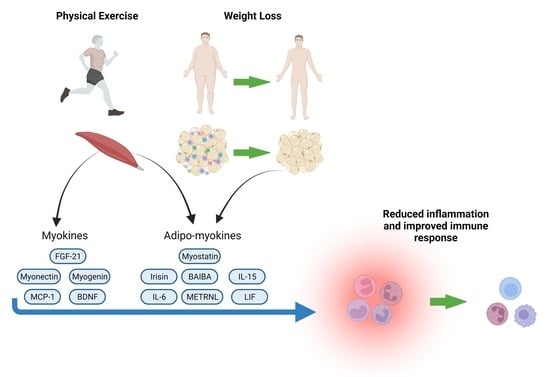
Influence of Nutritional Status and Physical Exercise on Immune Response in Metabolic Syndrome
Highlights
- Role of Muscle Tissue as an Endocrine Organ: The review explores the role of skeletal muscle as an endocrine organ, capable of modulating immune and metabolic function through the release of myokines during exercise.
- Interaction of Nutrition, Exercise and Inflammation: Examines how adequate nutrition and exercise can attenuate the chronic low-grade inflammation associated with metabolic syndrome.
- Innovative Perspective on Adipo-Myokines: Highlights the importance of adipo-myokines as key mediators in the communication between skeletal muscle and adipose tissue, with implications in immune metabolic control.
- Clinical Implications for Viral Infections: Analyses the role of obesity-related inflammation and metabolic syndrome in worsening viral infections, including H1N1 and SARS-CoV-2.
- Summary of Recent Evidence: Provides an up-to-date overview of data on the immunology of exercise and metabolic modulation in health and disease.
Abstract
1. Introduction
2. Metabolic Syndrome and Viral Infections
3. Adipose Tissue Dysfunction and Immunomodulation in MetS
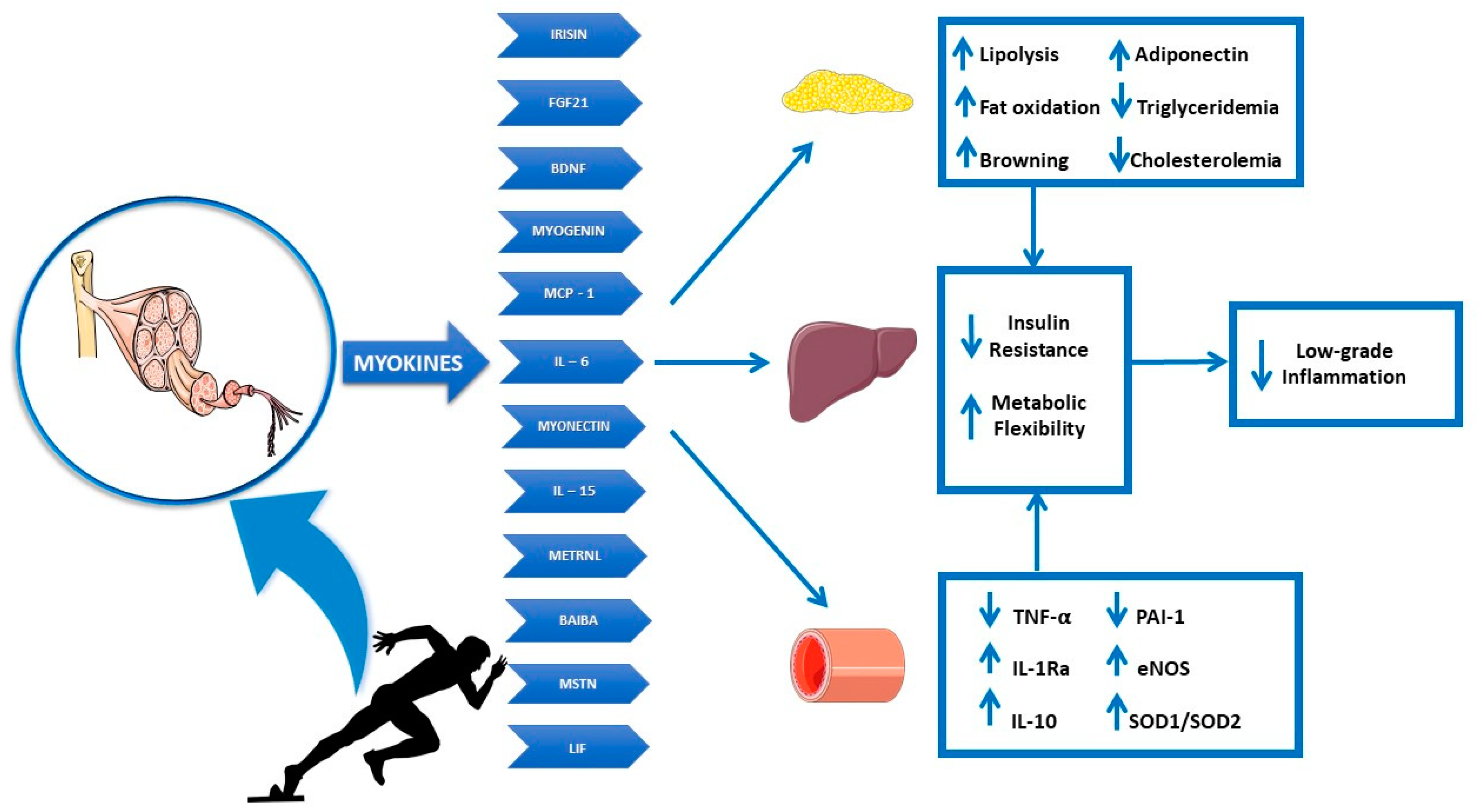
4. Skeletal Muscle and Exercise Immunology
5. Myokines
6. Adipo-Myokines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Council of the Obesity Society. Obesity as a disease: The Obesity Society Council resolution. Obesity (Silver Spring) 2008, 16, 1151. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Garrey, W.E.; Bryan, W.R. Variations in White Blood Cell Counts. Physiol. Rev. 1935, 15, 597–638. [Google Scholar] [CrossRef]
- Hoffman-Goetz, L.; Keir, R.; Thorne, R.; Houston, M.E.; Young, C. Chronic exercise stress in mice depresses splenic T lymphocyte mitogenesis in vitro. Clin. Exp. Immunol. 1986, 66, 551–557. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Tvede, N.; Pedersen, B.K.; Hansen, F.R.; Bendix, T.; Christensen, L.D.; Galbo, H.; Halkjaer-Kristensen, J. Effect of physical exercise on blood mononuclear cell subpopulations and in vitro proliferative responses. Scand J. Immunol. 1989, 29, 383–389. [Google Scholar] [CrossRef]
- Mackinnon, L.T.; Chick, T.W.; van As, A.; Tomasi, T.B. The effect of exercise on secretory and natural immunity. Adv. Exp. Med. Biol. 1987, 216A, 869–876. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. (1985) 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Thorne, R.J.; Houston, M.E. Splenic immune responses following treadmill exercise in mice. Can. J. Physiol. Pharmacol. 1988, 66, 1415–1419. [Google Scholar] [CrossRef]
- Smith, R.; Mathis, A.D.; Ventura, D.; Prince, J.T. Proteomics, lipidomics, metabolomics: A mass spectrometry tutorial from a computer scientist’s point of view. BMC Bioinform. 2014, 15 (Suppl. S7), S9. [Google Scholar] [CrossRef] [PubMed]
- Moffa, S.; Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Impronta, F.; Sorice, G.P.; Santoro, A.; Di Giuseppe, G.; Pontecorvi, A.; Giaccari, A. The Interplay between Immune System and Microbiota in Diabetes. Mediat. Inflamm. 2019, 2019, 9367404. [Google Scholar] [CrossRef]
- Pratesi, A.; Tarantini, F.; Di Bari, M. Skeletal muscle: An endocrine organ. Clin. Cases Miner. Bone Metab. 2013, 10, 11–14. [Google Scholar] [CrossRef]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Feraco, A.; Gorini, S.; Armani, A.; Camajani, E.; Rizzo, M.; Caprio, M. Exploring the Role of Skeletal Muscle in Insulin Resistance: Lessons from Cultured Cells to Animal Models. Int. J. Mol. Sci. 2021, 22, 9327. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Lombardo, M.; Bellia, C.; Moletto, C.; Aulisa, G.; Padua, E.; Della-Morte, D.; Caprio, M.; Bellia, A. Effects of Quality and Quantity of Protein Intake for Type 2 Diabetes Mellitus Prevention and Metabolic Control. Curr. Nutr. Rep. 2020, 9, 329–337. [Google Scholar] [CrossRef]
- Xuan, S.; Szabolcs, M.; Cinti, F.; Perincheri, S.; Accili, D.; Efstratiadis, A. Genetic analysis of type-1 insulin-like growth factor receptor signaling through insulin receptor substrate-1 and -2 in pancreatic beta cells. J. Biol. Chem. 2010, 285, 41044–41050. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Mezza, T.; Severi, I.; Suleiman, M.; Cefalo, C.M.A.; Sorice, G.P.; Moffa, S.; Impronta, F.; Quero, G.; Alfieri, S.; et al. Noradrenergic fibers are associated with beta-cell dedifferentiation and impaired beta-cell function in humans. Metabolism 2021, 114, 154414. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Ryoo, S.G. Prevalence of Diabetes in the 2009 Influenza A (H1N1) and the Middle East Respiratory Syndrome Coronavirus: A Systematic Review and Meta-Analysis. J. Public Health Res. 2016, 5, 733. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Syrjanen, J. Obesity and the outcome of infection. Lancet Infect. Dis. 2010, 10, 442–443. [Google Scholar] [CrossRef]
- Milner, J.J.; Beck, M.A. The impact of obesity on the immune response to infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection-from underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T.; California Pandemic Working, G. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef]
- Kwong, J.C.; Campitelli, M.A.; Rosella, L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: A cohort study. Clin. Infect. Dis. 2011, 53, 413–421. [Google Scholar] [CrossRef]
- Allard, R.; Leclerc, P.; Tremblay, C.; Tannenbaum, T.N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010, 33, 1491–1493. [Google Scholar] [CrossRef]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Sohn, S.H.; Lee, S.Y.; Park, H.L.; Park, Y.W.; Kim, H.; Nam, J.H. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ. Toxicol. Pharmacol. 2015, 40, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Hertz, T.; Johnson, C.; Mehle, A.; Krammer, F.; Schultz-Cherry, S. Obesity Outweighs Protection Conferred by Adjuvanted Influenza Vaccination. mBio 2016, 7, e01144-16. [Google Scholar] [CrossRef] [PubMed]
- Knoll, R.; Schultze, J.L.; Schulte-Schrepping, J. Monocytes and Macrophages in COVID-19. Front. Immunol. 2021, 12, 720109. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, L.E.; Roche, H.M.; Sheedy, F.J. Obesity, COVID-19 and innate immunometabolism. Br. J. Nutr. 2021, 125, 628–632. [Google Scholar] [CrossRef]
- Chu, Y.; Yang, J.; Shi, J.; Zhang, P.; Wang, X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 64. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Siswanto, B.B.; Meyer, M. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab. 2021, 47, 101178. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Zhu, G.; Wang, Q.; Lv, Q.; Huang, Y.; Yu, Y.; Si, X.; Yi, H.; Wang, C.; et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: A retrospective cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001476. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pr. 2020, 167, 108382. [Google Scholar] [CrossRef]
- Cariou, B.; Pichelin, M.; Goronflot, T.; Gonfroy, C.; Marre, M.; Raffaitin-Cardin, C.; Thivolet, C.; Wargny, M.; Hadjadj, S.; Gourdy, P.; et al. Phenotypic characteristics and prognosis of newly diagnosed diabetes in hospitalized patients with COVID-19: Results from the CORONADO study. Diabetes Res. Clin. Pr. 2021, 175, 108695. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lavie, C.J.; Mehra, M.R.; Henry, B.M.; Lippi, G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin. Proc. 2020, 95, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- de Heredia, F.P.; Gomez-Martinez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H. Body fat distribution, body composition, and respiratory function in elderly men. Am. J. Clin. Nutr. 2005, 82, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Zammit, C.; Liddicoat, H.; Moonsie, I.; Makker, H. Obesity and respiratory diseases. Int. J. Gen. Med. 2010, 3, 335–343. [Google Scholar] [CrossRef]
- Huttunen, R.; Karppelin, M.; Syrjanen, J. Obesity and nosocomial infections. J. Hosp. Infect. 2013, 85, 8–16. [Google Scholar] [CrossRef]
- Le, N.T.; Robinson, J.; Lewis, S.J. Obese patients and radiography literature: What do we know about a big issue? J. Med. Radiat. Sci. 2015, 62, 132–141. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus white adipose tissue: A mini-review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Mondini, E.; Cinti, F.; Cinti, S.; Sebert, S.; Savolainen, M.J.; Salonurmi, T. Fto-Deficiency Affects the Gene and MicroRNA Expression Involved in Brown Adipogenesis and Browning of White Adipose Tissue in Mice. Int. J. Mol. Sci. 2016, 17, 1851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Slezak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Elffers, T.W.; de Mutsert, R.; Lamb, H.J.; de Roos, A.; Willems van Dijk, K.; Rosendaal, F.R.; Jukema, J.W.; Trompet, S. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS ONE 2017, 12, e0185403. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Vitiello, L.; Ferraro, E.; De Simone, S.; Gatta, L.; Feraco, A.; Racioppi, L.; Rosano, G. CXCL12 prolongs naive CD4+ T lymphocytes survival via activation of PKA, CREB and Bcl2 and BclXl up-regulation. Int. J. Cardiol. 2016, 224, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Fukuhara, A.; Onodera, T.; Kita, S.; Yokoyama, C.; Otsuki, M.; Shimomura, I. SDF-1 Is an Autocrine Insulin-Desensitizing Factor in Adipocytes. Diabetes 2018, 67, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef]
- Ozata, M.; Ozdemir, I.C.; Licinio, J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999, 84, 3686–3695. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lim, J.H.; Choi, S.W.; Kim, M.; Kim, S.T.; Kim, M.S.; Cho, Y.S.; Chun, E.; Lee, K.Y. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 2010, 394, 562–568. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, M.; Ogawa, Y.; Masuzaki, H.; Tanaka, M.; Ozaki, S.; Nakao, K.; Mimori, T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin. Exp. Immunol. 2002, 128, 21–26. [Google Scholar] [CrossRef]
- Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sanchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef]
- De Rosa, V.; Procaccini, C.; Cali, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- Mancuso, P.; Curtis, J.L.; Freeman, C.M.; Peters-Golden, M.; Weinberg, J.B.; Myers, M.G., Jr. Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of Streptococcus pneumoniae and alveolar macrophage bactericidal function. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L78–L86. [Google Scholar] [CrossRef] [PubMed]
- Tsiotra, P.C.; Boutati, E.; Dimitriadis, G.; Raptis, S.A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. BioMed Res. Int. 2013, 2013, 487081. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Lombardo, M.; Boaria, A.; Aulisa, G.; Padua, E.; Annino, G.; Pratesi, A.; Caprio, M.; Iellamo, F.; Bellia, A. Sarcopenic obesity: Etiology and lifestyle therapy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7152–7162. [Google Scholar] [CrossRef]
- Bernardi, S.; Marcuzzi, A.; Piscianz, E.; Tommasini, A.; Fabris, B. The Complex Interplay between Lipids, Immune System and Interleukins in Cardio-Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 4058. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Yates, T.; Baker, L.A.; Zaccardi, F.; Smith, A.C. Sarcopenic obesity and the risk of hospitalization or death from coronavirus disease 2019: Findings from UK Biobank. JCSM Rapid Commun. 2021, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Ferrari, N. Metabolic Health-The Role of Adipo-Myokines. Int. J. Mol. Sci. 2019, 20, 6159. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin--mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Kruse, R.; Vienberg, S.G.; Vind, B.F.; Andersen, B.; Hojlund, K. Effects of insulin and exercise training on FGF21, its receptors and target genes in obesity and type 2 diabetes. Diabetologia 2017, 60, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, S.H.; Min, Y.K.; Yang, H.M.; Lee, J.B.; Lee, M.S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Meza-Arana, C.E.; Brito-Cordova, G.; Gomez-Perez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, P. Effect of coculturing on the myogenic and adipogenic marker gene expression. Appl. Biochem. Biotechnol. 2014, 173, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, B.; Fathi, R.; Rezazadeh Valojerdi, M.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J. 2017, 18, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Fortino, S.A.; Baker, J.M.; Snijders, T.; Joanisse, S.; McGlory, C.; McKay, B.R.; Kumbhare, D.; Parise, G. Consistent expression pattern of myogenic regulatory factors in whole muscle and isolated human muscle satellite cells after eccentric contractions in humans. J. Appl. Physiol. (1985) 2019, 127, 1419–1426. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- Fang, J.; Feng, C.; Chen, W.; Hou, P.; Liu, Z.; Zuo, M.; Han, Y.; Xu, C.; Melino, G.; Verkhratsky, A.; et al. Redressing the interactions between stem cells and immune system in tissue regeneration. Biol. Direct 2021, 16, 18. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef] [PubMed]
- Menshikova, E.V.; Ritov, V.B.; Toledo, F.G.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am. J. Physiol. Metab. 2005, 288, E818–E825. [Google Scholar] [CrossRef] [PubMed]
- Nordby, P.; Saltin, B.; Helge, J.W. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: A role for muscle oxidative capacity? Scand. J. Med. Sci. Sports 2006, 16, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Toloza, F.J.K.; Mantilla-Rivas, J.O.; Perez-Matos, M.C.; Ricardo-Silgado, M.L.; Morales-Alvarez, M.C.; Pinzon-Cortes, J.A.; Perez-Mayorga, M.; Arevalo-Garcia, M.L.; Tolosa-Gonzalez, G.; Mendivil, C.O. Plasma Levels of Myonectin but Not Myostatin or Fibroblast-Derived Growth Factor 21 Are Associated with Insulin Resistance in Adult Humans without Diabetes Mellitus. Front. Endocrinol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Pourranjbar, M.; Arabnejad, N.; Naderipour, K.; Rafie, F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life 2018, 11, 381–386. [Google Scholar] [CrossRef]
- De Assis, G.G.; Gasanov, E.V.; de Sousa, M.B.C.; Kozacz, A.; Murawska-Cialowicz, E. Brain derived neutrophic factor, a link of aerobic metabolism to neuroplasticity. J. Physiol. Pharmacol. 2018, 69, 351–358. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A. The effect of physical activity on the brain derived neurotrophic factor: From animal to human studies. J. Physiol. Pharmacol. 2010, 61, 533–541. [Google Scholar]
- Kim, S.; Choi, J.Y.; Moon, S.; Park, D.H.; Kwak, H.B.; Kang, J.H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 491–505. [Google Scholar] [CrossRef]
- Takei, N.; Furukawa, K.; Hanyu, O.; Sone, H.; Nawa, H. A possible link between BDNF and mTOR in control of food intake. Front. Psychol. 2014, 5, 1093. [Google Scholar] [CrossRef]
- Vega, J.A.; Garcia-Suarez, O.; Hannestad, J.; Perez-Perez, M.; Germana, A. Neurotrophins and the immune system. J. Anat. 2003, 203, 1–19. [Google Scholar] [CrossRef]
- Kozlov, E.M.; Grechko, A.V.; Chegodaev, Y.S.; Wu, W.K.; Orekhov, A.N. Contribution of Neurotrophins to the Immune System Regulation and Possible Connection to Alcohol Addiction. Biology 2020, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 2008, 59 (Suppl. S7), 119–132. [Google Scholar] [PubMed]
- Nonomura, T.; Tsuchida, A.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Int. J. Exp. Diabetes Res. 2001, 2, 201–209. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Seelaender, M.; Silva, B.S.A.; Cunha, E.; Deus, M.C.; Vasconcellos, F.T.F.; Marqueze, L.F.B.; Gadotti, A.C.; Baena, C.P.; Pereira, T.; et al. COVID-19 Outcome Relates with Circulating BDNF, According to Patient Adiposity and Age. Front. Nutr. 2021, 8, 784429. [Google Scholar] [CrossRef]
- Brunelli, S.; Rovere-Querini, P. The immune system and the repair of skeletal muscle. Pharmacol. Res. 2008, 58, 117–121. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Nomura, S.; Shouzu, A.; Omoto, S.; Nishikawa, M.; Fukuhara, S. Significance of chemokines and activated platelets in patients with diabetes. Clin. Exp. Immunol. 2000, 121, 437–443. [Google Scholar] [CrossRef]
- Troseid, M.; Lappegard, K.T.; Claudi, T.; Damas, J.K.; Morkrid, L.; Brendberg, R.; Mollnes, T.E. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur. Heart J. 2004, 25, 349–355. [Google Scholar] [CrossRef]
- Yakeu, G.; Butcher, L.; Isa, S.; Webb, R.; Roberts, A.W.; Thomas, A.W.; Backx, K.; James, P.E.; Morris, K. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: Roles of PPARgamma and Th2 cytokines. Atherosclerosis 2010, 212, 668–673. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529 Pt 1, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engstrom-Ruud, L.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Bruning, J.C. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Yargic, M.P.; Torgutalp, S.; Akin, S.; Babayeva, N.; Torgutalp, M.; Demirel, H.A. Acute long-distance trail running increases serum IL-6, IL-15, and Hsp72 levels. Appl. Physiol. Nutr. Metab. 2019, 44, 627–631. [Google Scholar] [CrossRef]
- Lin, J.X.; Leonard, W.J. The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028449. [Google Scholar] [CrossRef]
- Krolopp, J.E.; Thornton, S.M.; Abbott, M.J. IL-15 Activates the Jak3/STAT3 Signaling Pathway to Mediate Glucose Uptake in Skeletal Muscle Cells. Front. Physiol. 2016, 7, 626. [Google Scholar] [CrossRef]
- Ikeda, S.I.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef]
- Barra, N.G.; Palanivel, R.; Denou, E.; Chew, M.V.; Gillgrass, A.; Walker, T.D.; Kong, J.; Richards, C.D.; Jordana, M.; Collins, S.M.; et al. Interleukin-15 modulates adipose tissue by altering mitochondrial mass and activity. PLoS ONE 2014, 9, e114799. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, M.F.; Huang, J.N.; Li, J.M.; Jiang, J.; Wang, J.A. Role of interleukin-15 in cardiovascular diseases. J. Cell. Mol. Med. 2020, 24, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Hingorjo, M.R.; Zehra, S.; Saleem, S.; Qureshi, M.A. Serum Interleukin-15 and its relationship with adiposity Indices before and after short-term endurance exercise. Pak. J. Med. Sci. 2018, 34, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Nicola, N.A.; Babon, J.J. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Brandt, N.; O’Neill, H.M.; Kleinert, M.; Schjerling, P.; Vernet, E.; Steinberg, G.R.; Richter, E.A.; Jorgensen, S.B. Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am. J. Physiol. Metab. 2015, 309, E142–E153. [Google Scholar] [CrossRef][Green Version]
- Broholm, C.; Mortensen, O.H.; Nielsen, S.; Akerstrom, T.; Zankari, A.; Dahl, B.; Pedersen, B.K. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J. Physiol. 2008, 586, 2195–2201. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernandez-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Colaianni, G.; Sanesi, L.; Storlino, G.; Brunetti, G.; Colucci, S.; Grano, M. Irisin and Bone: From Preclinical Studies to the Evaluation of Its Circulating Levels in Different Populations of Human Subjects. Cells 2019, 8, 451. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Guo, J.; Chen, Y.; Meng, C. The Neuroprotective Effect of Irisin in Ischemic Stroke. Front. Aging Neurosci. 2020, 12, 588958. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhang, F.; Wen, J.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Jiang, S. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. 2017, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Wolff, C.A.; Suer, M.K.; Harber, M.P. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R461–R468. [Google Scholar] [CrossRef]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhao, X.Y.; Lin, J.D. The brown fat secretome: Metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 2015, 26, 231–237. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- AlKhairi, I.; Cherian, P.; Abu-Farha, M.; Madhoun, A.A.; Nizam, R.; Melhem, M.; Jamal, M.; Al-Sabah, S.; Ali, H.; Tuomilehto, J.; et al. Increased Expression of Meteorin-Like Hormone in Type 2 Diabetes and Obesity and Its Association with Irisin. Cells 2019, 8, 1283. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Fromenty, B. Effects of beta-aminoisobutyric acid on leptin production and lipid homeostasis: Mechanisms and possible relevance for the prevention of obesity. Fundam. Clin. Pharmacol. 2010, 24, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Stautemas, J.; Van Kuilenburg, A.B.P.; Stroomer, L.; Vaz, F.; Blancquaert, L.; Lefevere, F.B.D.; Everaert, I.; Derave, W. Acute Aerobic Exercise Leads to Increased Plasma Levels of R- and S-beta-Aminoisobutyric Acid in Humans. Front. Physiol. 2019, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Olza, J.; Aguilera, C.M.; Gil, A.; Ruiz, J.R. Role of Exercise in the Activation of Brown Adipose Tissue. Ann. Nutr. Metab. 2015, 67, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Global Recommendations on Physical Activity for Health; WHO Guidelines Approved by the Guidelines Review Committee. Available online: https://www.who.int/publications/i/item/9789241599979 (accessed on 15 March 2022).
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 16, 297–298. [Google Scholar] [CrossRef]
| Metabolic Alterations and Viral Infection Outcomes | ||||
|---|---|---|---|---|
| First Author | Viral Infection | Results | Type of Publication | Country |
| Louie, J.K., 2011 [27] | H1N1 Influenza | Half of 534 adult case patients hospitalized with 2009 H1N1 infection were obese. Extreme obesity (BMI ≥ 40 kg/m2) was associated with increased odds of death, thus representing an independent risk factor for mortality. | Article | California, USA |
| Kwong, J.C., 2011 [28] | H1N1 Influenza | Logistic regression to examine the association between BMI and hospitalization for selected respiratory diseases in a cohort of 82,545 adults over 12 influenza seasons (1996–1997 through 2007–2008) indicates that severely obese individuals (Class II or III, BMI > 35 kg/m2) with and without chronic conditions are at increased risk for respiratory hospitalizations during influenza seasons. | Article | Canada |
| Allard, R., 2010 [29] | H1N1 Influenza | Diabetes triples the risk of hospitalization after influenza A (H1N1) p and quadruples the risk of ICU admission once hospitalized. | Article | Canada |
| Pranata, R., 2021 [36] | SARS-CoV-2 | A total of 34,390 patients from 12 studies were included in this meta-analysis. Increased BMI was associated with increased poor outcome in patients with COVID-19. | Meta-Analysis | Several countries |
| Guo, W., 2020 [37] | SARS-CoV-2 | COVID-19 patients with diabetes (n = 24) were at higher risk of severe pneumonia, release of tissue injury-related enzymes, excessive uncontrolled inflammation responses and hypercoagulable state associated with dysregulation of glucose metabolism. Diabetes should be considered as a risk factor for a rapid progression and bad prognosis of COVID-19. | Article | China |
| Wu, J., 2020 [38] | SARS-CoV-2 | Elevation of admission blood glucose level was an independent risk factor for progression to critical cases/death among non-critical cases in a cohort of 2041 consecutive hospitalized patients with COVID-19. | Article | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Feraco, A.; Bellia, C.; Prisco, L.; D’Ippolito, I.; Padua, E.; Storz, M.A.; Lauro, D.; Caprio, M.; Bellia, A. Influence of Nutritional Status and Physical Exercise on Immune Response in Metabolic Syndrome. Nutrients 2022, 14, 2054. https://doi.org/10.3390/nu14102054
Lombardo M, Feraco A, Bellia C, Prisco L, D’Ippolito I, Padua E, Storz MA, Lauro D, Caprio M, Bellia A. Influence of Nutritional Status and Physical Exercise on Immune Response in Metabolic Syndrome. Nutrients. 2022; 14(10):2054. https://doi.org/10.3390/nu14102054
Chicago/Turabian StyleLombardo, Mauro, Alessandra Feraco, Chiara Bellia, Luigi Prisco, Ilenia D’Ippolito, Elvira Padua, Maximilian Andreas Storz, Davide Lauro, Massimiliano Caprio, and Alfonso Bellia. 2022. "Influence of Nutritional Status and Physical Exercise on Immune Response in Metabolic Syndrome" Nutrients 14, no. 10: 2054. https://doi.org/10.3390/nu14102054
APA StyleLombardo, M., Feraco, A., Bellia, C., Prisco, L., D’Ippolito, I., Padua, E., Storz, M. A., Lauro, D., Caprio, M., & Bellia, A. (2022). Influence of Nutritional Status and Physical Exercise on Immune Response in Metabolic Syndrome. Nutrients, 14(10), 2054. https://doi.org/10.3390/nu14102054