Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Study Outcomes and Sample Collection
2.3. SARS-CoV-2-Binding IgG and IgA Antibody Levels and Cytokine Level Measurements
2.4. Statistical Analysis
3. Results
3.1. Study Data, Compliance and Baseline Characteristics of the Subjects
3.2. COVID-19 Infection Incidence, Severity, and Duration
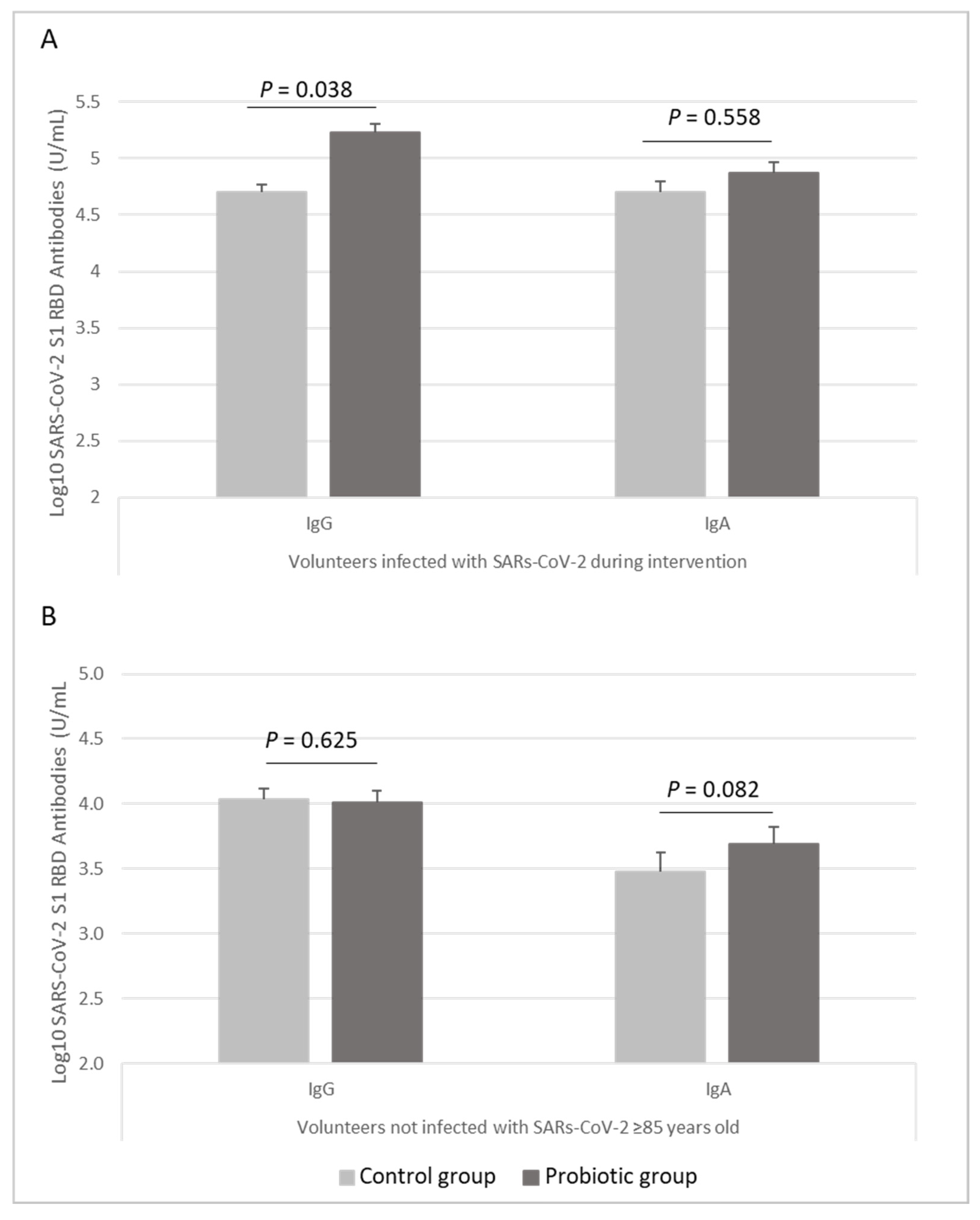
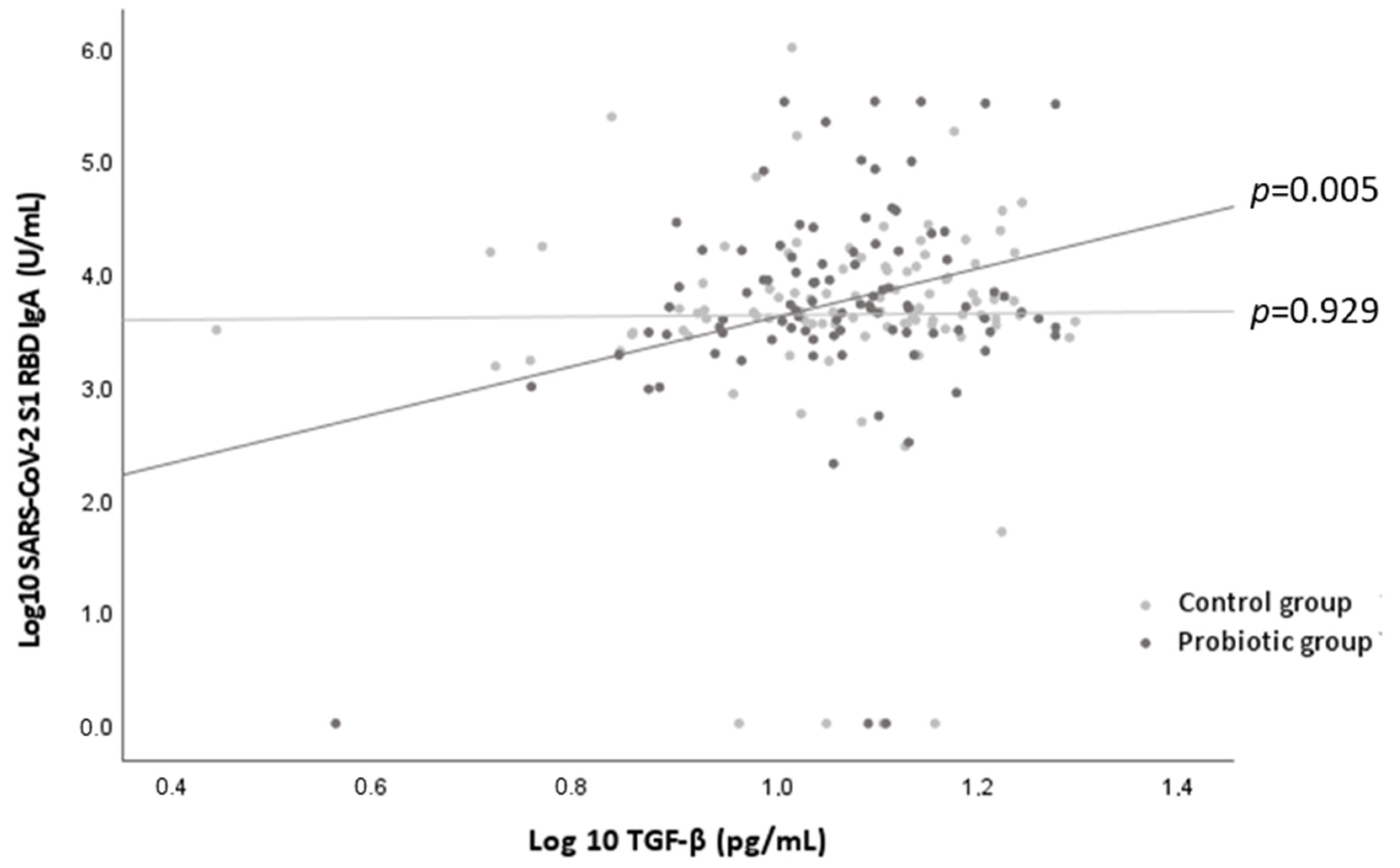
3.3. Immune Response to the COVID-19 Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO COVID-19 Dashboard. World Health Organization: Geneva, 2020. Available online: https://covid19.who.int/ (accessed on 15 October 2021).
- Bellino, S. COVID-19 Vaccines Approved in the European Union: Current Evidence and Perspectives. Expert Rev. Vaccines 2021, 20, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Fortner, A.; Schumacher, D. First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization. Discoveries 2021, 9, e122. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why Does COVID-19 Disproportionately Affect Older People? Aging (Albany N. Y.) 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.; Harrison, E.; Green, C.; Hardwick, H.; Pius, R.; Norman, L.; Holden, K.; Read, J.; Dondelinger, F.; Carson, G. Features of 16,749 Hospitalised UK Patients with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol. 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7243036/ (accessed on 15 October 2021).
- Yanez, N.D.; Weiss, N.S.; Romand, J.-A.; Treggiari, M.M. COVID-19 Mortality Risk for Older Men and Women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- Garnier-Crussard, A.; Forestier, E.; Gilbert, T.; Krolak-Salmon, P. Novel Coronavirus (COVID-19) Epidemic: What Are the Risks for Older Patients? J. Am. Geriatr. Soc. 2020, 68, 939–940. [Google Scholar] [CrossRef]
- Tisminetzky, M.; Delude, C.; Hebert, T.; Carr, C.; Goldberg, R.J.; Gurwitz, J.H. Age, Multiple Chronic Conditions, and COVID-19: A Literature Review. J. Gerontol. Ser. A 2020. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Olmedo, C.; Alejos, B.; Lapeña, M.F.; Sierra, M.J.; Limia, A. Direct and Indirect Effectiveness of MRNA Vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 in Long-Term Care Facilities, Spain. Emerg. Infect. Dis. 2021, 27, 2595. [Google Scholar] [CrossRef]
- Kissling, E.; Hooiveld, M.; Martín, V.S.; Martínez-Baz, I.; William, N.; Vilcu, A.-M.; Mazagatos, C.; Domegan, L.; de Lusignan, S.; Meijer, A. Vaccine Effectiveness against Symptomatic SARS-CoV-2 Infection in Adults Aged 65 Years and Older in Primary Care: I-MOVE-COVID-19 Project, Europe, December 2020 to May 2021. Eurosurveillance 2021, 26, 2100670. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.; Lim, E.; Touzier, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Heterogeneity in Immune Responses to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Schwarz, T.; Tober-Lau, P.; Hillus, D.; Helbig, E.T.; Lippert, L.J.; Thibeault, C.; Koch, W.; Landgraf, I.; Michel, J.; Bergfeld, L.; et al. Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany. Emerg. Infect. Dis. 2021, 27, 2174–2178. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-Dependent Immune Response to the Biontech/Pfizer BNT162b2 COVID-19 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and Human Vaccine Immune Responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Hevia, A.; Delgado, S.; Sánchez, B.; Margolles, A. Molecular Players Involved in the Interaction between Beneficial Bacteria and the Immune System. Front. Microbiol. 2015, 6, 1285. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Sierra, S.; Lara-Villoslada, F.; Fonollá, J.; Navas, M.; Rodríguez, J.M.; Xaus, J. Oral Intake of Lactobacillus Fermentum CECT5716 Enhances the Effects of Influenza Vaccination. Nutrition 2007, 23, 254–260. [Google Scholar] [CrossRef]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A Probiotic Fermented Dairy Drink Improves Antibody Response to Influenza Vaccination in the Elderly in Two Randomised Controlled Trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, J.A.; Martín, R.; Rodríguez, J.M.; Xaus, J. The Consumption of Two New Probiotic Strains, Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711, Boosts the Immune System of Healthy Humans. Int. Microbiol. 2006, 9, 47–52. [Google Scholar]
- Lara-Villoslada, F.; Sierra, S.; Boza, J. Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y lactobacillus gasseri CECT5714. Nutr. Hosp. 2007, 22, 496–502. [Google Scholar] [PubMed]
- Martínez-Cañavate, A.; Sierra, S.; Lara-Villoslada, F.; Romero, J.; Maldonado, J.; Boza, J.; Xaus, J.; Olivares, M. A Probiotic Dairy Product Containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 Induces Immunological Changes in Children Suffering from Allergy. Pediatr. Allergy Immunol. 2009, 20, 592–600. [Google Scholar] [CrossRef]
- Redondo, N.; Nova, E.; Gheorghe, A.; Díaz, L.E.; Hernández, A.; Marcos, A. Evaluation of Lactobacillus Coryniformis CECT5711 Strain as a Coadjuvant in a Vaccination Process: A Randomised Clinical Trial in Healthy Adults. Nutr. Metab. 2017, 14, 2. [Google Scholar] [CrossRef][Green Version]
- Fonollá, J.; Gracián, C.; Maldonado-Lobón, J.A.; Romero, C.; Bédmar, A.; Carrillo, J.C.; Martín-Castro, C.; Cabrera, A.L.; García-Curiel, J.M.; Rodríguez, C.; et al. Effects of Lactobacillus Coryniformis K8 CECT5711 on the Immune Response to Influenza Vaccination and the Assessment of Common Respiratory Symptoms in Elderly Subjects: A Randomized Controlled Trial. Eur. J. Nutr. 2019, 58, 83–90. [Google Scholar] [CrossRef]
- Akatsu, H. Exploring the Effect of Probiotics, Prebiotics, and Postbiotics in Strengthening Immune Activity in the Elderly. Vaccines 2021, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Morelli, L. Probiotics as Adjuvants in Vaccine Strategy: Is There More Room for Improvement? Vaccines 2021, 9, 811. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, G.; Martin, D.C. Sample Size as a Function of Coefficient of Variation and Ratio of Means. Am. Stat. 1993, 47, 165–167. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 (coronavirus disease): Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 27 September 2021).
- Ciabattini, A.; Garagnani, P.; Santoro, F.; Rappuoli, R.; Franceschi, C.; Medaglini, D. Shelter from the Cytokine Storm: Pitfalls and Prospects in the Development of SARS-CoV-2 Vaccines for an Elderly Population. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Nunes, B.; Rodrigues, A.P.; Kislaya, I.; Cruz, C.; Peralta-Santos, A.; Lima, J.; Leite, P.P.; Sequeira, D.; Dias, C.M.; Machado, A. MRNA Vaccine Effectiveness against COVID-19-Related Hospitalisations and Deaths in Older Adults: A Cohort Study Based on Data Linkage of National Health Registries in Portugal, February to August 2021. Eurosurveillance 2021, 26, 2100833. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W. Effectiveness of Pfizer-BioNTech and Moderna Vaccines against COVID-19 among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Urbanowicz, R.A.; Tsoleridis, T.; Jackson, H.J.; Cusin, L.; Duncan, J.D.; Chappell, J.G.; Tarr, A.W.; Nightingale, J.; Norrish, A.R.; Ikram, A.; et al. Two Doses of the SARS-CoV-2 BNT162b2 Vaccine Enhance Antibody Responses to Variants in Individuals with Prior SARS-CoV-2 Infection. Sci. Transl. Med. 2021, 13, eabj0847. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Lee, H.K.; Knabl, L.; Knabl, L.; Kapferer, S.; Pateter, B.; Walter, M.; Furth, P.A.; Hennighausen, L. Robust Immune Response to the BNT162b MRNA Vaccine in an Elderly Population Vaccinated 15 Months after Recovery from COVID-19. Infectious Diseases (except HIV/AIDS). 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.09.08.21263284v1 (accessed on 21 October 2021).
- Demaret, J.; Corroyer-Simovic, B.; Alidjinou, E.K.; Goffard, A.; Trauet, J.; Miczek, S.; Vuotto, F.; Dendooven, A.; Huvent-Grelle, D.; Podvin, J.; et al. Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People. Front Immunol. 2021, 12, 778679. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Hybrid Immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Moradi-Kalbolandi, S.; Majidzadeh-A, K.; Abdolvahab, M.H.; Jalili, N.; Farahmand, L. The Role of Mucosal Immunity and Recombinant Probiotics in SARS-CoV2 Vaccine Development. Probiotics Antimicrob Proteins 2021, 13, 1239–1253. [Google Scholar] [CrossRef]
- Markewitz, R.; Pauli, D.; Dargvainiene, J.; Steinhagen, K.; Engel, S.; Herbst, V.; Zapf, D.; Krüger, C.; Sharifzadeh, S.; Schomburg, B.; et al. The Temporal Course of T- and B-Cell-Responses to Vaccination with BNT162b2 and MRNA-1273. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Canaday, D.H.; Carias, L.; Oyebanji, O.A.; Keresztesy, D.; Wilk, D.; Payne, M.; Aung, H.; St. Denis, K.; Lam, E.C.; Wilson, B.; et al. Reduced BNT162b2 Messenger RNA Vaccine Response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Naive Nursing Home Residents. Clin. Infect. Dis. 2021, 73, 2112–2211. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; Vynck, M.; De Vriese, A.S.; Reynders, M. Dynamics of the Cellular and Humoral Immune Response after BNT162b2 MRNA Covid-19 Vaccination in Covid-19 Naive Nursing Home Residents. J. Infect. Dis. 2021, 224, 1690–1693. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and Cellular Immunogenicity of the BNT162b2 MRNA Covid-19 Vaccine in Nursing Home Residents. Clin. Infect. Dis. 2021, 73, 2145–2147. [Google Scholar] [CrossRef]
- Takahashi, T.; Fukudome, H.; Ueno, H.M.; Watanabe-Matsuhashi, S.; Nakano, T.; Kobayashi, T.; Ishimaru, K.; Nakao, A. Effects of Probiotic Supplementation on TGF-Β1, TGF-Β2, and IgA Levels in the Milk of Japanese Women: An Open-Label Pilot Study. Front. Nutr. 2019, 6, 128. [Google Scholar] [CrossRef]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus Gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the Scientific Requirements for Health Claims Related to the Immune System, the Gastrointestinal Tract and Defence against Pathogenic Microorganisms. EFSA J. 2016, 14, 4369. [CrossRef]
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukhopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594. [Google Scholar] [CrossRef]
- Singh, K.; Rao, A. Probiotics: A Potential Immunomodulator in COVID-19 Infection Management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment? Microb. Pathog. 2020, 148, 104452. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Attar, A.; Papizadeh, S.; Jeda, A.S.; Hosseini-Fard, S.R.; Jamasbi, E.; Kazemi, S.; Amerkani, S.; Talei, G.R.; Moradi, P.; et al. The Emerging Role of Probiotics as a Mitigation Strategy against Coronavirus Disease 2019 (COVID-19). Arch. Virol. 2021, 166, 1819–1840. [Google Scholar] [CrossRef] [PubMed]
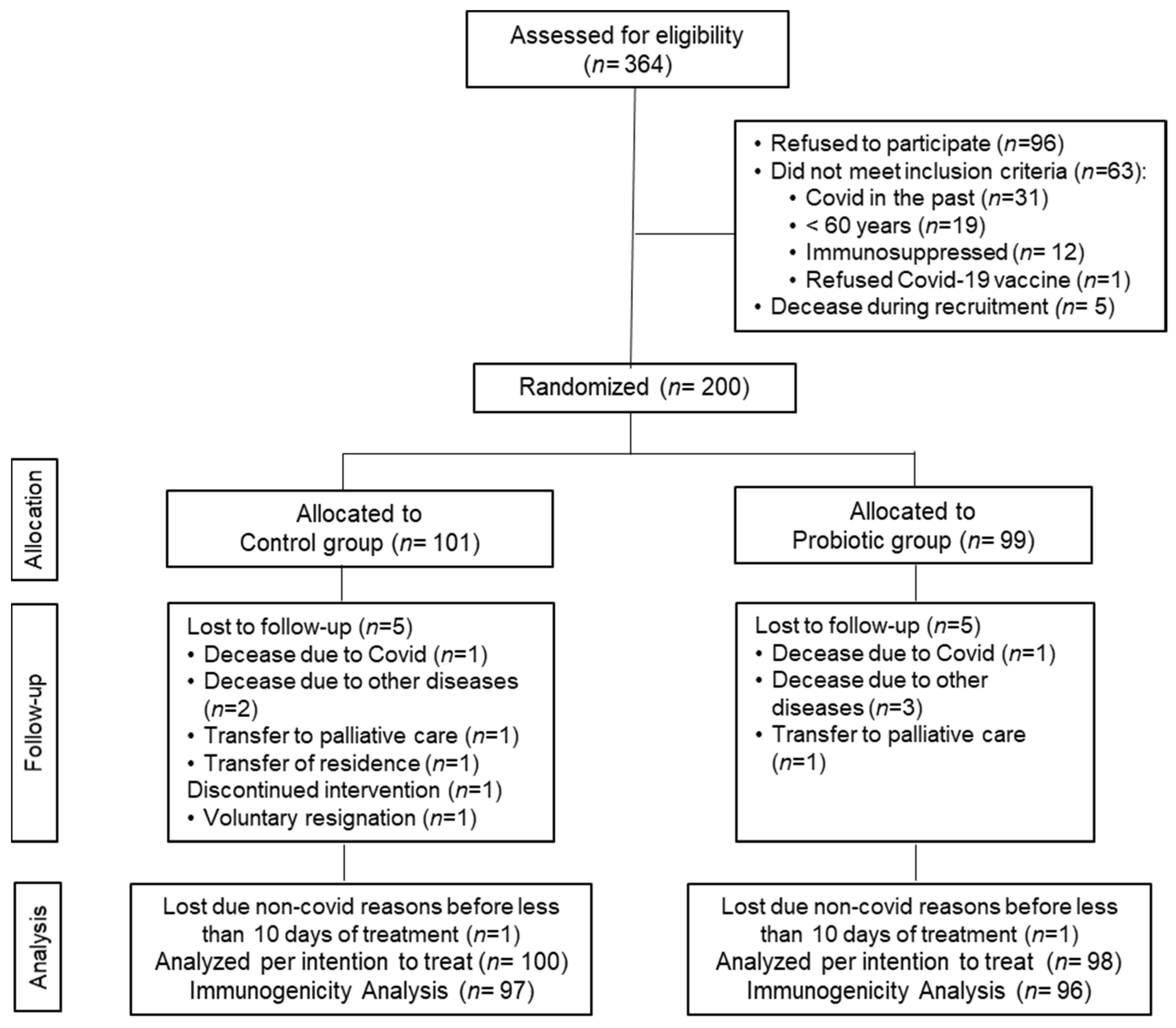
| All Volunteers (n = 198) | Control Group (n = 100) | Probiotic Group (n = 98) | p between-Groups | ||
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 83.13 ± 9.13 | 83.97 ±8.99 | 82.28 ± 9.22 | 0.192 |
| Median (IQR) | 85 (77–90) | 86 (77–90) | 84 (77–90) | ||
| Nursing home | 0.992 | ||||
| Sta Olalla | n (%) | 39 (19.7%) | 20 (20.0%) | 19 (19.4%) | |
| San Marcos | n (%) | 91 (46%) | 46 (46.0%) | 45 (45.9%) | |
| San Simon | n (%) | 68 (34.3%) | 34 (34.0%) | 34 (34.7%) | |
| Sex | 0.356 | ||||
| Men | n (%) | 73 (36.9%) | 40 (40.0%) | 33 (33.7%) | |
| Women | n (%) | 125 (63.1%) | 60 (60.0%) | 65 (66.3%) | |
| Postural control | 0.345 | ||||
| Stand upright | n (%) | 138 (69.7%) | 65 (65.0%) | 73 (74.5%) | |
| Non-stand upright | n (%) | 21 (10.6%) | 12 (12.0%) | 9 (9.2%) | |
| Bedridden patient | n (%) | 39 (19.7%) | 23 (23.0%) | 16 (16.3%) | |
| BMI (kg/m2) 1 | Mean ± SD | 26.63 ± 5.11 | 26.25 ± 4.92 | 26.96 ± 5.28 | 0.411 |
| Obesity | n (%) | 42 (21.2%) | 21 (21.0%) | 21 (21.4%) | 0.941 |
| Low weight | n (%) | 34 (17.2%) | 15 (15.0%) | 19 (19.4%) | 0.413 |
| Smokers | n (%) | 18 (9.1%) | 8 (8.0%) | 10 (10.2%) | 0.590 |
| Former alcoholics | n (%) | 10 (5.1%) | 4 (4.0%) | 6 (6.1%) | 0.495 |
| Dyslipidemia | n (%) | 80 (40.4%) | 38 (38.0%) | 42 (42.9%) | 0.486 |
| Hypertension | n (%) | 125 (63.1%) | 63 (63.0%) | 62 (63.3%) | 0.969 |
| Diabetes Mellitus | n (%) | 47 (23.7%) | 23 (23.0%) | 24 (24.5%) | 0.805 |
| Cardiovascular Disease | n (%) | 77 (38.9%) | 41 (41.0%) | 36 (36.7%) | 0.538 |
| Cognitive Diseases 2 | n (%) | 91 (46.0%) | 47 (47.0%) | 44 (44.9%) | 0.767 |
| Chronic Lung Disease | n (%) | 27 (13.6%) | 15 (15.0%) | 12 (12.2%) | 0.572 |
| Renal disease | n (%) | 21 (10.6%) | 12 (12.0%) | 9 (9.2%) | 0.520 |
| Hepatic disease | n (%) | 7 (3.5%) | 4 (4.0%) | 3 (3.1%) | 0.721 |
| Previous cancer diagnosis | n (%) | 18 (9.1%) | 3 (3.0%) | 15 (15.3%) | 0.003 |
| Rheumatic diseases 3 | n (%) | 57 (28.8%) | 27 (27.0%) | 30 (30.4%) | 0.562 |
| Psychiatric diseases 4 | n (%) | 82 (41.4%) | 36 (36.0%) | 46 (46.9%) | 0.118 |
| Disease Index 5 | Mean ± SD | 3.20 ± 1.62 | 3.09 ± 1.66 | 3.32 ± 1.58 | 0.328 |
| Median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | ||
| Number of habitual medications | Median (IQR) | 8 (5–11) | 7.5 (5–10) | 9 (6–11) | 0.108 |
| All Patients (n = 19) | Control Group (n = 8) | Probiotic Group (n = 11) | p between-Groups | ||
|---|---|---|---|---|---|
| Classification of severity | 0.189 | ||||
| Asymptomatic Infection | n (%) | 5 (26.3%) | 1 (12.5%) | 4 (36.4%) | |
| Mild Illness | n (%) | 3 (15.8%) | 2 (25%) | 1 (9.1%) | |
| Moderate Illness | n (%) | 2 (10.5%) | 1 (12.5%) | 1 (9.1%) | |
| Severe Illness | n (%) | 7 (36.8%) | 3 (37.5%) | 4 (36.4%) | |
| Critical Illness | n (%) | 2 (10.5%) | 1 (12.5%) | 1 (9.1%) | |
| Time to symptom resolution (days) 1 | Mean ± SD | 7.47 ± 1.79 | 6.13 ± 4.22 | 8.45 ± 9.69 | 0.587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ferreiro, A.; Formigo-Couceiro, F.J.; Veiga-Gutierrez, R.; Maldonado-Lobón, J.A.; Hermida-Cao, A.M.; Rodriguez, C.; Bañuelos, O.; Olivares, M.; Blanco-Rojo, R. Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial. Nutrients 2022, 14, 228. https://doi.org/10.3390/nu14010228
Fernández-Ferreiro A, Formigo-Couceiro FJ, Veiga-Gutierrez R, Maldonado-Lobón JA, Hermida-Cao AM, Rodriguez C, Bañuelos O, Olivares M, Blanco-Rojo R. Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial. Nutrients. 2022; 14(1):228. https://doi.org/10.3390/nu14010228
Chicago/Turabian StyleFernández-Ferreiro, Anxo, Francisco J. Formigo-Couceiro, Roi Veiga-Gutierrez, Jose A. Maldonado-Lobón, Ana M. Hermida-Cao, Carlos Rodriguez, Oscar Bañuelos, Mónica Olivares, and Ruth Blanco-Rojo. 2022. "Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial" Nutrients 14, no. 1: 228. https://doi.org/10.3390/nu14010228
APA StyleFernández-Ferreiro, A., Formigo-Couceiro, F. J., Veiga-Gutierrez, R., Maldonado-Lobón, J. A., Hermida-Cao, A. M., Rodriguez, C., Bañuelos, O., Olivares, M., & Blanco-Rojo, R. (2022). Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial. Nutrients, 14(1), 228. https://doi.org/10.3390/nu14010228







