Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome
Abstract
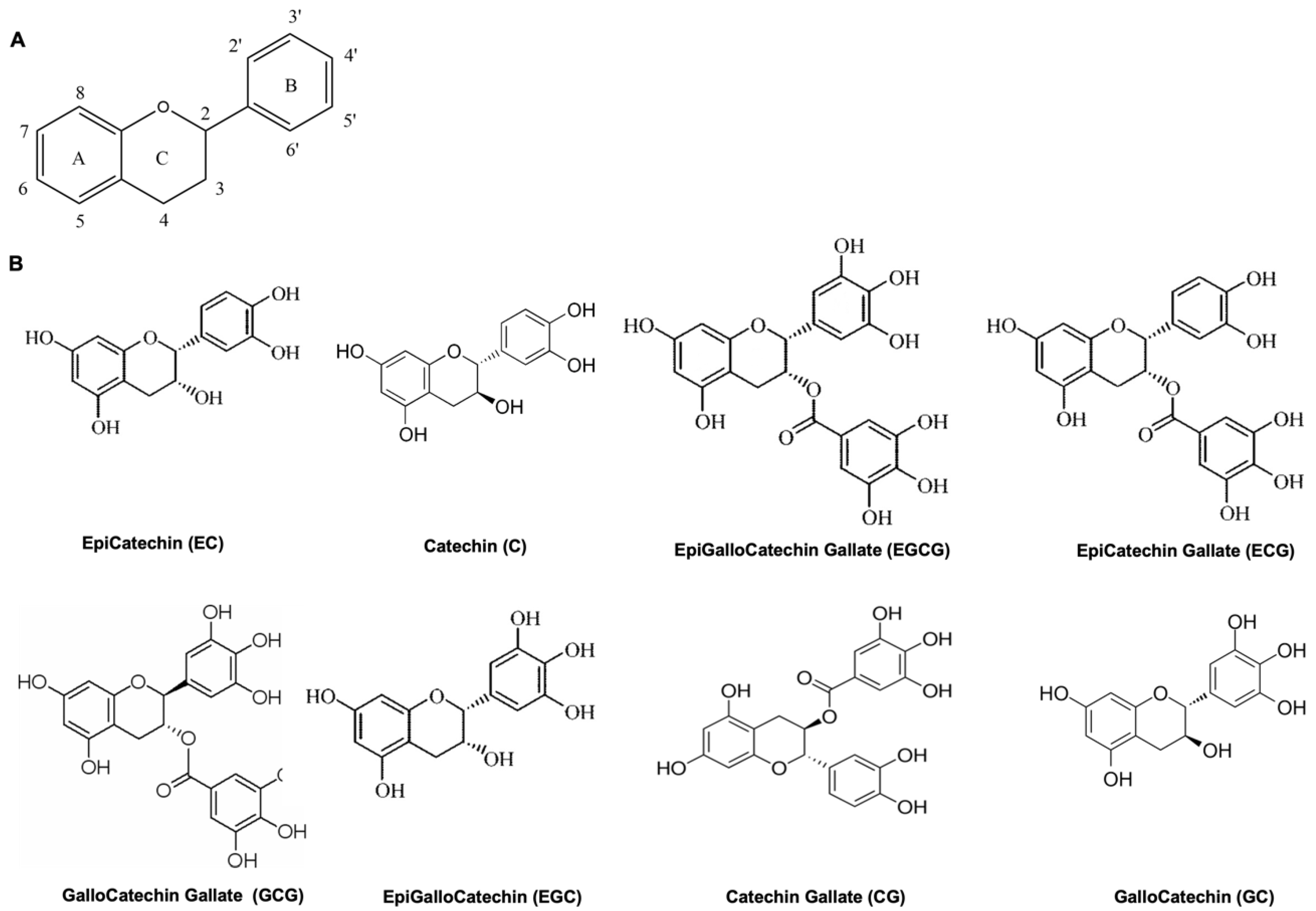
:1. Introduction
1.1. Polyphenols and Health Promoting Effects
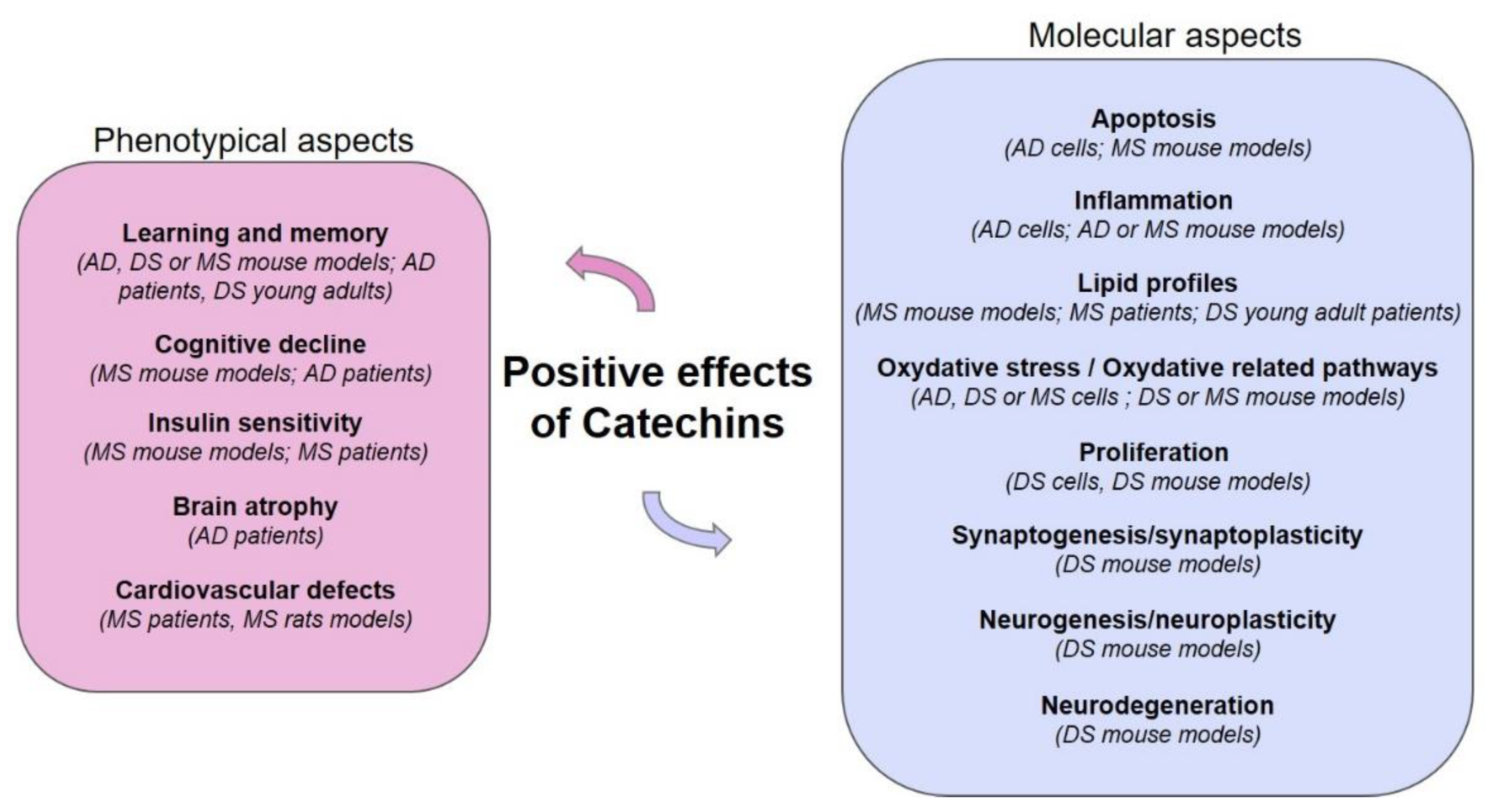
1.2. Catechins, Oxidative Stress and Inflammation
2. Down Syndrome and Catechins
2.1. Down Syndrome
2.2. Catechins in Molecular Effects of Down Syndrome
3. Alzheimer’s Disease and Catechins
3.1. Alzheimer’s Disease
3.2. Catechins in Molecular Effects of Alzheimer’s Disease
4. Metabolic Syndrome, Microbiota and Catechins
5. DYRK1A and Catechins
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 1, ES60–ES77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Bergland, A.K.; Soennesyn, H.; Dalen, I.; Rodriguez-Mateos, A.; Berge, R.K.; Giil, L.M.; Rajendran, L.; Siow, R.; Tassotti, M.; Aarsland, D.; et al. Effects of Anthocyanin Supplementation on Serum Lipids, Glucose, Markers of Inflammation and Cognition in Adults with Increased Risk of Dementia—A Pilot Study. Front. Genet. 2019, 10, 536. [Google Scholar] [CrossRef]
- Chai, S.C.; Jerusik, J.; Davis, K.; Wright, R.S.; Zhang, Z. Effect of Montmorency tart cherry juice on cognitive performance in older adults: A randomized controlled trial. Food Funct. 2019, 10, 4423–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jin, C.H.; Ho, R.K. Separation of catechin compounds from different teas. Biotechnol. J. 2006, 1, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.; Kim, E.M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, E173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Teapolyphenolsinpromotionofhumanhealth. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.Y.; Kim, E.; Hwang, K.; Ratan, Z.A.; Hwang, H.; Kim, E.-M.; Kim, D.; Park, J.; Cho, J.Y. Cytoprotective effect of epigallocatechin gallate (EGCG)-5′-O-α-glucopyranoside, a novel EGCG derivative. Int. J. Mol. Sci. 2018, 19, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Goncalves, C.A. Green tea (−) epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentão, P. Glutathione and the Antioxidant Potential of Binary Mixtures with Flavonoids: Synergisms and Antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef] [Green Version]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns. Colitis. 2012, 6, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.Y.; Lee, C.; Park, G.H.; Jang, J.H. Neuroprotective Effect of Epigallocatechin-3-gallate against β-Amyloid-induced Oxidative and Nitrosative Cell Death via Augmentation of Antioxidant Defense Capacity. Arch. Pharm. Res. 2009, 32, 869–881. [Google Scholar] [CrossRef]
- Marinovic, M.P.; Morandi, A.C.; Otton, R. Green tea catechins alone or in combination alter functional parameters of human neutrophils via suppressing the activation of TLR-4/NFκB p65 signal pathway. Toxicol. In Vitro 2015, 29, 1766–1778. [Google Scholar] [CrossRef]
- Nadim, M.; Auriol, D.; Lamerant-Faye, L.N.; Lefèvre, F.; Dubanet, L.; Redziniak, G.; Kieda, C.; Grillon, C. Improvement of polyphenol properties upon glucosylation in a UV-induced skin cell ageing model. Int. J. Cosmet. Sci. 2014, 36, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Fang, Y.; Wei, S.M. Effect and mechanism of epigallocatechin-3-gallate (EGCG) against the hydrogen peroxide-induced oxidative damage in human dermal fibroblasts. J. Cosmet. Sci. 2013, 64, 35–44. [Google Scholar] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Hayakawa, S.; Nakano, S.; Ito, S.; Oishi, Y.; Suzuki, Y.; Isemura, M. In Vitro and In Silico Studies of the Molecular Interactions of Epigallocatechin-3-O-gallate (EGCG) with Proteins That Explain the Health Benefits of Green Tea. Molecules 2018, 23, 1295. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Lu, T.H.; Su, C.C.; Lay, I.S.; Lin, H.Y.; Fang, K.M.; Ho, T.J.; Chen, K.L.; Su, Y.C.; Chiang, W.C.; et al. Lotus Leaf (Nelumbo nucifera) and its Active Constituents Prevent Inflammatory Responses in Macrophages via JNK/NF-κB Signaling Pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Huang, Q.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (–)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Feng, W.Y. Metabolism of Green Tea Catechins: An Overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Madrid-Gambin, F.; Garcia-Aby, M.; Andres-Lacueva, C.; Logue, C.; Gallagher, A.M.; Mack, C.; Kulling, S.E.; Gao, Q.; Pratici, G.; et al. Biomarkers of intake for coffee, tea, and sweetened beverages. Genes Nutr. 2018, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzillotta, C.; Di Domenico, F. Stress Responses in Down Syndrome Neurodegeneration: State of the Art and Therapeutic Molecules. Biomolecules 2021, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Langdon, J.; Down, H. Observations on an ethnic classification of idiots. Heredity 1866, 21, 695–697. [Google Scholar] [CrossRef]
- Lejeune, J.; Gautier, M.; Turpin, R. Study of somatic chromosomes from 9 mongoloid children. Comptes Rendus Hebd. Seances l’Acad. Des Sci. 1959, 248, 1721–1722. [Google Scholar]
- Vraneković, J.; Božović, I.B.; Grubić, Z.; Wagner, J.; Pavlinić, D.; Dahoun, S.; Bena, F.; Čulić, V.; Brajenović-Milić, B. Down Syndrome: Parental Origin, Recombination, and Maternal Age. Genet. Test. Mol. Biomark. 2012, 16, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Dierssen, M. Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 2012, 13, 844–858. [Google Scholar] [CrossRef]
- Akhtar, F.; Bokhari, S.R.A. Down Syndrome. In StatPearls; StatPearls Publishing: Teasure Island, FL, USA, 2021. [Google Scholar]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer's disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 9, 758–766. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Di Domenico, F.; Head, E.; Butterfield, D.A.; Perluigi, M.; Barone, E. Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol Dis. 2020, 137, 104772. [Google Scholar] [CrossRef]
- Sinet, P.M.; Théophile, D.; Rahmani, Z.; Chettouh, Z.; Blouin, J.L.; Prieur, M.; Noel, B.; Delabar, J.M. Mapping of the Down syndrome phenotype on chromosome 21 at the molecular level. Biomed. Pharmacother. 1994, 48, 247–252. [Google Scholar] [CrossRef]
- Lyle, R.; Béna, F.; Gagos, S.; Gehrig, C.; Lopez, G.; Schinzel, A.; Lespinasse, J.; Bottani, A.; Dahoun, S.; Taine, L. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur. J. Hum. Genet. 2009, 17, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, L.E.; Roper, R.J.; Sengstaken, C.L.; Peterson, E.A.; Aquino, V.; Galdzicki, Z.; Siarey, R.; Pletnikov, M.; Moran, T.H.; Reeves, R.H. Trisomy for the Down syndrome « critical region » is necessary but not sufficient for brain phenotypes of trisomic mice. Hum. Mol. Genet. 2007, 16, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Dhanasekaran, A.R.; Gardiner, K.J. Mouse models of Down syndrome: Gene content and consequences. Mamm. Genome 2016, 27, 11–12. [Google Scholar] [CrossRef]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Yu, E.; Brault, V. Rodent models in Down syndrome research: Impact and future opportunities. Dis. Models Mech. 2017, 10, 1165–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.; Joost, H.-G. Structural and functional characteristics of dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol. 1998, 62, 1–17. [Google Scholar]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Tejedor, F.J.; Hämmerle, B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 2010, 278, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Thomazeau, A.; Lassalle, O.; Lafrati, J.; Souchet, B.; Guedj, F.; Janel, N.; Chavis, P.; Delabar, J.; Manzoni, O.J. Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a. J. Neurosci. 2014, 34, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- De Toma, I.; Ortega, M.; Aloy, P.; Sabidó, E.; Dierssen, M. DYRK1A Overexpression Alters Cognition and Neural-Related Proteomic Pathways in the Hippocampus That Are Rescued by Green Tea Extract and/or Environmental Enrichment. Front. Mol. Neurosci. 2019, 12, 272. [Google Scholar] [CrossRef]
- Atas-Ozcan, H.; Brault, V.; Duchon, A.; Herault, Y. Dyrk1a from Gene Function in Development and Physiology to Dosage Correction across Life Span in Down Syndrome. Genes 2021, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cué, C.; Rueda, N. Signalling Pathways Implicated in Alzheimer’s Disease Neurodegeneration in Individuals with and without Down Syndrome. Int. J. Mol. Sci. 2020, 21, 6906. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Arena, A.; Head, E.; Butterfield, D.A.; Perluigi, M. Disturbance of redox homeostasis in Down Syndrome: Role of iron dysmetabolism. Free Radic. Biol. Med. 2018, 114, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermak, G.; Sojitra, S.; Yin, F.; Cadenas, E.; Cuervo, A.M.; Davies, K.J. Chronic expression of RCAN1-1L protein induces mitochondrial autophagy and metabolic shift from oxidative phosphorylation to glycolysis in neuronal cells. J. Biol. Chem. 2012, 287, 14088–14098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, D.; Manente, G.A.; Moro, L.; Marra, E.; Vacca, R.A. Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: Involvement of the cAMP/PKA signalling pathway. Biochem. J. 2011, 435, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, D.; De Rasmo, D.; Signorile, A.; Rossi, L.; de Bari, L.; Scala, I.; Granese, B.; Papa, S.; Vacca, R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta 2013, 1832, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Valenti, D.; de Bari, L.; de Rasmo, D.; Signorile, A.; Henrion-Caude, A.; Contestabile, A.; Vacca, R.A. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim. Biophys. Acta. 2016, 1862, 1093–1104. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Xu, X.; Song, M.; Tao, H.; Bai, Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012, 56, 1292–1303. [Google Scholar] [CrossRef]
- Hibaoui, Y.; Grad, I.; Letourneau, A.; Sailani, M.R.; Dahoun, S.; Santoni, F.A.; Gimelli, S.; Guipponi, M.; Pelte, M.F.; Béna, F.; et al. Modelling and rescuing neurodevelopmental defect of Down syndrome using induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. EMBO Mol. Med. 2014, 6, 259–277. [Google Scholar] [CrossRef]
- Gu, Y.; Moroy, G.; Paul, J.-L.; Rebillat, A.-S.; Dierssen, M.; De La Torre, R.; Cieuta-Walti, C.; Dairou, J.; Janel, N. Molecular Rescue of Dyrk1A Overexpression Alterations in Mice with Fontup® Dietary Supplement: Role of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1404. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, R.; De Sola, S.; Pons, M.; Duchon, A.; de Lagran, M.M.; Farré, M.; Fitó, M.; Benejam, B.; Langohr, K.; Rodriguez, J.; et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 2014, 58, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Notredame, C.; Gonzalez, J.R.; Dierssen, M. Principal Component Analysis of the Effects of Environmental Enrichment and (-)-epigallocatechin-3-gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome. Front. Behav. Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Gonzalez, J.R.; Notredame, C.; Dierssen, M. Combined Treatment with Environmental Enrichment and (-)-Epigallocatechin-3-Gallate Ameliorates Learning Deficits and Hippocampal Alterations in a Mouse Model of Down Syndrome. eNeuro 2016, 3, 103–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagni, F.; Giacomini, A.; Emili, M.; Trazzi, S.; Guidi, S.; Sassi, M.; Ciani, E.; Rimondini, R.; Bartesaghi, R. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 2016, 333, 277–301. [Google Scholar] [CrossRef] [PubMed]
- De Toma, I.; Ortega, M.; Catuara-Solarz, S.; Sierra, C.; Sabidó, E.; Dierssen, M. Re-establishment of the epigenetic state and rescue of kinome deregulation in Ts65Dn mice upon treatment with green tea extract and environmental enrichment. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McElyea, S.D.; Starbuck, J.M.; Tumbleson-Brink, D.M.; Harrington, E.; Blazek, J.D.; Ghoneima, A.; Kula, K.; Roper, R.J. Influence of prenatal EGCG treatment and Dyrk1a dosage reduction on craniofacial features associated with Down syndrome. Hum. Mol. Genet. 2016, 25, 4856–4869. [Google Scholar]
- Jamal, R.; LaCombe, J.; Patel, R.; Blackwell, M.; Thomas, J.R.; Sloan, K.; Wallace, J.M.; Roper, R.J. Increased dosage and treatment time of Epigallocatechin-3-gallate (EGCG) negatively affects skeletal parameters in normal mice and Down syndrome mouse models. PLoS ONE 2022, 17, e0264254. [Google Scholar] [CrossRef]
- Cué, C.M.; Dierssen, M. Plasticity as a therapeutic target for improving cognition and behavior in Down syndrome. Chang. Brains Appl. Brain Plast. Adv. Recover Hum. Abil. 2020, 251, 269–302. [Google Scholar]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Becker, W.; Sippl, W. Activation, regulation, and inhibition of dyrk1a. FEBS J. 2011, 278, 246–256. [Google Scholar] [CrossRef]
- Guedj, F.; Sébrié, C.; Rivals, I.; Ledru, A.; Paly, E.; Bizot, J.C.; Smith, D.; Rubin, E.; Gillet, B.; Arbones, M.; et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS ONE 2009, 4, e4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souchet, B.; Guedj, F.; Penke-Verdier, Z.; Daubigney, F.; Duchon, A.; Herault, Y.; Bizot, J.-C.; Janel, N.; Créau, N.; Delatour, B.; et al. Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front. Behav. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souchet, B.; Duchon, A.; Gu, Y.; Dairou, J.; Chevalier, C.; Daubigney, F.; Nalesso, V.; Creau, N.; Yu, Y.; Janel, N.; et al. Prenatal treatment with EGCG enriched green tea extract rescues GAD67 related developmental and cognitive defects in Down syndrome mouse models. Sci. Rep. 2019, 9, 3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, I.; Valenti, D.; Scotto D’Aniello, V.; Marino, M.; Riccio, M.P.; Bravaccio, C.; Vacca, R.A.; Strisciuglio, P. Epigallocatechin-3-Gallate Plus Omega-3 Restores the Mitochondrial Complex I and F0F1-ATP Synthase Activities in PBMCs of Young Children with Down Syndrome: A Pilot Study of Safety and Efficacy. Antioxidants 2021, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with down’s syndrome (TESDAD): A double-blind randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef]
- Starbuck, J.M.; Llambrich, S.; Gonzàlez, R.; Albaigès, J.; Sarlé, A.; Wouters, J.; González, A.; Sevillano, X.; Sharpe, J.; De La Torre, R.; et al. Green tea extracts containing epigallocatechin-3-gallate modulate facial development in Down syndrome. Sci. Rep. 2021, 11, 4715. [Google Scholar] [CrossRef]
- Xicota, L.; Rodriguez, J.; Langohr, K.; Fito, M.; Dierssen, M.; de la Torre, R.; TESDAD Study Group. Effect of epigallocatechin gallate on the body composition and lipid profile of down syndrome individuals: Implications for clinical management. Clin. Nutr. 2020, 39, 1292–1300. [Google Scholar] [CrossRef]
- Stringer, M.; Abeysekera, I.; Dria, K.J.; Roper, R.J.; Goodlett, C.R. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacol.Biochem. Behav. 2015, 138, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Stringer, M.; Abeysekera, I.; Thomas, J.; Lacombe, J.; Stancombe, K.; Stewart, R.; Dria, K.; Wallace, J.M.; Goodlett, C.R.; Roper, R. Epigallocatechin-3-gallate (EGCG) consumption in the Ts65Dn model of down syndrome fails to improve behavioral deficits and is detrimental to skeletal phenotypes. Physiol. Behav. 2017, 177, 230–241. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Stringer, M.; LaCombe, J.; Patel, R.; Wallace, J.M.; Roper, R.J. Evaluation of the therapeutic potential of Epigallocatechin-3-gallate (EGCG) via oral gavage in young adult Down syndrome mice. Sci. Rep. 2020, 10, 10426. [Google Scholar] [CrossRef]
- Yang, L.; Jin, X.; Yan, J.; Jin, Y.; Yu, W.; Wu, H.; Xu, S. Prevalence of dementia, cognitive status and associated risk factors among elderly of Zhejiang province, China in 2014. Age Ageing 2016, 45, 708–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef]
- Paula-Lima, A.C.; Brito-Moreira, J.; Ferreira, S.T. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. J. Neurochem. 2013, 126, 191–202. [Google Scholar] [CrossRef]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Domenico, F.; Cenini, G.; Sultana, R.; Perluigi, M.; Uberti, D.; Memo, M.; Butterfield, D.A. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer’s disease brain: Implications for AD pathogenesis. Neurochem. Res. 2009, 34, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.M.; Katsouri, L.; Sastre, M. Modulation of inflammation in transgenic models of Alzheimer’s disease. J. Neuroinflamm. 2014, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age-related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2018, 218, 165–184. [Google Scholar] [CrossRef]
- Ma, Q.P.; Huang, C.; Cui, Q.Y.; Yang, D.J.; Sun, K.; Chen, X.; Li, X.H. Meta-Analysis of the Association between Tea Intake and the Risk of Cognitive Disorders. PLoS ONE 2016, 11, e0165861. [Google Scholar] [CrossRef]
- Kimura-Ohba, S.; Yang, Y. Oxidative DNA Damage Mediated by Intranuclear MMP Activity Is Associated with Neuronal Apoptosis in Ischemic Stroke. Oxid. Med. Cell. Longev. 2016, 2016, 6927328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Choi, D.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013, 24, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Mérillon, J.-M.; Ramawat, K.G. Bioactive Molecules in Food, 1st ed.; Springer Nature: Cham, Switzerland, 2019; pp. 230–256. [Google Scholar]
- Bahia, P.K.; Rattray, M.; Williams, R.J. Dietary flavonoid (–)-epicatechin stimulates phosphatidylinositol 3-kinase-dependent antioxidant response element activity and up-regulates glutathione in cortical astrocytes. J. Neurochem. 2008, 106, 2194–2204. [Google Scholar]
- Levites, Y.; Amit, T.; Youdim, M.B.; Mandel, S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002, 277, 30574–30580. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, F.; Jin, H.; Li, R.; Wang, Y.; Zhang, W.; Wang, H.; Chen, W. Involvement of PKCα and ERK1/2 signaling pathways in EGCG’s protection against stress-induced neural injuries in Wistar rats. Neuroscience 2017, 346, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.-F.; Nie, Y.-X.; Tong, Q.-Z.; Tang, Y.-L.; Zeng, Y.; Jing, K.-Q.; Zheng, X.-L.; Liao, D.-F. Epigallocatechin-3-Gallate Attenuates Impairment of Learning and Memory in Chronic Unpredictable Mild Stress-Treated Rats by Restoring Hippocampal Autophagic Flux. PLoS ONE 2014, 9, e112683. [Google Scholar] [CrossRef]
- Unno, K.; Takabayashi, F.; Yoshida, H.; Choba, D.; Fukutomi, R.; Kikunaga, N.; Kishido, T.; Oku, N.; Hoshino, M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology 2007, 8, 89–95. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Li, Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1-42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience 2009, 163, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Xicota, L.; Rodriguez-Morato, J.; Dierssen, M.; de la Torre, R. Potential Role of (−)-Epigallocatechin-3-Gallate (EGCG) in the Secondary Prevention of Alzheimer Disease. Curr. Drug Targets 2017, 18, 174–195. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Liu, M.Y.; Wang, S.; Tang, Q.S.; Yao, W.F.; Zhao, H.S.; Wei, M.J. Research on EGCG improving the degenerative changes of the brain in AD model mice induced with chemical drugs. Zhong Yao Cai 2012, 35, 1641–1644. [Google Scholar] [PubMed]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Liu, W.; Zhou, H.-Y.; Gui, Y.-R.; Yang, Y.-H.; Wu, M.-J.; Xiao, Y.-F.; Shang, J.-T.; Long, G.-F.; Shu, X.-J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green tea (−)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef]
- Giunta, B.; Hou, H.; Zhu, Y.; Salemi, J.; Ruscin, A.; Shytle, R.D.; Tan, J. Fish Oil enhances anti-amyloidogenic properties of Green Tea EGCG in Tg2576 mice. Neurosci. Lett. 2010, 471, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Levites, Y.; Amit, T.; Mandel, S.; Youdim, M.B. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003, 17, 952–954. [Google Scholar] [CrossRef]
- Kao, Y.-H.; Hiipakka, R.A.; Liao, S. Modulation of Endocrine Systems and Food Intake by Green Tea Epigallocatechin Gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef]
- Bao, S.; Cao, Y.; Fan, C.; Fan, Y.; Bai, S.; Teng, W.; Shan, Z. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rats. Mol. Nutr. Food Res. 2014, 58, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bao, S.; Yang, W.; Zhang, J.; Li, L.; Shan, Z.; Teng, W. Epigallocatechin gallate prevents inflammation by reducing macrophage infiltration and inhibiting tumor necrosis factor-alpha signaling in the pancreas of rats on a high-fat diet. Nutr. Res. 2014, 34, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Honma, K.; Yoshinari, O.; Nanjo, F.; Hara, Y. Effects of Dietary Catechins on Glucose Tolerance, Blood Pressure and Oxidative Status in Goto-Kakizaki Rats. J. Nutr. Sci. Vitaminol. 2007, 53, 496–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. 2020, 246, 163–176. [Google Scholar] [CrossRef]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (-)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef] [Green Version]
- Gancar, M.; Kurin, E.; Bednarikova, Z.; Marek, J.; Mucaji, P.; Nagy, M.; Gazova, Z. Amyloid Aggregation of Insulin: An Interaction Study of Green Tea Constituents. Sci. Rep. 2020, 10, 9115. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, X.-L.; Wu, J.-M.; Tang, Y.; Qiu, W.-Q.; Shen, X.; Teng, J.-F.; Pan, R.; Zhao, Y.; Yu, L.; et al. Polyphenols Isolated from Lychee Seed Inhibit Alzheimer’s Disease-Associated Tau through Improving Insulin Resistance via the IRS-1/PI3K/Akt/GSK-3β Pathway. J. Ethnopharmacol. 2020, 251, 112548. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-Gallate Protects against Diabetic Cardiomyopathy through Modulating the Cardiometabolic Risk Factors, Oxidative Stress, Inflammation, Cell Death and Fibrosis in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Biomed. Pharmacother. 2017, 94, 362–373. [Google Scholar] [CrossRef]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; Feo, V.D.; Zia-Ul-Haq, M.; Abroma Augusta, L. (Malvaceae) Leaf Extract Attenuates Diabetes Induced Nephropathy and Cardiomyopathy via Inhibition of Oxidative Stress and Inflammatory Response. J. Transl. Med. 2015, 13, 6. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS Signaling and ER Stress in Cardiovascular Disease. Mol. Aspects Med. 2018, 63, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxid Med. Cell Longev. 2020, 2020, 6569728. [Google Scholar] [CrossRef] [PubMed]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kwon, D.; Kim, M.; Kim, H.K.; Lee, Y.C.; Park, S.J.; Yoo, W.H.; Chae, S.; Chung, M.; Kim, H.; et al. The Involvement of Endoplasmic Reticulum Stress in Flavonoid-induced Protection on Cardiac Cell Death Caused by Ischaemia/Reperfusion. J. Pharm. Pharmacol. 2010, 62, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Wang, S.; Liu, M.; Feng, Y.; Wang, X. Icariin Protects Rat Cardiac H9c2 Cells from Apoptosis by Inhibiting Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2013, 14, 17845–17860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Lin, H.; Wang, Q.; Hou, J.-W.; Mao, Z.-J.; Li, Y.-G. Protective Role of Silibinin against Myocardial Ischemia/Reperfusion Injury-Induced Cardiac Dysfunction. Int. J. Biol. Sci. 2020, 16, 1972–1988. [Google Scholar] [CrossRef]
- Zhao, R.; Xie, X.; Le, K.; Li, W.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Endoplasmic Reticulum Stress in Diabetic Mouse, or Glycated LDL-Treated Endothelial Cells: Protective Effect of Saskatoon Berry Powder and Cyanidin Glycans. J. Nutritional. Biochem. 2015, 26, 1248–1253. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Hu, T.; Shi, J.-J.; Fang, J.; Wang, Q.; Chen, Y.-B.; Zhang, S.-J. Quercetin Ameliorates Diabetic Encephalopathy through SIRT1/ER Stress Pathway in Db/Db Mice. Aging Albany NY 2020, 12, 7015–7029. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.-P.; Moreira, R.J.; Costa, J.; Mariné-Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry Proanthocyanidins and Anthocyanins Improve Metabolic Health through a Gut Microbiota-Dependent Mechanism in Diet-Induced Obese Mice. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance, and Intestinal Inflammation in Association with Increased Akkermansia spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, X.; Lu, Q.; Zhang, L.; Wang, X.; Liu, R. C-Ring Cleavage Metabolites of Catechin and Epicatechin Enhanced Antioxidant Activities through Intestinal Microbiota. Food Res. Int. Ott. Ont. 2020, 135, 109271. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2015, 60, 160–174. [Google Scholar] [CrossRef]
- Zhou, J.; Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Bay, B.; Yen, P.M. Epigallocatechin-3-Gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE 2014, 9, e87161. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.; Bolin, A.P.; Cardoso, C.A.; Otton, R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur. J. Nutr. 2015, 55, 2231–2244. [Google Scholar] [CrossRef]
- Grove, K.A.; Sae-Tan, S.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity 2012, 20, 2311–2313. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Dong, S.; Liu, Y.; Liu, Y. Molecular interactions between (−)-Epigallocatechin gallate analogs and pancreatic lipase. PLoS ONE 2014, 9, e111143. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutrition 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.-M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A Single Supplement of a Standardised Bilberry (Vaccinium myrtillus L.) Extract (36% Wet Weight Anthocyanins) Modifies Glycaemic Response in Individuals with Type 2 Diabetes Controlled by Diet and Lifestyle. J. Nutritional Sci. 2013, 2, e22. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.-X.; Xu, N.; Bao, B.; Liu, J. Quercetin Reduces Obesity-Associated ATM Infiltration, and Inflammation in Mice: A Mechanism Including AMPKα1/SIRT1[S]. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid Inhibition of Sodium-Dependent Vitamin C Transporter 1 (SVCT1) and Glucose Transporter Isoform 2 (GLUT2), Intestinal Transporters for Vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.; Gross, R.; Petit, P.; et al. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic Β-cells against Oxidative Damage via the ERK1/2 Pathway. Brit. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Buya, M.; Qin, W.; Sun, C.; Cai, H.; Xie, Q.; Xu, B.; Wu, Y. Anthocyanins from Chinese Bayberry Extract Activate Transcription Factor Nrf2 in β Cells and Negatively Regulate Oxidative Stress-Induced Autophagy. J. Agr. Food Chem. 2013, 61, 8765–8772. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.Á.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa Flavonoid Epicatechin Protects Pancreatic Beta Cell Viability and Function against Oxidative Stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the Brain: Interactions at the Blood–Brain Barrier and Their Physiological Effects on the Central Nervous System. Free Radical Bio. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Ibars, M.; Ardid-Ruiz, A.; Suárez, M.; Muguerza, B.; Bladé, C.; Aragonès, G. Proanthocyanidins Potentiate Hypothalamic Leptin/STAT3 Signalling and Pomc Gene Expression in Rats with Diet-Induced Obesity. Int. J. Obes. 2017, 41, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG Ameliorates High-fat– and High-fructose-induced Cognitive Defects by Regulating the IRS/AKT and ERK/CREB/BDNF Signaling Pathways in the CNS. Faseb J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kek, H.C.; Lim, J.; Gelling, R.W.; Han, W. Green Tea (-)-Epigallocatechin-3-Gallate Counteracts Daytime Overeating Induced by High-Fat Diet in Mice. Mol. Nutr. Food Res. 2016, 60, 2565–2575. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef]
- Quiroga, R.; Villarreal, M.A. Vinardo: A Scoring Function Based on Autodock Vina Improves Scoring, Docking, and Virtual Screening. PLoS ONE 2016, 11, e0155183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, S.; Kii, I.; Sumida, Y.; Kato-Sumida, T.; Ogawa, Y.; Ito, N.; Nakamura, M.; Sonamoto, R.; Kataoka, N.; Hosoya, T.; et al. Design and synthesis of a potent inhibitor of class 1 DYRK kinases as a suppressor of adipogenesis. Bioorgan. Med. Chem. 2015, 23, 4434–4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Taylor, B.; Jin, Q.; Nguyen-Tran, T.V.; Meeusen, S.; Zhnag, Y.Q.; Kamireddy, A.; Swafford, A.; Powers, A.F.; Walker, J.; et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun. 2015, 6, 8372. [Google Scholar] [CrossRef] [PubMed]
- Souchet, B.; Audrain, M.; Billard, J.M.; Dairou, J.; Fol, R.; Orefice, N.S.; Tada, S.; Gu, Y.; Dufayet-Chaffaud, G.; Limanton, E.; et al. Inhibition of DYRK1A proteolysis modifies its kinase specificity and rescues Alzheimer phenotype in APP/PS1 mice. Acta Neuropathol. Commun. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, X.; Tian, L.; Gao, Y.; Liu, W.; Chen, H.; Jiang, X.; Xu, Z.; Ding, H.; Zhao, Q. Design, synthesis and biological evaluation of harmine derivatives as potent GSK-3beta/DYRK1A dual inhibitors for the treatment of Alzheimer's disease. Eur. J. Med. Chem. 2021, 222, 113554. [Google Scholar] [CrossRef]
- Yates, A.A.; Erdman, J.W.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101. [Google Scholar] [CrossRef]

| Compound | Dyrk1A-ΔC Dyrk1A Remaining Activity at 0.1 μM (%) | Dyrk1A-ΔC Dyrk1A Remaining Activity at 1 μM (%) | Dyrk1A-ΔC Dyrk1A Remaining Activity at 10 μM (%) | Dyrk1A-ΔC Dyrk1A Remaining Activity at 100 μM (%) |
|---|---|---|---|---|
| CG | 50.9 ± 4.4 | 5.7 ± 0.7 | 2.2 ± 0.2 | 0.9 ± 0.3 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| 75.3 ± 13.3 | 5.1 ± 1 | 1.5 ± 0.2 | 0.6 ± 0.03 | |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| EGCG | 71.3 ± 4.6 | 16.3 ± 2.3 | 6.4 ± 1.1 | 3.5 ± 0.8 |
| (n = 13) | (n = 13) | (n = 13) | (n = 13) | |
| 67.5 ± 9.4 | 10 ± 2 | 3.4 ± 0.6 | 2.1 ± 0.5 | |
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | |
| GCG | 48.2 ± 2.9 | 5.5 ± 0.6 | 3.2 ± 0.8 | 1.6 ± 0.4 |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | |
| 78.2 ± 17.4 | 4.8 ± 1.5 | 1.4 ± 0.1 | 0.4 ± 0.1 | |
| (n = 3) | (n = 3) | (n = 3) | (n = 3) |
| Compound | Dyrk1A-ΔC Dyrk1A Remaining Activity with 200 μM ATP (%) | Dyrk1A-ΔC Dyrk1A Remaining Activity with 400 μM ATP (%) | Dyrk1A-ΔC Dyrk1A Remaining Activity with 800 μM ATP (%) |
|---|---|---|---|
| CG (10 μM) | 6.1 ± 1.2 | 3 ± 0.5 | 3.5 ± 1.5 |
| (n = 4) | (n = 4) | (n = 4) | |
| 2.5 ± 0.4 | 2 ± 0.3 | 1.7 ± 0.4 | |
| (n = 4) | (n = 4) | (n = 4) | |
| EGCG (10 μM) | 11 ± 3.1 | 9.6 ± 2.1 | 5.4 ± 1.3 |
| (n = 4) | (n = 4) | (n = 4) | |
| 6.3 ± 0.9 | 5.1 ± 0.9 | 4 ± 0.7 | |
| (n = 4) | (n = 4) | (n = 4) | |
| GCG (10 μM) | 4.7 ± 0.8 | 4.1 ± 1.2 | 3.7 ± 0.9 |
| (n = 4) | (n = 4) | (n = 4) | |
| 2.3 ± 0.4 | 1.7 ± 0.3 | 1.5 ± 0.3 | |
| (n = 4) | (n = 4) | (n = 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noll, C.; Kandiah, J.; Moroy, G.; Gu, Y.; Dairou, J.; Janel, N. Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome. Nutrients 2022, 14, 2039. https://doi.org/10.3390/nu14102039
Noll C, Kandiah J, Moroy G, Gu Y, Dairou J, Janel N. Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome. Nutrients. 2022; 14(10):2039. https://doi.org/10.3390/nu14102039
Chicago/Turabian StyleNoll, Christophe, Janany Kandiah, Gautier Moroy, Yuchen Gu, Julien Dairou, and Nathalie Janel. 2022. "Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome" Nutrients 14, no. 10: 2039. https://doi.org/10.3390/nu14102039
APA StyleNoll, C., Kandiah, J., Moroy, G., Gu, Y., Dairou, J., & Janel, N. (2022). Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome. Nutrients, 14(10), 2039. https://doi.org/10.3390/nu14102039







