Serum Leptin Levels, Nutritional Status, and the Risk of Healthcare-Associated Infections in Hospitalized Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Data Collection
2.3. Nutritional Assessment
2.4. Invasive Procedures
2.5. Assessment of HAIs
2.6. Laboratory Variables
2.7. Data Analysis
Independent Relationships between the Baseline Leptin Level and the Occurrence of HAIs
3. Results
3.1. Characteristics of the Study Population
3.2. Associations between the Serum Leptin Level and Patient Characteristics
3.3. Factors Associated with HAIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.; Borhani, A.A.; Dunn, M.A.; Andrzejewski, M.; Martin, K.; Behari, J. Serum Leptin Is a Biomarker of Malnutrition in Decompensated Cirrhosis. PLoS ONE 2016, 11, e0159142. [Google Scholar] [CrossRef]
- Amirkalali, B.; Sharifi, F.; Fakhrzadeh, H.; Mirarefein, M.; Ghaderpanahi, M.; Badamchizadeh, Z.; Larijani, B. Low serum leptin serves as a biomarker of malnutrition in elderly patients. Nutr. Res. 2010, 30, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Golmard, J.-L.; Coussieu, C.; Noel, M.R.; Durand, D.J.; Piette, F.; Nivet-Antoine, V. Leptin a new biological marker for evaluating malnutrition in elderly patients. Eur. J. Clin. Nutr. 2006, 61, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Arner, P.; Palmblad, J. Low circulating leptin levels in protein-energy malnourished chronically ill elderly patients. J. Intern. Med. 1997, 242, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Akın, S.; Tufan, F.; Bulut, L.; Genç, S.; Bahat, G.; Saka, B.; Erten, N.; Karan, M.A. Limited utility of adipokine levels in the diagnosis of malnutrition in the elderly. Aging Clin. Exp. Res. 2013, 26, 229–234. [Google Scholar] [CrossRef]
- Matarese, G.; Moschos, S.; Mantzoros, C.S. Leptin in Immunology. J. Immunol. 2005, 174, 3137–3142. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. 20 YEARS OF LEPTIN: Human disorders of leptin action. J. Endocrinol. 2014, 223, T63–T70. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef] [Green Version]
- Laurent, M.; Oubaya, N.; David, J.-P.; Engels, C.; Canoui-Poitrine, F.; Corsin, L.; Liuu, E.; Audureau, E.; Bastuji-Garin, S.; Paillaud, E. Functional decline in geriatric rehabilitation ward; is it ascribable to hospital acquired infection? A prospective cohort study. BMC Geriatr. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, F.; Hulstaert, F.; Devriese, S.; van de Sande, S. Hospital-acquired infections in Belgian acute-care hospitals: An estimation of their global impact on mortality, length of stay and healthcare costs. Epidemiol. Infect. 2012, 140, 126–136. [Google Scholar] [CrossRef]
- Laurent, M.; Bastuji-Garin, S.; Plonquet, A.; Bories, P.; Le Thuaut, A.; Audureau, E.; Lang, P.; Nakib, S.; Liuu, E.; Canoui-Poitrine, F.; et al. Interrelations of immunological parameters, nutrition, and healthcare-associated infections: Prospective study in elderly in-patients. Clin. Nutr. 2015, 34, 79–85. [Google Scholar] [CrossRef] [PubMed]
- NanZhu, Y.; Xin, L.; Xianghua, Y.; Jun, C.; Min, L. Risk factors analysis of nosocomial pneumonia in elderly patients with acute cerebral infraction. Medicine 2019, 98, e15045. [Google Scholar] [CrossRef]
- Carlsson, M.; Håglin, L.; Rosendahl, E.; Gustafson, Y. Poor nutritional status is associated with urinary tract infection among older people living in residential care facilities. J. Nutr. Health Aging 2012, 17, 186–191. [Google Scholar] [CrossRef]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.-M.; Jambou, P.; Filippi, J.; Van Obberghen, E.; Hebuterne, X. Malnutrition is an independent factor associated with nosocomial infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, K.J.; Hernandez, B.; Blancato, R.; Blankenship, J.; Mitchell, K. Impact of an interdisciplinary malnutrition quality improvement project at a large metropolitan hospital. BMJ Open Qual. 2020, 9, e000735. [Google Scholar] [CrossRef] [Green Version]
- Laurent, M.; Bories, P.N.; Le Thuaut, A.; Liuu, E.; Ledudal, K.; Bastuji-Garin, S.; Paillaud, E. Impact of Comorbidities on Hospital-Acquired Infections in a Geriatric Rehabilitation Unit: Prospective Study of 252 Patients. J. Am. Med Dir. Assoc. 2012, 13, 760.e7–760.e12. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The index of Adl: A standardized measure of biological and phychological funcation. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.; Mulsant, B.; Reynolds, C.F. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485. [Google Scholar] [PubMed]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Plonquet, A.; Bastuji-Garin, S.; Tahmasebi, F.; Brisacier, C.; Ledudal, K.; Farcet, J.; Paillaud, E. Immune risk phenotype is associated with nosocomial lung infections in elderly in-patients. Immun. Ageing 2011, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.; Micic, D.; Chenoweth, E.; Deng, L.; Galecki, A.T.; Ring, C.; Young, V.B.; Aronoff, D.; Malani, P.N.; Galecki, A.T.; et al. Poor Functional Status as a Risk Factor for SevereClostridium difficileInfection in Hospitalized Older Adults. J. Am. Geriatr. Soc. 2013, 61, 1738–1742. [Google Scholar] [CrossRef] [Green Version]
- Santucci, N.E.; D’Attilio, L.; Besedovsky, H.; Del Rey, A.; Bay, M.L.; Bottasso, O. A Clinical Correlate of the Dysregulated Immunoendocrine Response in Human Tuberculosis. Neuroimmunomodulation 2010, 17, 184–187. [Google Scholar] [CrossRef]
- Loosen, S.H.; Koch, A.; Tacke, F.; Roderburg, C.; Luedde, T. The Role of Adipokines as Circulating Biomarkers in Critical Illness and Sepsis. Int. J. Mol. Sci. 2019, 20, 4820. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, B.; Xu, Y.; Deng, Z.; Xue, H.; Wang, L.; He, L. Diagnostic value of serum leptin and a promising novel diagnostic model for sepsis. Exp. Ther. Med. 2014, 7, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, A.; Weiskirchen, R.; Zimmermann, H.W.; Sanson, E.; Trautwein, C.; Tacke, F. Relevance of Serum Leptin and Leptin-Receptor Concentrations in Critically Ill Patients. Mediat. Inflamm. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hillenbrand, A.; Knippschild, U.; Weiss, M.; Schrezenmeier, H.; Henne-Bruns, D.; Huber-Lang, M.; Wolf, A.M. Sepsis induced changes of adipokines and cytokines-septic patients compared to morbidly obese patients. BMC Surg. 2010, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Tzanela, M.; Orfanos, S.E.; Tsirantonaki, M.; Kotanidou, A.; Sotiropoulou, C.; Christophoraki, M.; Vassiliadi, D.; Thalassinos, N.C.; Roussos, C. Leptin alterations in the course of sepsis in humans. In Vivo 2006, 20, 565–570. [Google Scholar]
- Bracho-Riquelme, R.; Reyes-Romero, M.; Pescador, N.; Flores-García, A. A Leptin Serum Concentration Less than 10 ng/ml Is a Predictive Marker of Outcome in Patients with Moderate to Severe Secondary Peritonitis. Eur. Surg. Res. 2008, 41, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.A.; Silva, R.C.; Correa-Costa, M.; Hiyane, M.I.; Carvalho, M.H.C.; Landgraf, R.G.; Câmara, N.O. Leptin Downregulates LPS-Induced Lung Injury: Role of Corticosterone and Insulin. Cell. Physiol. Biochem. 2014, 33, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Negrin, L.; Jahn, A.; van Griensven, M. Leptin Protects against Mortality and Organ Dysfunction in A Two-Hit Trauma/Sepsis Model and is IL-6-Dependent. Shock 2017, 48, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-Y.; Xu, M.; Ji, Z.-Y.; Wang, Y.-X.; Dong, M.-Q.; Liu, M.-L.; Xu, D.-Q.; Zhao, P.-T.; Liu, Y.; Luo, Y.; et al. Leptin Attenuates Lipopolysaccharide or Oleic Acid-Induced Acute Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Karin, M.; Bogut, A.; Hojsak, I.; Babić, E.; Volarić, M.; Bevanda, M. Nutritional status and its effect on complications in patients with colorectal cancer. Wien. Klin. Wochenschr. 2020, 132, 431–437. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, J.J.G.-R.A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.; Abdelhafiz, D.; Abdelhafiz, A.H. Sarcopenic obesity as a determinant of cardiovascular disease risk in older people-a systematic review. Postgrad. Med. 2021. just-accepted. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gomez-Ambrosi, J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001, 15, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Lord, G.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, G.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Huffnagle, G.B.; Olszewski, M.A.; Phipps, J.; Peters-Golden, M. Leptin Corrects Host Defense Defects after Acute Starvation in Murine Pneumococcal Pneumonia. Am. J. Respir. Crit. Care Med. 2006, 173, 212–218. [Google Scholar] [CrossRef]
- Faggioni, R.; Moser, A.; Feingold, K.R.; Grunfeld, C. Reduced Leptin Levels in Starvation Increase Susceptibility to Endotoxic Shock. Am. J. Pathol. 2000, 156, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Matarese, G.; La Rocca, C.; Moon, H.-S.; Huh, J.Y.; Brinkoetter, M.T.; Chou, S.; Perna, F.; Greco, D.; Kilim, H.P.; Gao, C.; et al. Selective capacity of metreleptin administration to reconstitute CD4+T-cell number in females with acquired hypoleptinemia. Proc. Natl. Acad. Sci. USA 2013, 110, E818–E827. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Anbar, R.; Nevo, R.F.G.; Schlesinger, A.; Frishman, S.; Salai, M.; Beloosesky, Y. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin. Nutr. 2016, 35, 1053–1058. [Google Scholar] [CrossRef]
- Marshall, S.; Young, A.; Bauer, J.; Isenring, E. Malnutrition in Geriatric Rehabilitation: Prevalence, Patient Outcomes, and Criterion Validity of the Scored Patient-Generated Subjective Global Assessment and the Mini Nutritional Assessment. J. Acad. Nutr. Diet. 2016, 116, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| MD | Total Population (N = 232) | Women (N = 168) | Men (N = 64) | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 84.8 [81.2–90.1] | 84.5 [81–90] | 84 [79–87] | |
| ADL score | 8 [4–12] | 8 [5–12] | 8 [3.5–11] | |
| CIRS-G score | 11 [9–14] | 11 [9–14] | 13 [10–15] | |
| Invasive procedures | 70 (30.2) | 27 (42.2) | 43 (25.6) | |
| MMSE score | 6 | 21 [16–25] | 21 [16–25] | 21 [17–26] |
| Body mass index (kg/m2) | ||||
| <22 | 78 (33.6) | 62 (36.9) | 16 (25) | |
| 22–29.9 | 112 (48.3) | 74 (44.05) | 38 (59.4) | |
| ≥30 | 42 (18.1) | 32 (19.05) | 10 (15.6) | |
| MNA | ||||
| Malnutrition, <17 | 50 (21.6) | 38 (22.6) | 12 (18.8) | |
| At risk, 17–23.5 | 125 (53.9) | 90 (53.6) | 35 (54.7) | |
| Normal, 24–30 | 57 (24.6) | 40 (23.8) | 17 (26.6) | |
| Serum albumin (g/L) | 1 | 33.9 [30.9–37.4] | 34 [30.9–37.3] | 33.7 [30.8–37.5] |
| Serum C-reactive protein (mg/L) | 1 | 6 [2.5–17] | 5 [2.5–13] | 8.5 [2.5–31] |
| Serum leptin (μg/L) | 9.3 [3.7–21.6] | 10.5 [4–24.4] | 7.3 [2.5–12.6] | |
| Immunological factors | ||||
| CD4/CD8 T-cell ratio | 7 | 2.5 [1.4–3.5] | 2.4 [1.4–3.5] | 2.7 [1.4–3.5] |
| CD4+ T-cells | 7 | |||
| Naïve CD45RA+CD62L+ (%) | 17.5 [13.3–22.9] | 17.1 [12.9–22.1] | 18.8 [15–24.5] | |
| CD8+ T-cells | ||||
| Naïve CD45RA+CD62L+ (%) | 7 | 5 [3.3–7.3] | 5.1 [3.5–7.1] | 4.7 [3–7.8] |
| Peripheral memory CD45RA−CD62L− (%) | 7 | 2.7 [1.5–5] | 2.6 [1.4–5.4] | 2.9 [1.8–4.5] |
| Terminal effector CD28− (%) | 8 | 56 [38.5–69] | 56 [37–69] | 55 [42–66] |
| 3-month outcome Occurrence of a healthcare-associated infection | 89 (38.4) | 63 (37.5) | 26 (40.6) | |
| Discharge to home | 127 (55.7) | 36 (57.1) | 91 (55.2) | |
| Discharge to an institution | 57 (25.0) | 14 (22.2) | 43 (26.1) | |
| Still in hospital | - | 30 (13.2) | 7 (11.1) | 23 (13.9) |
| Deceased | - | 14 (6.1) | 6 (9.5) | 8 (4.9) |
| Leptin Level (μg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Women (N = 168) | Men (N = 64) | Total Population (N = 232) | ||||||||
| 10.6 [4–24.4] | 7.3 [2.5–12.6] | 9.3 [3.7–21.6] | ||||||||
| Spearman’s rho | p a | Spearman’s rho | p a | Spearman’s rho | p b | |||||
| Baseline characteristics | ||||||||||
| Age (years) | −0.15 | 0.048 | −0.09 | 0.50 | ||||||
| Serum albumin (g/L) | 0.20 | 0.01 | −0.02 | 0.89 | ||||||
| Serum C reactive protein (mg/L) | 0.10 | 0.20 | −0.15 | 0.25 | ||||||
| CD4/CD8 T-cell ratio | −0.102 | 0.20 | −0.015 | 0.91 | −0.9 | 0.16 | ||||
| CD4+ T-cells | ||||||||||
| Naïve (CD45RA+CD62L+) (%) | −0.019 | 0.81 | 0.27 | 0.034 | ||||||
| CD8+ T-cells | ||||||||||
| Naïve (CD45RA+CD62L+) (%) | 0.08 | 0.28 | −0.013 | 0.92 | 0.08 | 0.23 | ||||
| Peripheral memory (CD45RA−CD62L−) (%) | −0.028 | 0.72 | 0.04 | 0.78 | −0.008 | 0.91 | ||||
| Terminal effector CD28− (%) | 0.15 | 0.06 | −0.13 | 0.31 | 0.09 | 0.20 | ||||
| N | Median [IQR] | pa | N | Median [IQR] | pa | N | Median [IQR] | pb | ||
| Body mass index | <22 | 62 | 3.5 [2.1–5.6] | <0.000 | 16 | 2.5 [1.1–4.8] | <0.000 | 3.3 [1.6–5.1] | <0.000 | |
| 22–29.9 | 74 | 13.9 [8.6–25.2] | 38 | 7.5 [2.8–11.9] | 11 [5.1–21] | |||||
| ≥30 | 32 | 45.9 [21.7–72.6] | 10 | 21.4 [12.9–48] | 38.1 [20.4–66] | |||||
| MNA | <17 | 38 | 3.8 [1.9–8.5] | <0.000 | 12 | 2.4 [0.95–6.3] | <0.000 | 50 | 3.5 [1.5–7.1] | <0.000 |
| 17–23.5 | 90 | 10.7 [4.2–25.2] | 35 | 5.7 [2.4–12.4] | 125 | 9.5 [4–22.1] | ||||
| 24–30 | 40 | 21.8 [10.3–47] | 17 | 12.4 [9.2–15] | 57 | 16.4 [9.3–33] | ||||
| Outcome | ||||||||||
| Healthcare-associated infection | No | 105 | 11.8 [4.6–26.3] | 0.01 | 38 | 6.4 [2.5–11.9] | 0.35 | |||
| Yes | 63 | 5.9 [2.6–17.7] | 26 | 7.9 [2.4–12.9] | ||||||
| HAI+ | HAI− | Univariate Analysis | ||
|---|---|---|---|---|
| N = 63 | N = 105 | p a | OR [95%CI] b | |
| Age (years) | 85.5 [82–89] | 85 [81–90] | 0.78 | - |
| ADL | 7 [3–10] | 9 [6–12] | 0.001 | 0.88 [0.82–0.95] |
| CIRS-Gc | 13 [10–16] | 10 [8–12] | <0.000 | 2.02 [1.39–2.94] |
| Invasive procedure | 39 (52.9) | 11 (9.7) | <0.000 | 10.43 [4.77–22.83] |
| Body mass index (kg/m2) | 0.29 | |||
| <22 | 28 (44.4) | 34 (32.4) | 1.61 [0.81–3.23] | |
| 22–29.9 | 25 (39.7) | 49 (46.7) | Ref. | |
| ≥30 | 10 (15.9) | 22 (21) | 0.90 [0.37–2.17] | |
| MNA | 0.001 | |||
| <17 | 24 (38.1) | 14 (13.3) | 5.90 [2.19–15.93] | |
| 17–23.5 | 30 (47.6) | 60 (57.1) | 1.72 [0.73–4.08] | |
| 24–30 | 9 (14.3) | 31 (29.5) | Ref | |
| Albumin d (g/L) | 31.6 [28.7–34.1] | 35.7 [31.9–37.8] | <0.000 | 0.39 [0.25–0.59] |
| C-reactive protein (mg/L) c | 8 [2.5–20] | 2.5 [2.4–11] | 0.01 | 1.65 [1.20–2.28] |
| CD4/CD8 ratio d | 2.0 [1.29–3.16] | 2.56 [1.55–3.77] | 0.07 | 0.75 [0.54–1.04] |
| Naïve CD4+ T-cells (CD45RA+CD62L+) (%) | 16.5 [12.7–23.1] | 17.25 [13.2–21.6] | 0.53 | - |
| CD8+ T-cells | ||||
| Naïve (CD45RA+CD62L+) (%) d | 4.8 [3.2–7] | 5.25 [3.95–7.55] | 0.05 | 0.69 [0.49–0.96] |
| Peripheral memory (CD45RA−CD62L−) (%) c | 2.9 [1.5–10.4] | 2.35 [1.1–4.65] | 0.03 | 1.53 [1.07–2.18] |
| Terminal effector CD28− (%) c | 62.5 [30–73] | 52 [37.5–66] | 0.09 | 1.09 [0.89–1.50] |
| Leptin level (μg/L) d | 5.9 [2.6–17.7] | 11.8 [4.6–26.3] | 0.01 | 0.67 [0.49–0.90] |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR [95%CI] | p Value | OR [95%CI] | p Value | OR [95%CI] | p Value | |
| CIRS-G a | 1.58 [1.04–2.39] | 0.03 | 1.54 [1.01–2.34] | 0.044 | ||
| Invasive procedure | 6.30 [2.66–14.92] | <0.001 | 6.44 [2.73–15.20] | <0.001 | 6.23 [2.61–14.87] | <0.001 |
| Naïve CD8+ T-cells (CD45RA+CD62L+)% b | 0.68 [0.46–0.99] | 0.043 | 0.70 [0.49–1.03] | 0.07 | 0.64 [0.43–0.94] | 0.02 |
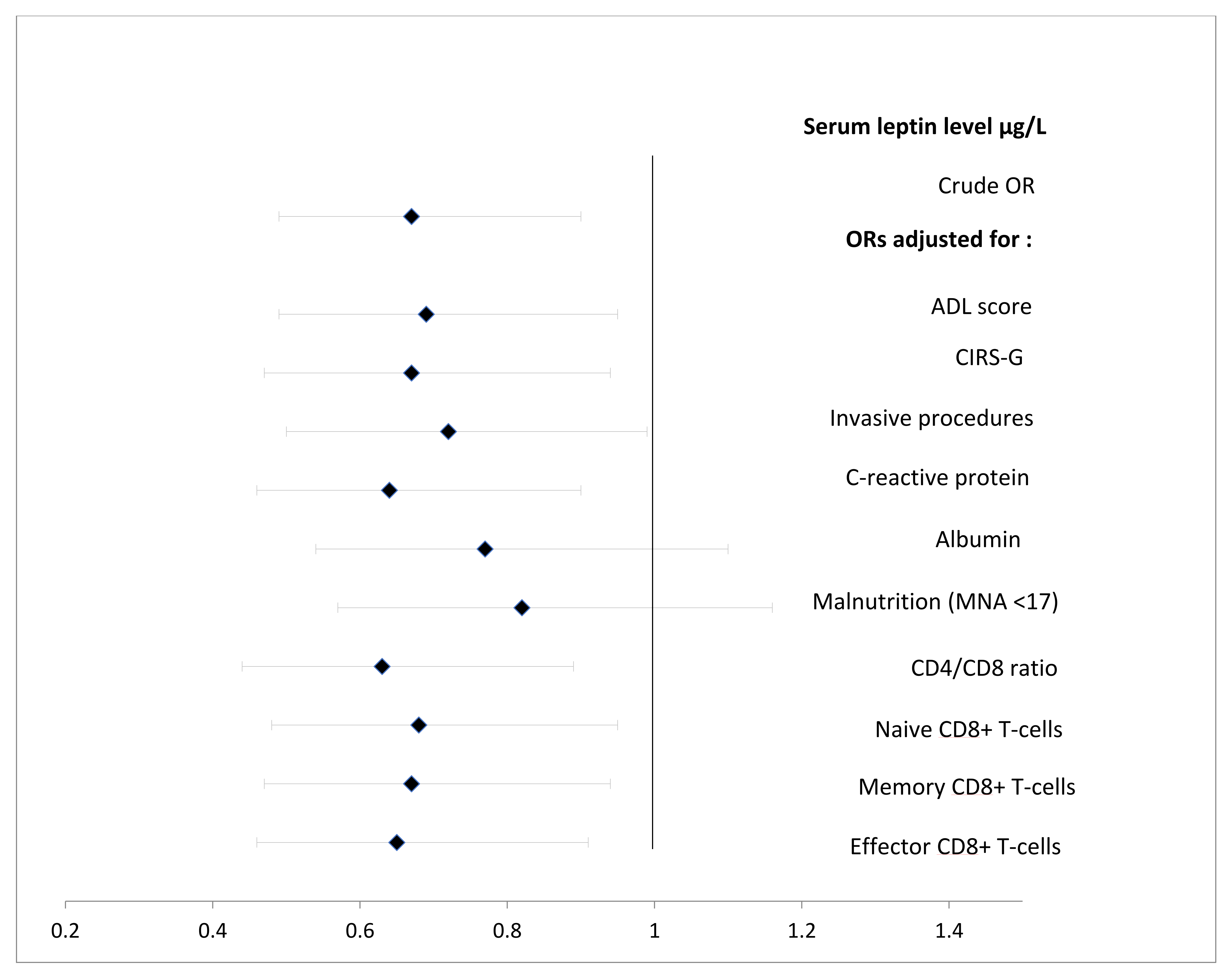
| Serum leptin level (μg/L) b | 0.70 [0.49–0.97] | 0.048 | ||||
| Albumin | 0.46 [0.29–0.74] | 0.001 | ||||
| MNA < 17 | 3.22 [1.36–7.63] | 0.008 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillaud, E.; Poisson, J.; Granier, C.; Ginguay, A.; Plonquet, A.; Conti, C.; Broussier, A.; Raynaud-Simon, A.; Bastuji-Garin, S. Serum Leptin Levels, Nutritional Status, and the Risk of Healthcare-Associated Infections in Hospitalized Older Adults. Nutrients 2022, 14, 226. https://doi.org/10.3390/nu14010226
Paillaud E, Poisson J, Granier C, Ginguay A, Plonquet A, Conti C, Broussier A, Raynaud-Simon A, Bastuji-Garin S. Serum Leptin Levels, Nutritional Status, and the Risk of Healthcare-Associated Infections in Hospitalized Older Adults. Nutrients. 2022; 14(1):226. https://doi.org/10.3390/nu14010226
Chicago/Turabian StylePaillaud, Elena, Johanne Poisson, Clemence Granier, Antonin Ginguay, Anne Plonquet, Catherine Conti, Amaury Broussier, Agathe Raynaud-Simon, and Sylvie Bastuji-Garin. 2022. "Serum Leptin Levels, Nutritional Status, and the Risk of Healthcare-Associated Infections in Hospitalized Older Adults" Nutrients 14, no. 1: 226. https://doi.org/10.3390/nu14010226
APA StylePaillaud, E., Poisson, J., Granier, C., Ginguay, A., Plonquet, A., Conti, C., Broussier, A., Raynaud-Simon, A., & Bastuji-Garin, S. (2022). Serum Leptin Levels, Nutritional Status, and the Risk of Healthcare-Associated Infections in Hospitalized Older Adults. Nutrients, 14(1), 226. https://doi.org/10.3390/nu14010226







