Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids—A Randomized Control Cross-Over Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Protein Supplement
2.4. Protein AA Profile and Analysis
2.5. Blood Samples
2.6. Serum Amino Acid Concentration Determined by 1H NMR Spectroscopy
2.7. Visual Analogue Scale
2.8. Dual-Energy X-ray Absorptiometry
2.9. Statistics
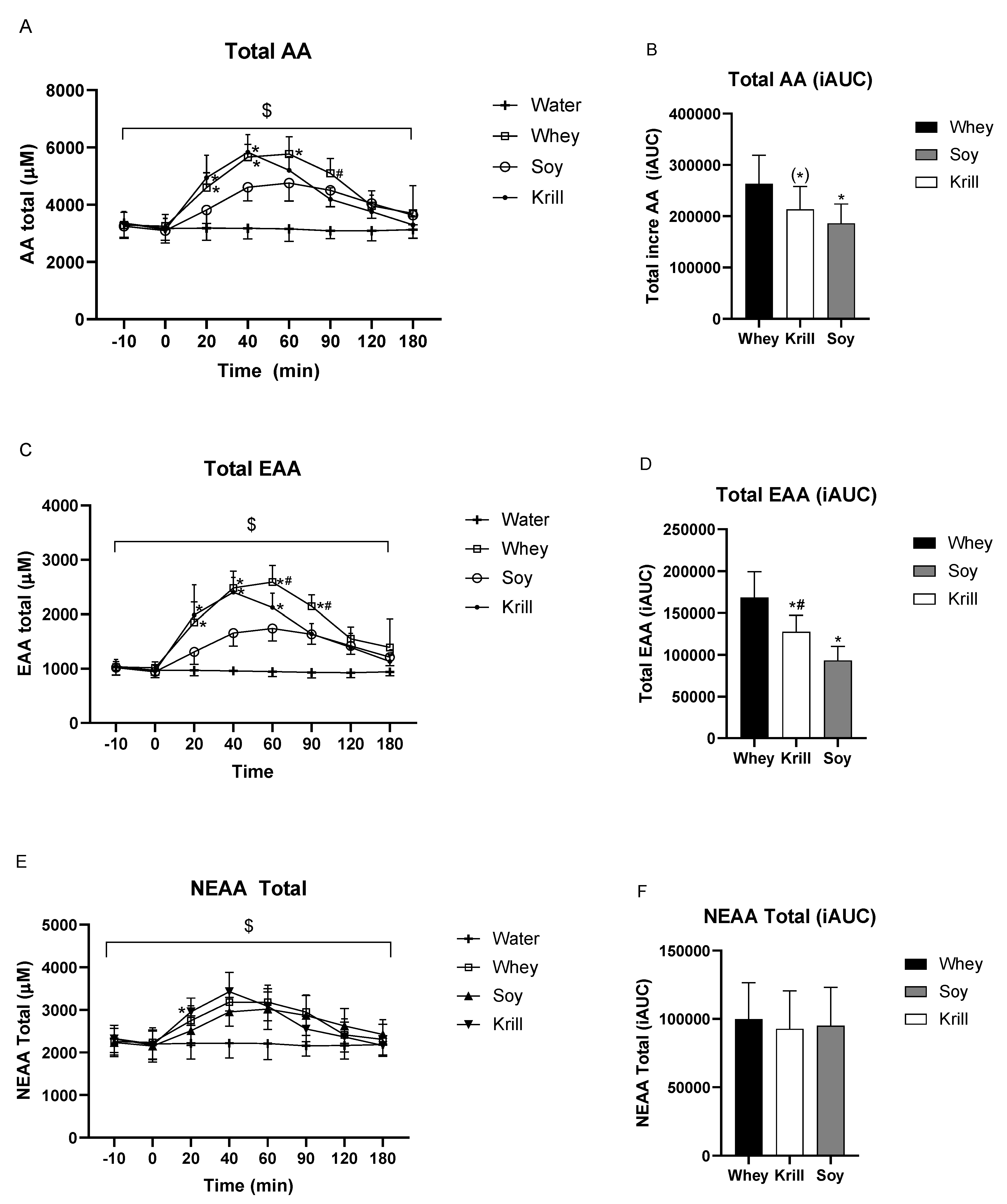
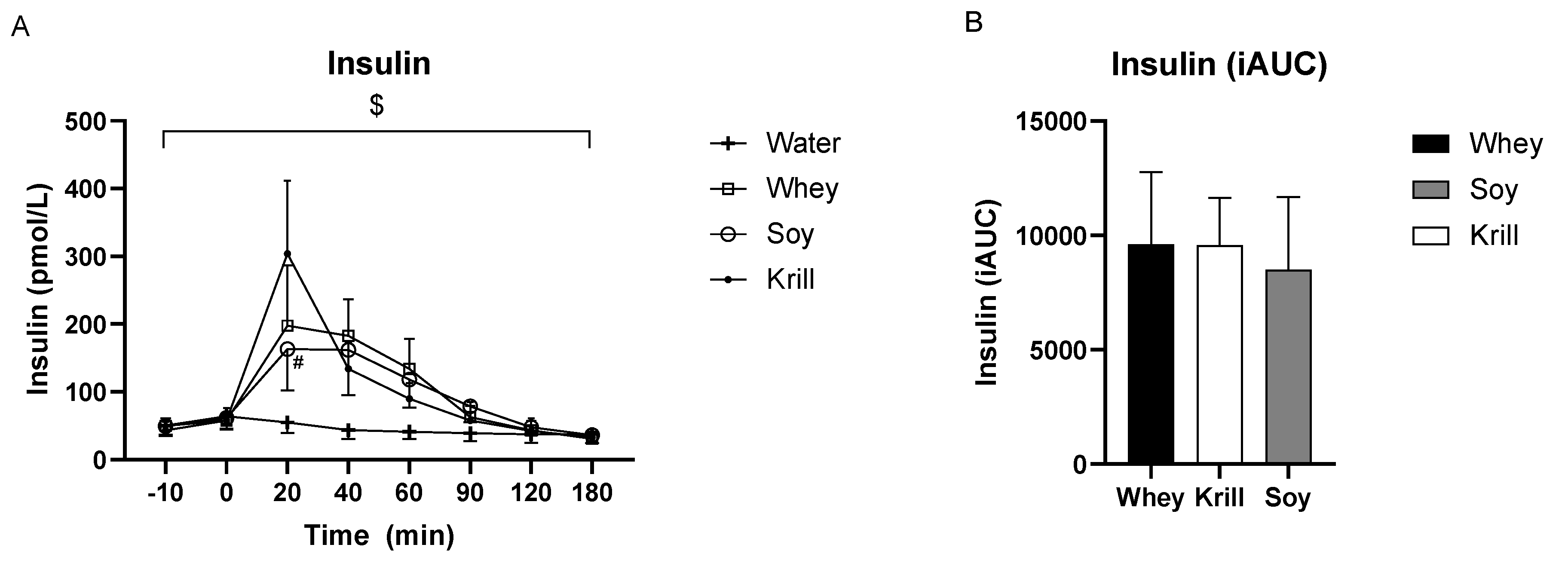
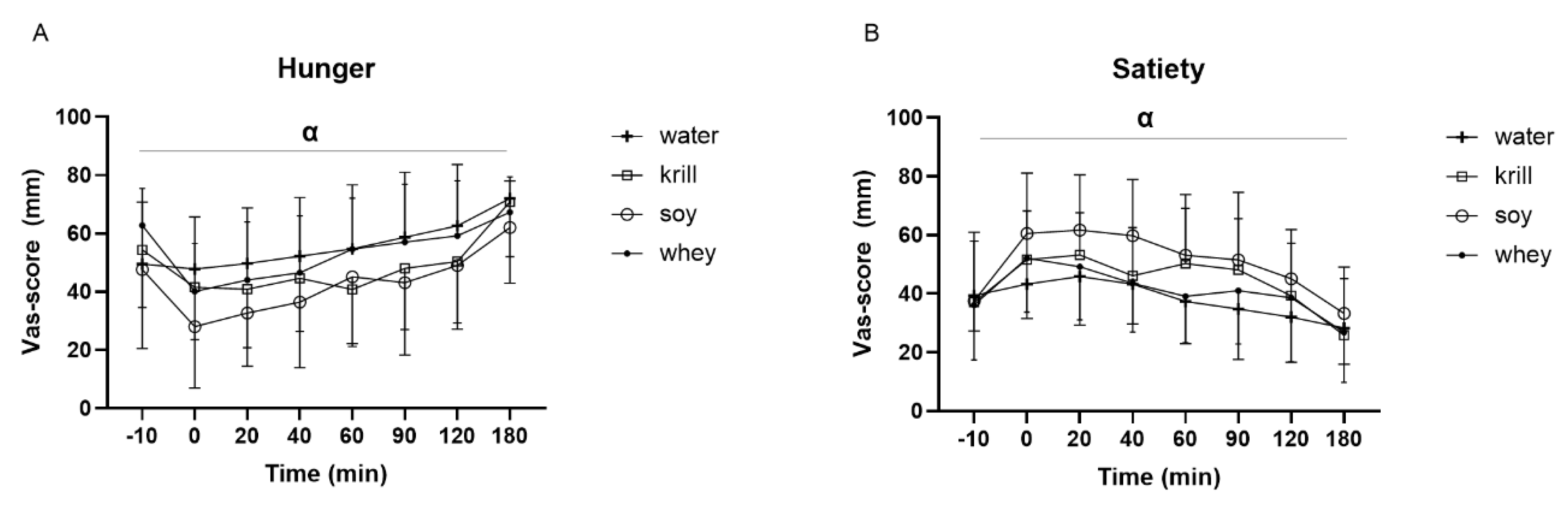
3. Results
3.1. Amino Acid Concentrations
3.2. Insulin
3.3. VAS Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO’s Director-General on How to Feed the World in 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009.
- Tipton, K.D. Protein for adaptations to exercise training. Eur. J. Sport Sci. 2008, 8, 107–118. [Google Scholar] [CrossRef]
- Becker, W.; Anderssen, S.A.; Fogelholm, M.; Gunnarsdottir, I.; Hursti, U.K.K.; Meltzer, H.M.; Pedersen, A.N.; Schwab, U.; Tetens, I.; Wirfalt, E. NNR 2012: Nordic nutrition recommendations—Integrating nutrition and physical activity. Ann. Nutr. Metab. 2013, 63, 897. [Google Scholar]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016, 13, 64. [Google Scholar] [CrossRef] [Green Version]
- Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation. FAO Food Nutr. Pap. 2013, 92, 1–66.
- WHO; FAO; UNUEC. Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Tipton, K.D.; Gurkin, B.E.; Matin, S.; Wolfe, R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J. Nutr. Biochem. 1999, 10, 89–95. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. S1), A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Fearnside, P.M. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 2001, 28, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Froehlich, H.E.; Runge, C.A.; Gentry, R.R.; Gaines, S.D.; Halpern, B.S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl. Acad. Sci. USA 2018, 115, 5295–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krafft, B.; Bakkeplass, K.; Berge, T.; Biuw, M.; Erices, J.; Jones, E.; Knutsen, T.; Kubilius, R.; Kvalsund, M.; Lindstrøm, U.; et al. Report from a Krill Focused Survey with Rv Kronprins Haakon and Land-Based Predator Work in Antarctica During 2018/2019; Havforskningsinstituttet: Bergen, Norway, 2019. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Wyse, C.; Gray, S.R.; Harris, W.S.; Lazzerini, K. Effects of dietary supplementation with krill meal on serum pro-inflammatory markers after the Iditarod sled dog race. Res. Vet. Sci. 2018, 121, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Mørkøre, T.; Moreno, H.; Borderías, J.; Larsson, T.; Hellberg, H.; Hatlen, B.; Romarheim, O.H.; Ruyter, B.; Lazado, C.C.; Jiménez-Guerrero, R. Dietary inclusion of Antarctic krill meal during the finishing feed period improves health and fillet quality of Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2020, 124, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, A.J.; Sabry-Neto, H.; Oliveira-Neto, S.; Burri, L. Feed preference and growth response of juvenile Litopenaeus vannamei to supplementation of marine chemoattractants in a fishmeal-challenged diet. J. World Aquac. Soc. 2019, 50, 1048–1063. [Google Scholar] [CrossRef]
- Burri, L.; Johnsen, L. Krill Products: An Overview of Animal Studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [Green Version]
- Ursoniu, S.; Sahebkar, A.; Serban, M.-C.; Antal, D.; Mikhailidis, D.P.; Cicero, A.; Athyros, V.; Rizzo, M.; Rysz, J.; Banach, M. Lipid-modifying effects of krill oil in humans: Systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2017, 75, 361–373. [Google Scholar] [CrossRef]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.-S.; Ahn, C.-B.; Ahn, G. In vivo hepatoprotective effects of a peptide fraction from krill protein hydrolysates against alcohol-induced oxidative damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef] [Green Version]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef]
- Ramsvik, M.S.; Bjorndal, B.; Vik, R.; Bruheim, I.; Skorve, J.; Berge, R.K. Krill protein hydrolysate reduces plasma triacylglycerol level with concurrent increase in plasma bile acid level and hepatic fatty acid catabolism in high-fat fed mice. Funct. Foods Health Dis. 2013, 3, 428–440. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, L.; Tao, J.; Chi, C.-F.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vangsoe, M.T.; Thogersen, R.; Bertram, H.C.; Heckmann, L.-H.L.; Hansen, M. Ingestion of Insect Protein Isolate Enhances Blood Amino Acid Concentrations Similar to Soy Protein in A Human Trial. Nutrients 2018, 10, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of Different Sources and Degrees of Hydrolysis of Dietary Protein: Effect on Plasma Amino Acids, Dipeptides, and Insulin Responses in Human Subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Skov, K.; Oxfeldt, M.; Thøgersen, R.; Hansen, M.; Bertram, H.C. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate-A Randomized Controlled Trial. Nutrients 2019, 11, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkkinen, E.S.; Savolainen, M.J.; Taurio, J.; Marvola, T.; Bruheim, I. Prospective, randomized, double-blinded, placebo-controlled study on safety and tolerability of the krill powder product in overweight subjects with moderately elevated blood pressure. Lipids Health Dis. 2018, 17, 287. [Google Scholar] [CrossRef] [Green Version]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E628–E634. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Yang, J.; Chi, Y.; Burkhardt, B.R.; Guan, Y.; Wolf, B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010, 68, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimble, G.; Rees, R.; Keohane, P.; Cartwright, T.; Desreumaux, M.; Silk, D. Effect of peptide chain length on absorption of egg protein hydrolysates in the normal human jejunum. Gastroenterology 1987, 92, 136–142. [Google Scholar] [CrossRef]
- Grimble, G.; Sarda, M.G.; Sessay, H.; Marrett, A.; Kapadia, S.; Bowling, T.; Silk, D. The influence of whey hydrolysate peptide chain length on nitrogen and carbohydrate absorption in the perfused human jejunum. Clin. Nutr. 1994, 13, 46. [Google Scholar] [CrossRef]
- Calbet, J.A.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Farnfield, M.M.; Trenerry, C.; Carey, K.A.; Cameron-Smith, D. Plasma amino acid response after ingestion of different whey protein fractions. Int. J. Food Sci. Nutr. 2009, 60, 476–486. [Google Scholar] [CrossRef]
- Power, O.; Hallihan, A.; Jakeman, P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 2009, 37, 333–339. [Google Scholar] [CrossRef]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef]
- Nakayama, K.; Tagawa, R.; Saito, Y.; Sanbongi, C. Effects of whey protein hydrolysate ingestion on post-exercise muscle protein synthesis compared with intact whey protein in rats. Nutr. Metab. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels-Ansdell, D.; Schünemann, H.J. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar] [CrossRef]
- Skov, A.R.; Toubro, S.; Rønn, B.; Holm, L.; Astrup, A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1999, 23, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.H.; Moore, S.E. Dietary Proteins in the Regulation of Food Intake and Body Weight in Humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [CrossRef]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein Source, Quantity, and Time of Consumption Determine the Effect of Proteins on Short-Term Food Intake in Young Men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef] [Green Version]
- Mellinkoff, S.M.; Frankland, M.; Boyle, D.; Greipel, M. Relationship between serum amino acid concentration and fluctuations in appetite (Reprinted). Obes. Res. 1997, 5, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.B.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.; Westerterp, K.R.; Engelen, M.; Brummer, R.J.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Gachon, P.; Beaufrère, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]


| Subjects (n = 10 Men) | ||
|---|---|---|
| Age (years) | 27 | ±2 |
| Weight (kg) | 81.5 | ±10.0 |
| Height (cm) | 186 | ±6 |
| BMI (kg/m2) | 23.6 | ±2.7 |
| Activity level (hours/week) | 3.5 | ±2.4 |
| Krill Protein Hydrolysate (35.1 g Powder) | Whey Protein Isolate (35.3 g Powder) | Soy Protein Isolate (39.6 g Powder) | |
|---|---|---|---|
| Macronutrient composition | |||
| Energy (kcal) | 132 | 130 | 155 |
| Protein (g) ¤ | 32.7 | 30.7 | 34.8 |
| Fat (g) | <0.4 | 0.3 | 1.5 |
| Carbohydrates (g) | 0.0 | 1.1 | 0.0 |
| Alanine, g | 2.02 | 1.83 | 1.47 |
| Arginine, g | 2.17 | 0.67 | 2.54 |
| Asparagine, g | 4.08 | 3.78 | 4.20 |
| Cysteine, g | 0.27 | 0.76 | 0.40 |
| Glutamine, g | 5.20 | 6.17 | 6.62 |
| Glycine, g | 1.57 | 0.48 | 1.44 |
| Histidine, g * | 0.85 | 0.52 | 0.91 |
| Isoleucine, g *,# | 1.77 | 2.17 | 1.57 |
| Leucine, g *,# | 2.87 | 3.63 | 2.73 |
| Lysine, g * | 3.28 | 3.26 | 2.16 |
| Methionine, g * | 1.02 | 0.82 | 0.47 |
| Phenylalanine, g * | 1.62 | 0.97 | 1.81 |
| Proline, g | 1.27 | 2.18 | 1.84 |
| Serine, g | 1.44 | 1.62 | 1.86 |
| Threonine, g * | 1.75 | 2.55 | 1.38 |
| Tyrosine, g * | 1.52 | 1.02 | 1.44 |
| Valine, g *,# | 1.89 | 1.98 | 1.65 |
| Tryptophan, g | 0.42 | 0.59 | 0.50 |
| TAA | 35.00 | 35.00 | 35.00 |
| EAA * | 16.56 | 16.91 | 14.13 |
| NEAA | 18.44 | 18.09 | 20.87 |
| BCAA # | 6.53 | 7.78 | 5.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thøgersen, R.; Bertram, H.C.; Vangsoe, M.T.; Hansen, M. Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids—A Randomized Control Cross-Over Trial. Nutrients 2021, 13, 3187. https://doi.org/10.3390/nu13093187
Thøgersen R, Bertram HC, Vangsoe MT, Hansen M. Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids—A Randomized Control Cross-Over Trial. Nutrients. 2021; 13(9):3187. https://doi.org/10.3390/nu13093187
Chicago/Turabian StyleThøgersen, Rebekka, Hanne Christine Bertram, Mathias T. Vangsoe, and Mette Hansen. 2021. "Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids—A Randomized Control Cross-Over Trial" Nutrients 13, no. 9: 3187. https://doi.org/10.3390/nu13093187
APA StyleThøgersen, R., Bertram, H. C., Vangsoe, M. T., & Hansen, M. (2021). Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids—A Randomized Control Cross-Over Trial. Nutrients, 13(9), 3187. https://doi.org/10.3390/nu13093187







