, placebo controlled, double-blind study | obesity (BMI ≥ 30 kg/m2), metabolic syndrome | Resveratrol potentiated beneficial effects of diet and physical training; ↓ VLDL and the total cholesterol in blood plasma | [52] |
| Compound | Phytochemical Classification | Pancreatic Lipase Inhibitory Effects | References | |
|---|---|---|---|---|
| IC50 for the Examined Compound | IC50 for Orlistat | |||
| Caffeic acid | Phenolic acids | 401.5 μM | 4.0 μM | [64] |
| Chlorogenic acid | 110.0 μM 114.0 μM | 0.23 μM ND | [65] | |
| p-Coumaric acid | 170.2 μM | 4.0 μM | [64] | |
| Ellagic acid | 44.78 μM | 0.23 μM | [65] | |
| Ferulic acid | 2.49 μM | 4.0 μM | [65] | |
| Cyanidin-3-rutinoside | Anthocyanidins and their derivatives | 188.28 μM | ND | [67] |
| 59.4 μM | 31.7 μM | [68] | ||
| Delphinidin-3-glucoside | 223.26 μM | ND | [68] | |
| Procyanidin B2 | Proanthocyanidins | 7.96 μM | ND | [69] |
| cis-Piceid | Stilbene derivatives | 76.1 μM | 0.7 μM | [70] |
| trans-Piceid | 121.5 μM | 0.7 μM | [70] | |
| trans-Resveratrol | >200 μM | 0.7 μM | [70] | |
| Kaempferol-3-O-rutinoside | Flavonoids and their glycosides | 2.9 μM | 1.45 μM | [71] |
| Quercetin | 421.1 μM | ND | [72] | |
| 146 μM | 1.45 μM | [71] | ||
| Quercetin-3-O-β-D-glucuronide | 94 μM | ND | [73] | |
| Rutin | 149 μM | 1.45 μM | [71] | |
| The Examined Rhubarb Compounds or Extracts | Experimental Model/Doses | Main Findings | References |
|---|---|---|---|
| desoxyrhapontigenin, emodin and chrysophanol, from roots of R. rhabarbarum | mice | ↓ postprandial hyperglycaemia by 35.8, 29.5, 42.3%, respectively | [109] |
| 70% ethanol Rhei Rhizoma extract | streptozotocin-induced diabetes in mice/5 mg/kg b.w. (body weight), 8 weeks | ↑ insulin-stimulated glucose uptake, ↓ carbohydrate digestion via inhibiting alpha-glucoamylase | [109] |
| decoction from R. turkestanicum rhizome | diabetic rats/200–600 mg/kg b.w, 3 weeks | no effects on serum glucose, ↓ serum triglyceride level | [113] |
| standardized extract from R. turkestanicum roots | streptozotocin-induced diabetes in rats/100, 200 and 300 mg/kg b.w., 4 weeks | ↓ blood glucose, ↓ diabetic changes in kidneys, liver and heart | [110] |
| R. emodi extract | rats treated with glucocorticoids/10, 20 and 30 g of rhubarb powder/kg of diet, 8 weeks | ↓ blood glucose and immunity markers | [111] |
| anthraquinone-glycoside preparation from R. palmatum | high-fat diet-induced type 2 diabetes mellitus in rats/100, 200, and 400 mg/kg b.w., 6 weeks | ↓ fasting blood glucose, ↓ total cholesterol and triglyceride levels, improvement in pathological changes in the liver, kidney and pancreatic tissues | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liudvytska, O.; Kolodziejczyk-Czepas, J. A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action. Nutrients 2022, 14, 2053. https://doi.org/10.3390/nu14102053
Liudvytska O, Kolodziejczyk-Czepas J. A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action. Nutrients. 2022; 14(10):2053. https://doi.org/10.3390/nu14102053
Chicago/Turabian StyleLiudvytska, Oleksandra, and Joanna Kolodziejczyk-Czepas. 2022. "A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action" Nutrients 14, no. 10: 2053. https://doi.org/10.3390/nu14102053
APA StyleLiudvytska, O., & Kolodziejczyk-Czepas, J. (2022). A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action. Nutrients, 14(10), 2053. https://doi.org/10.3390/nu14102053





