In Vitro Evaluation of Dietary Fiber Anti-Infectious Properties against Food-Borne Enterotoxigenic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Dietary-Fiber-Containing Products
2.2. ETEC Strain and Growth Conditions
2.3. Growth Assay
2.4. LT Toxin Overnight Production
2.5. Mucin Beads
2.6. Mucin Bead Adhesion Assay
2.7. Caco-2/HT29-MTX Cell Cultures
2.8. Adhesion Tests on Caco-2/HT29-MTX Co-Culture Model
3. Results
3.1. All Fiber-Containing Products Have No Effect on ETEC Growth in Complete Nutritive Medium
3.2. Lentil Fibers Decreases LT Toxin Production
3.3. Specific Yeast Cell Walls and Lentil Fibers Inhibit ETEC Adhesion to Mucin Beads
3.4. Most Fiber-Containing Products Inhibit ETEC Adhesion to Caco-2/HT29-MTX Co-Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Porter, N.T.; Martens, E.C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- de Paepe, K.; Verspreet, J.; Rezaei, M.N.; Hidalgo Martinez, S.; Meysman, F.; Van de Walle, D.; Dewettinck, K.; Raes, J.; Courtin, C.; Van de Wiele, T. Isolation of Wheat Bran-Colonizing and Metabolizing Species from the Human Fecal Microbiota. PeerJ 2019, 7, e6293. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, K.; Verspreet, J.; Courtin, C.M.; Van de Wiele, T. Microbial Succession during Wheat Bran Fermentation and Colonisation by Human Faecal Microbiota as a Result of Niche Diversification. ISME J. 2020, 14, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Sauvaitre, T.; Etienne-Mesmin, L.; Sivignon, A.; Mosoni, P.; Courtin, C.M.; Van de Wiele, T.; Blanquet-Diot, S. Tripartite Relationship between Gut Microbiota, Intestinal Mucus and Dietary Fibers: Towards Preventive Strategies against Enteric Infections. FEMS Microbiol. Rev. 2021, 45, fuaa052. [Google Scholar] [CrossRef] [PubMed]
- Chantarasataporn, P.; Tepkasikul, P.; Kingcha, Y.; Yoksan, R.; Pichyangkura, R.; Visessanguan, W.; Chirachanchai, S. Water-Based Oligochitosan and Nanowhisker Chitosan as Potential Food Preservatives for Shelf-Life Extension of Minced Pork. Food Chem. 2014, 159, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maestu, A.; Ma, Z.; Paik, S.-Y.-R.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K.C. Engineering of Chitosan-Derived Nanoparticles to Enhance Antimicrobial Activity against Foodborne Pathogen Escherichia coli O157:H7. Carbohydr. Polym. 2018, 197, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-Organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]
- Vardaka, V.D.; Yehia, H.M.; Savvaidis, I.N. Effects of Citrox and Chitosan on the Survival of Escherichia coli O157:H7 and Salmonella enterica in Vacuum-Packaged Turkey Meat. Food Microbiol. 2016, 58, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of Cholera Toxin by Human Milk Fractions and Sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T. Pectic Oligosaccharide Structure-Function Relationships: Prebiotics, Inhibitors of Escherichia coli O157:H7 Adhesion and Reduction of Shiga Toxin Cytotoxicity in HT29 Cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and Bioactivities of the Exopolysaccharide from a Probiotic Strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.; Liu, Z.; Almshawit, H.; Zisu, B.; Pillidge, C.; Rochfort, S.; Gill, H. Oligosaccharides in Goats’ Milk-Based Infant Formula and Their Prebiotic and Anti-Infection Properties. Br. J. Nutr. 2019, 122, 441–449. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Taylor, D.N.; Hamer, D.H.; Shlim, D.R. Medications for the Prevention and Treatment of Travellers’ Diarrhea. J. Travel Med. 2017, 24, S17–S22. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The Incidence, Aetiology, and Adverse Clinical Consequences of Less Severe Diarrhoeal Episodes among Infants and Children Residing in Low-Income and Middle-Income Countries: A 12-Month Case-Control Study as a Follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef] [PubMed]
- Fedor, A.; Bojanowski, I.; Korzeniewski, K. Gastrointestinal Infections in Returned Travelers. Int. Marit. Health 2019, 70, 244–251. [Google Scholar] [CrossRef]
- Stintzing, G.; Möllby, R. Colonization of the upper jejunum by enteropathogenic and enterotoxigenic Escherichia coli in paediatric diarrhoea. Acta Paediatr. 1982, 71, 457–465. [Google Scholar] [CrossRef]
- Allen, K.P.; Randolph, M.M.; Fleckenstein, J.M. Importance of Heat-Labile Enterotoxin in Colonization of the Adult Mouse Small Intestine by Human Enterotoxigenic Escherichia coli Strains. Infect. Immun. 2006, 74, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Luo, Q.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Fleckenstein, J.M. EatA, an Immunogenic Protective Antigen of Enterotoxigenic Escherichia coli, Degrades Intestinal Mucin. Infect. Immun. 2014, 82, 500–508. [Google Scholar] [CrossRef]
- Tapader, R.; Bose, D.; Pal, A. YghJ, the Secreted Metalloprotease of Pathogenic E. coli Induces Hemorrhagic Fluid Accumulation in Mouse Ileal Loop. Microb. Pathog. 2017, 105, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Vipin Madhavan, T.P.; Sakellaris, H. Colonization Factors of Enterotoxigenic Escherichia coli. Adv. Appl. Microbiol. 2015, 90, 155–197. [Google Scholar] [CrossRef]
- Qadri, F.; Svennerholm, A.-M.; Faruque, A.S.G.; Sack, R.B. Enterotoxigenic Escherichia coli in Developing Countries: Epidemiology, Microbiology, Clinical Features, Treatment, and Prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef]
- Turner, S.M.; Scott-Tucker, A.; Cooper, L.M.; Henderson, I.R. Weapons of Mass Destruction: Virulence Factors of the Global Killer Enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 2006, 263, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Idota, T.; Kawakami, H. Inhibitory Effects of Milk Gangliosides on the Adhesion of Escherichia coli to Human Intestinal Carcinoma Cells. Biosci. Biotechnol. Biochem. 1995, 59, 69–72. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, Å.V.; Parsons, B.N.; Prorok-Hamon, M.; Knight, P.; Winstanley, C.; O′Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Soluble Plantain Fibre Blocks Adhesion and M-Cell Translocation of Intestinal Pathogens. J. Nutr. Biochem. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Salcedo, J.; Barbera, R.; Matencio, E.; Alegría, A.; Lagarda, M.J. Gangliosides and Sialic Acid Effects upon Newborn Pathogenic Bacteria Adhesion: An in vitro Study. Food Chem. 2013, 136, 726–734. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary Fibre in Europe: Current State of Knowledge on Definitions, Sources, Recommendations, Intakes and Relationships to Health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Evans, D.J.; Evans, D.G.; DuPont, H.L.; Orskov, F.; Orskov, I. Patterns of Loss of Enterotoxigenicity by Escherichia coli Isolated from Adults with Diarrhea: Suggestive Evidence for an Interrelationship with Serotype. Infect. Immun. 1977, 17, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Fontes, C.F.; Ricci, L.C.; Oliveira, M.S.; Gatti, M.S.V.; Pestana de Castro, A.F. Evaluation of a Defined Medium for the Production of Both Thermolabile (LT) and Thermostable (ST) Enterotoxins OfEscherichia Coli. Med. Microbiol. Immunol. 1982, 171, 43–51. [Google Scholar] [CrossRef]
- Salimian, J.; Salmanian, A.; Khalesi, R.; Mohseni, M.; Moazzeni, S. Antibody against Recombinant Heat Labile Enterotoxin B Subunit (RLTB) Could Block LT Binding to Ganglioside M1 Receptor. Iran. J. Microbiol. 2010, 2, 120–127. [Google Scholar]
- Roussel, C.; de Paepe, K.; Galia, W.; de Bodt, J.; Chalancon, S.; Leriche, F.; Ballet, N.; Denis, S.; Alric, M.; Van de Wiele, T.; et al. Spatial and Temporal Modulation of Enterotoxigenic E. coli H10407 Pathogenesis and Interplay with Microbiota in Human Gut Models. BMC Biol. 2020, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, C.; Fournier, E.; Uriot, O.; Lajoie, F.; Verdier, C.; Comtet-Marre, S.; Thomas, M.; Kapel, N.; Cherbuy, C.; Alric, M.; et al. Comparative Methods for Fecal Sample Storage to Preserve Gut Microbial Structure and Function in an in vitro Model of the Human Colon. Appl. Microbiol. Biotechnol. 2020, 104, 10233–10247. [Google Scholar] [CrossRef]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G.; Lambourne, C.A. Trends in Dietary Fiber Intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Dietary Fibre (ID 744, 745, 746, 748, 749, 753, 803, 810, 855, 1415, 1416, 4308, 4330) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1735. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Nalin, D.R.; Hoover, D.L.; Bergquist, E.J.; Hornick, R.B.; Young, C.R. Immunity to Enterotoxigenic Escherichia coli. Infect. Immun. 1979, 23, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.E.; Suchindran, S.; Nicholson, B.P.; McClain, M.T.; Burke, T.; Ginsburg, G.S.; Harro, C.D.; Chakraborty, S.; Sack, D.A.; Woods, C.W.; et al. Transcriptomic Analysis of the Host Response and Innate Resilience to Enterotoxigenic Escherichia coli Infection in Humans. J. Infect. Dis. 2016, 213, 1495–1504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirhoseini, A.; Amani, J.; Nazarian, S. Review on Pathogenicity Mechanism of Enterotoxigenic Escherichia coli and Vaccines against It. Microb. Pathog. 2018, 117, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and Systemic Inflammation Induced by Symptomatic and Asymptomatic Enterotoxigenic E. coli Infection and Impact on Intestinal Colonization and ETEC Specific Immune Responses in an Experimental Human Challenge Model. Gut Microbes 2021, 13, 1891852. [Google Scholar] [CrossRef]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Forgie, A.J.; Fouhse, J.M.; Willing, B.P. Diet-Microbe-Host Interactions That Affect Gut Mucosal Integrity and Infection Resistance. Front. Immunol. 2019, 10, 1802. [Google Scholar] [CrossRef]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Roubos-van den Hil, P.J.; Schols, H.A.; Nout, M.J.R.; Zwietering, M.H.; Gruppen, H. First Characterization of Bioactive Components in Soybean Tempe That Protect Human and Animal Intestinal Cells against Enterotoxigenic Escherichia coli (ETEC) Infection. J. Agric. Food Chem. 2010, 58, 7649–7656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gänzle, M.G.; Schwab, C. Exopolysaccharide Synthesized by Lactobacillus reuteri Decreases the Ability of Enterotoxigenic Escherichia coli To Bind to Porcine Erythrocytes. AEM 2010, 76, 4863–4866. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, G.; Hermes, R.G.; Jiménez-Díaz, R.; Pérez, J.F.; Martín-Orúe, S.M. Screening of Extracts from Natural Feed Ingredients for Their Ability to Reduce Enterotoxigenic Escherichia coli (ETEC) K88 Adhesion to Porcine Intestinal Epithelial Cell-Line IPEC-J2. Vet. Microbiol. 2013, 167, 494–499. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Pérez, J.F.; Hermes, R.G.; Molist, F.; Jiménez-Díaz, R.; Martín-Orúe, S.M. Screening the Ability of Natural Feed Ingredients to Interfere with the Adherence of Enterotoxigenic Escherichia coli (ETEC) K88 to the Porcine Intestinal Mucus. Br. J. Nutr. 2014, 111, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; González-Ortiz, G.; Jiménez-Díaz, R.; Pérez-Trujillo, M.; Parella, T.; López-Colom, P.; Martín-Orúe, S.M. Exopolysaccharides from Olive Brines Could Reduce the Adhesion of ETEC K88 to Intestinal Epithelial Cells. Food Funct. 2018, 9, 3884–3894. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Guan, G.; Tan, J.; Al-Dhabi, N.A.; Wang, H.; Duraipandiyan, V.; Fang, J. Chitosan Modulates Inflammatory Responses in Rats Infected with Enterotoxigenic Escherichia coli. Mediat. Inflamm. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gutiérrez, I.; Martinez, A. Polysaccharide Hydrolysis with Engineered Escherichia coli for the Production of Biocommodities. J. Ind. Microbiol. Biotechnol. 2013, 40, 401–410. [Google Scholar] [CrossRef]
- Sarabia-Sainz, H.M.; Armenta-Ruiz, C.; Sarabia-Sainz, J.A.; Guzmán-Partida, A.M.; Ledesma-Osuna, A.I.; Vázquez-Moreno, L.; Ramos-Clamont Montfort, G. Adhesion of Enterotoxigenic Escherichia coli Strains to Neoglycans Synthesised with Prebiotic Galactooligosaccharides. Food Chem. 2013, 141, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Woodward, A.; Zijlstra, R.T.; Gänzle, M.G. Exopolysaccharides Synthesized by Lactobacillus reuteri Protect against Enterotoxigenic Escherichia coli in Piglets. Appl. Environ. Microbiol. 2014, 80, 5752–5760. [Google Scholar] [CrossRef]
- Roubos-van den Hil, P.J.; Nout, M.J.R.; Beumer, R.R.; van der Meulen, J.; Zwietering, M.H. Fermented Soya Bean (Tempe) Extracts Reduce Adhesion of Enterotoxigenic Escherichia coli to Intestinal Epithelial Cells. J. Appl. Microbiol. 2009, 106, 1013–1021. [Google Scholar] [CrossRef]
- Cilieborg, M.S.; Sangild, P.T.; Jensen, M.L.; Østergaard, M.V.; Christensen, L.; Rasmussen, S.O.; Mørbak, A.L.; Jørgensen, C.B.; Bering, S.B. A1,2-Fucosyllactose Does Not Improve Intestinal Function or Prevent Escherichia coli F18 Diarrhea in Newborn Pigs. J. Pediatric Gastroenterol. Nutr. 2017, 64, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in Vitro Exposure of Intestinal Epithelial Cells to E171 Food Additive Causes Oxidative Stress, Inducing Oxidation of DNA Bases but No Endoplasmic Reticulum Stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Vila, L.; Cortés, C.; Hernández, A.; Marcos, R. Exploring the Usefulness of the Complex in Vitro Intestinal Epithelial Model Caco-2/HT29/Raji-B in Nanotoxicology. Food Chem. Toxicol. 2018, 113, 162–170. [Google Scholar] [CrossRef]
- Gillois, K.; Stoffels, C.; Leveque, M.; Fourquaux, I.; Blesson, J.; Mils, V.; Cambier, S.; Vignard, J.; Terrisse, H.; Mirey, G.; et al. Repeated Exposure of Caco-2 versus Caco-2/HT29-MTX Intestinal Cell Models to (Nano)Silver in Vitro: Comparison of Two Commercially Available Colloidal Silver Products. Sci. Total Environ. 2021, 754, 142324. [Google Scholar] [CrossRef]
- Jansson, L.; Tobias, J.; Lebens, M.; Svennerholm, A.-M.; Teneberg, S. The Major Subunit, CfaB, of Colonization Factor Antigen I from Enterotoxigenic Escherichia coli Is a Glycosphingolipid Binding Protein. Infect. Immun. 2006, 74, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Lundgren, A.; Arifuzzaman, M.; Qadri, F.; Teneberg, S.; Svennerholm, A.-M. Children with the Le(A+b−) Blood Group Have Increased Susceptibility to Diarrhea Caused by Enterotoxigenic Escherichia coli Expressing Colonization Factor I Group Fimbriae. IAI 2009, 77, 2059–2064. [Google Scholar] [CrossRef]
- Madhavan, T.P.V.; Riches, J.D.; Scanlon, M.J.; Ulett, G.C.; Sakellaris, H. Binding of CFA/I Pili of Enterotoxigenic Escherichia coli to Asialo-GM1 Is Mediated by the Minor Pilin CfaE. Infect. Immun. 2016, 84, 1642–1649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheikh, A.; Rashu, R.; Begum, Y.A.; Kuhlman, F.M.; Ciorba, M.A.; Hultgren, S.J.; Qadri, F.; Fleckenstein, J.M. Highly Conserved Type 1 Pili Promote Enterotoxigenic E. coli Pathogen-Host Interactions. PLoS Negl. Trop. Dis. 2017, 11, e0005586. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Ghosal, A.; Sabui, S.; Chatterjee, N.S. Three Dimensional Modeling of C-Terminal Loop of CssA Subunit in CS6 of Enterotoxigenic Escherichia coli and Its Interaction with the 70 KDa Domain of Fibronectin. Bioinformation 2011, 6, 307–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
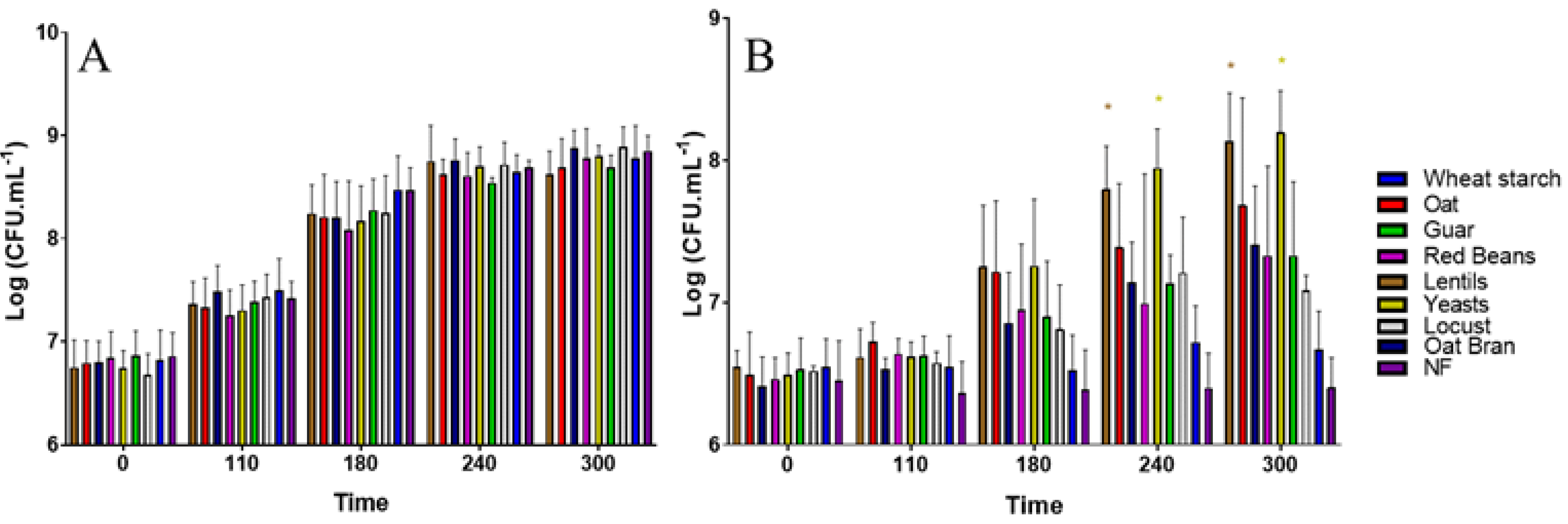
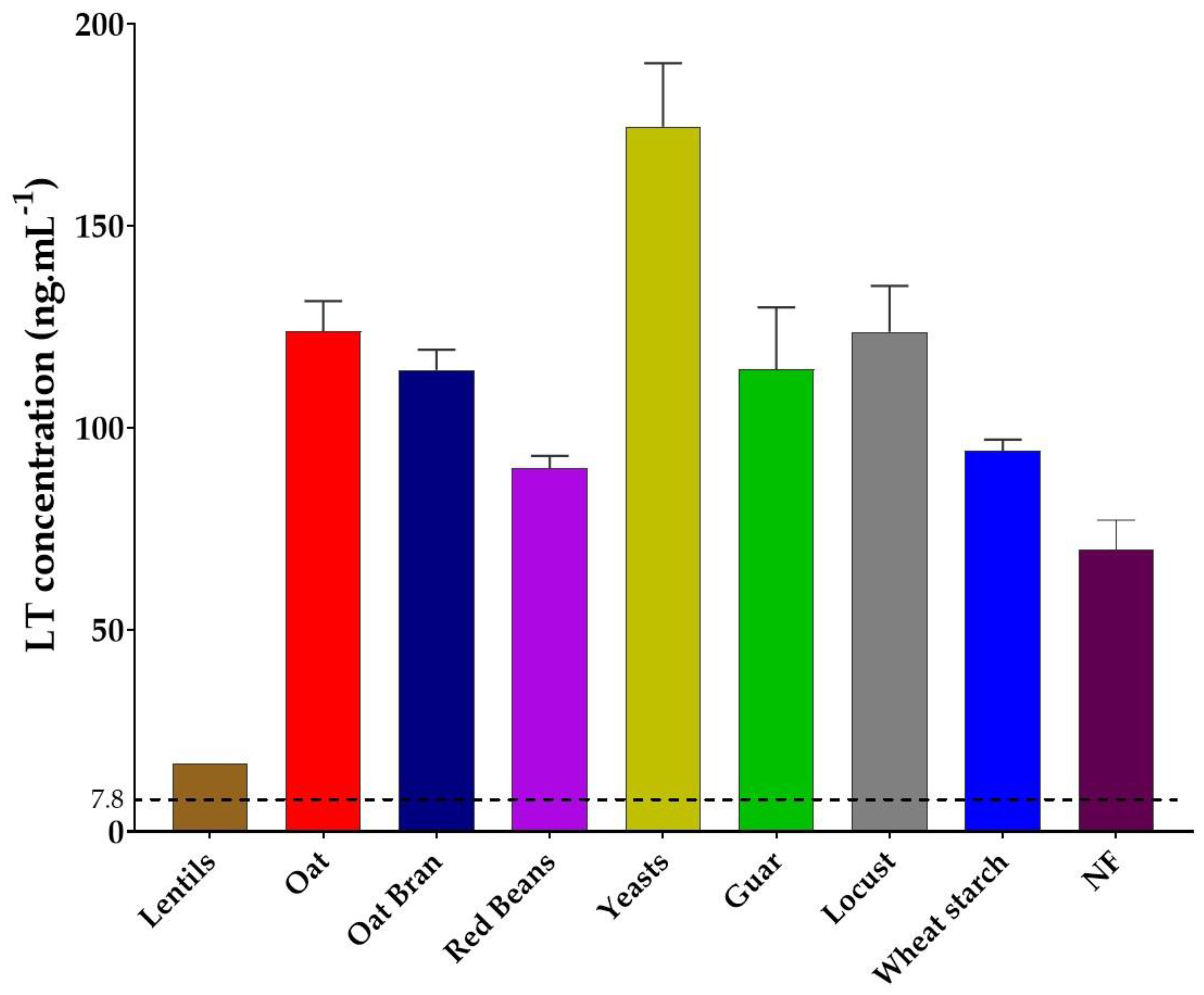
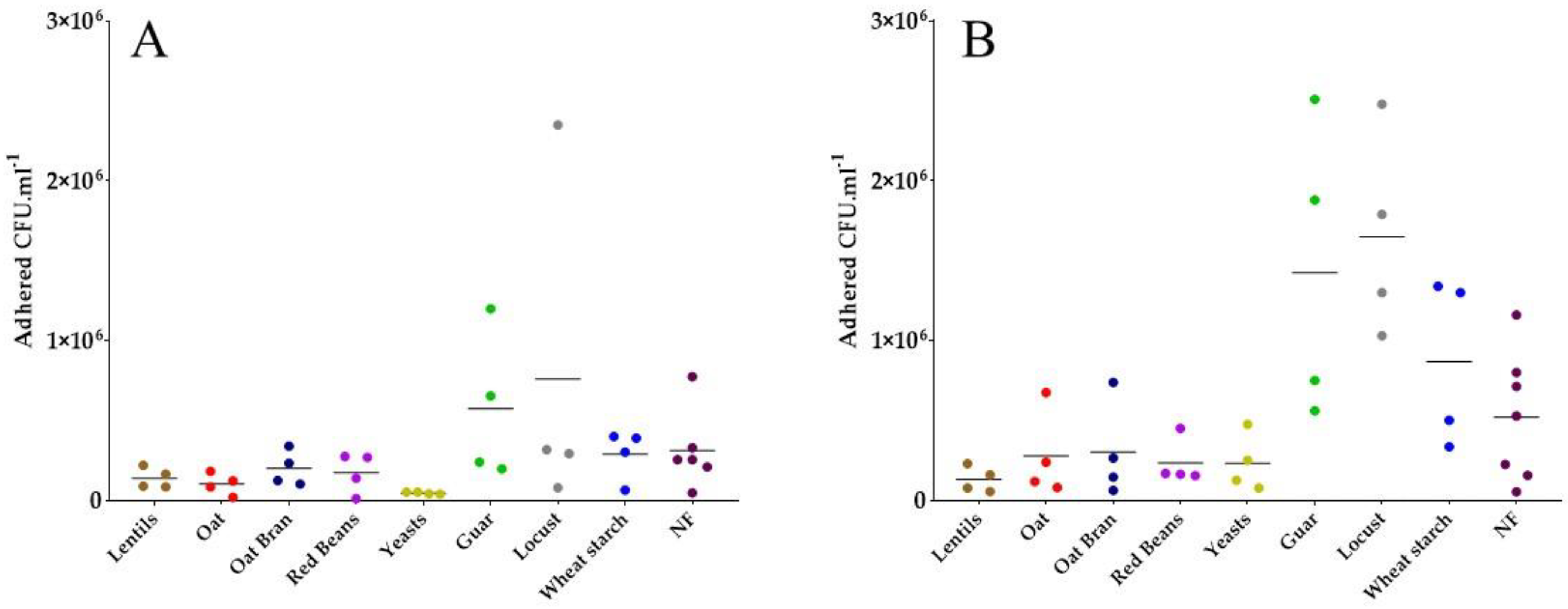
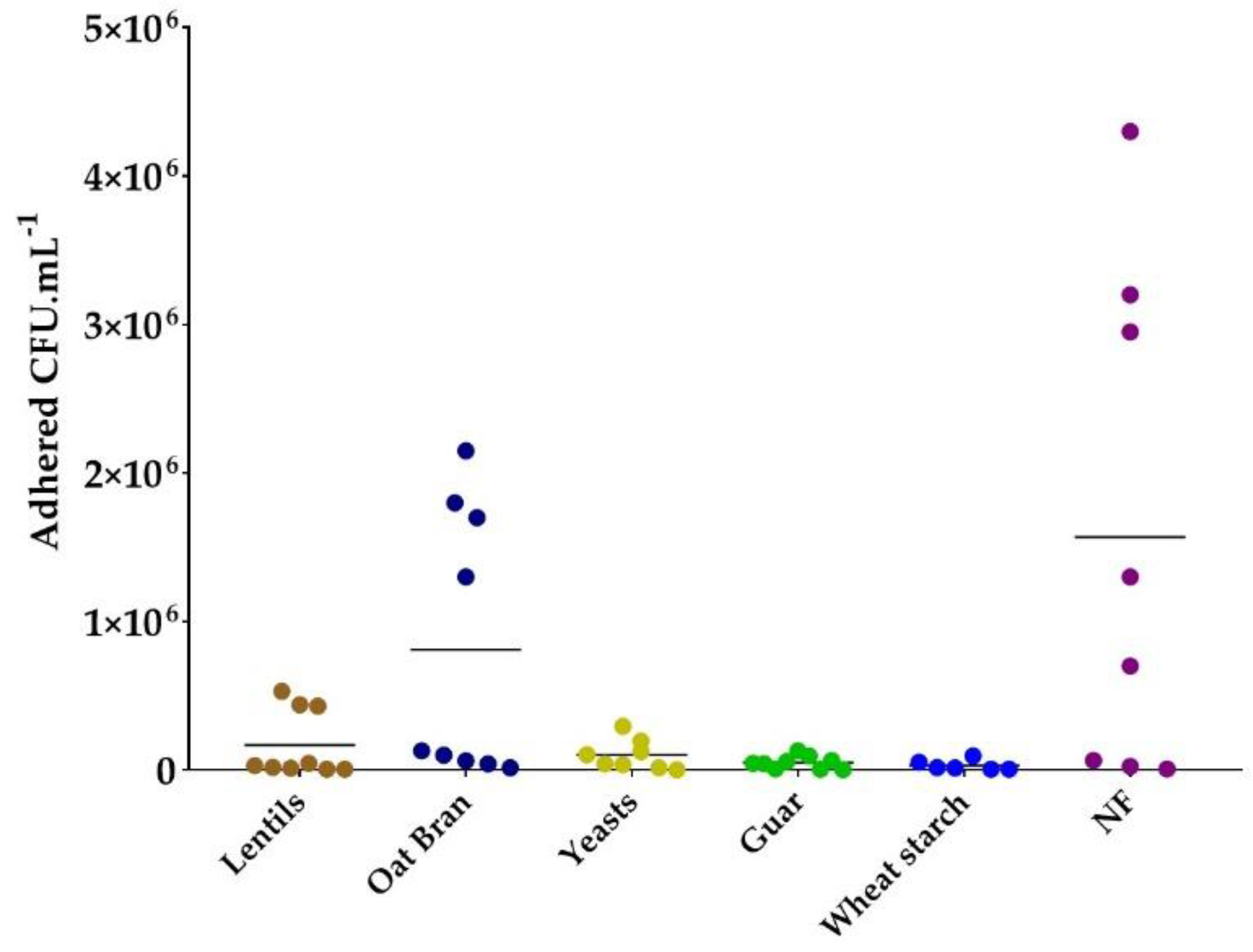
| Extract/Product | Origin | Product Source | Solubility at 2 g·L−1 in Water | Fiber Content (g·100 g−1) | Analysis Method |
|---|---|---|---|---|---|
| Green lentils | Plants | Home made | Insoluble | 41.4 | AOAC 985.29 |
| Guar gum | Plants | Commercially available | Soluble | 84.3 | AOAC 985.29 |
| Locus bean gum | Plants | Commercially available | Soluble | 83.3 | AOAC 985.29 |
| Oat | Plants | Home made | Insoluble | 19.8 | AOAC 985.29 |
| Oat bran | Plants | Provided by local companies | Insoluble | 44.4 | AOAC 985.29 |
| Red beans | Plants | Home made | Insoluble | 53 | AOAC 985.29 |
| Wheat starch | Plants | Provided by local companies | Soluble | 17 | Resistant starch content communicated by provider |
| Specific yeast cell walls (from Saccharomyces cerevisiae) | Microorganisms | Provided by local companies | Insoluble | 62.6 | AOAC 985.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauvaitre, T.; Durif, C.; Sivignon, A.; Chalancon, S.; Van de Wiele, T.; Etienne-Mesmin, L.; Blanquet-Diot, S. In Vitro Evaluation of Dietary Fiber Anti-Infectious Properties against Food-Borne Enterotoxigenic Escherichia coli. Nutrients 2021, 13, 3188. https://doi.org/10.3390/nu13093188
Sauvaitre T, Durif C, Sivignon A, Chalancon S, Van de Wiele T, Etienne-Mesmin L, Blanquet-Diot S. In Vitro Evaluation of Dietary Fiber Anti-Infectious Properties against Food-Borne Enterotoxigenic Escherichia coli. Nutrients. 2021; 13(9):3188. https://doi.org/10.3390/nu13093188
Chicago/Turabian StyleSauvaitre, Thomas, Claude Durif, Adeline Sivignon, Sandrine Chalancon, Tom Van de Wiele, Lucie Etienne-Mesmin, and Stéphanie Blanquet-Diot. 2021. "In Vitro Evaluation of Dietary Fiber Anti-Infectious Properties against Food-Borne Enterotoxigenic Escherichia coli" Nutrients 13, no. 9: 3188. https://doi.org/10.3390/nu13093188
APA StyleSauvaitre, T., Durif, C., Sivignon, A., Chalancon, S., Van de Wiele, T., Etienne-Mesmin, L., & Blanquet-Diot, S. (2021). In Vitro Evaluation of Dietary Fiber Anti-Infectious Properties against Food-Borne Enterotoxigenic Escherichia coli. Nutrients, 13(9), 3188. https://doi.org/10.3390/nu13093188







