Risk-Benefit Assessment of Cereal-Based Foods Consumed by Portuguese Children Aged 6 to 36 Months—A Case Study under the RiskBenefit4EU Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Relevant Components and Health Effects
2.2. Investigated Consumption Scenarios
- 100% BC scenario, simulating that all infants consume only BC;
- 100% IC scenario, simulating that all infants consume only IC;
- Optimal BC scenario, simulating that all infants consume only BC at an optimal composition; and
- Worst IC, simulating that all infants consume only IC with the worst composition.
2.3. Data Used in the Model
2.4. Exposure Assessment, DALY Calculation and Integration of Risks and Benefits
2.4.1. Total Fiber, Sodium and Free Sugars
2.4.2. Aflatoxins
2.4.3. Bacillus Cereus
2.4.4. Integration of DALY
3. Results
3.1. Intake and Exposure Assessment
3.2. Incidence and DALYs
3.3. Comparison to DRVs
4. Discussion
4.1. Consumption of Breakfast Cereals Instead of Infant Cereals Could Result in a Gain of Healthy-Life Years
4.2. Sources of Uncertainty
- Selection of relevant food components and associated health effects: Despite an extensive literature search being performed to support decisions in the selection of food components and associated health effects, other beneficial (e.g., micronutrients used for fortification or saturated fats) or hazardous (e.g., other mycotoxins such as deoxynivalenol and zearalenone, heavy metals, furans, acrylamide) components could be present in BC and IC; also, food allergies are relevant health outcomes when investigating the health impact of infant and toddler foods, but have been disregarded in this assessment.
- Data used in the exposure assessment: Concentration of B. cereus and aflatoxins in BC and IC were based on reported levels of analyzed samples or extracted from the Portuguese Food Composition Table (sodium, free sugars and fiber). These data are necessarily affected by uncertainty that impacted (over and/or underestimated) the exposure assessment.
- Risk of CVD and T2D later in life based on exposure that occurred in the first years of life: Due to the restrictions of the data, CVD and T2D cases were estimated, taking into account the available dose–response data. However, it was assumed that exposure occurring in the first years of life will contribute to and determine cases later in life.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
Appendix B. Fiber Intake and Risk Ratio Estimates
References
- Amezdroz, E.; Carpenter, L.; O’Callaghan, E.; Johnson, S.; Waters, E. Transition from milks to the introduction of solid foods across the first 2 years of life: Findings from an Australian birth cohort study. J. Hum. Nutr. Diet. 2015, 28, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.S.P.; Lopes, C.; Naska, A.; Trichopoulou, A.; de Almeida, M.D.V. Comparison of national food supply, household food availability and individual food consumption data in Portugal. J. Public Health 2007, 15, 447–455. [Google Scholar] [CrossRef]
- Collins, H.M.; Burton, R.A.; Topping, D.L.; Liao, M.-L.; Bacic, A.; Fincher, G.B. REVIEW: Variability in Fine Structures of Noncellulosic Cell Wall Polysaccharides from Cereal Grains: Potential Importance in Human Health and Nutrition. Cereal Chem. 2010, 87, 272–282. [Google Scholar] [CrossRef]
- Schwartz, M.B.; Vartanian, L.R.; Wharton, C.M.; Brownell, K.D. Examining the nutritional quality of breakfast cereals marketed to children. J. Am. Diet. Assoc. 2008, 108, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Vasco, E.; Nunes, B.; Loureiro, S.; Martins, C.; Alvito, P. Single-compound and cumulative risk assessment of mycotoxins present in breakfast cereals consumed by children from Lisbon region, Portugal. Food Chem. Toxicol. 2015, 86, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Martins, C.; Vasco, E.; Jager, A.; Oliveira, C.; Cunha, S.C.; Fernandes, J.O.; Nunes, B.; Loureiro, S.; Alvito, P. Portuguese children dietary exposure to multiple mycotoxins—An overview of risk assessment under MYCOMIX project. Food Chem. Toxicol. 2018, 118, 399–408. [Google Scholar] [CrossRef]
- Martins, C.; Assunção, R.; Cunha, S.C.; Fernandes, J.O.; Jager, A.; Petta, T.; Oliveira, C.A.; Alvito, P. Assessment of multiple mycotoxins in breakfast cereals available in the Portuguese market. Food Chem. 2018, 239, 132–140. [Google Scholar] [CrossRef]
- Zhang, K.; Flannery, B.M.; Oles, C.J.; Adeuya, A. Mycotoxins in infant/toddler foods and breakfast cereals in the U.S. retail market. Food Addit. Contam. Part B 2018, 11, 183–190. [Google Scholar] [CrossRef]
- Gardener, H.; Bowen, J.; Callan, S.P. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci. Total Environ. 2019, 651, 822–827. [Google Scholar] [CrossRef]
- Jean, J.; Sirot, V.; Hulin, M.; Le Calvez, E.; Zinck, J.; Noël, L.; Vasseur, P.; Nesslany, F.; Gorecki, S.; Guérin, T.; et al. Dietary exposure to cadmium and health risk assessment in children—Results of the French infant total diet study. Food Chem. Toxicol. 2018, 115, 358–364. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Chafey, C.; Testu, C.; Hulin, M.; Noël, L. Mercury in foods from the first French total diet study on infants and toddlers. Food Chem. 2018, 239, 920–925. [Google Scholar] [CrossRef]
- Tinggi, U.; Schoendorfer, N. Analysis of lead and cadmium in cereal products and duplicate diets of a small group of selected Brisbane children for estimation of daily metal exposure. J. Trace Elem. Med. Biol. 2018, 50, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Vasco, E.; Paixão, E.; Alvito, P. Total mercury in infant food, occurrence and exposure assessment in Portugal. Food Addit. Contam. Part B 2013, 6, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Inthavong, C.; Hommet, F.; Leblanc, J.-C.; Hulin, M.; Guérin, T. Levels of acrylamide in foods included in ‘the first French total diet study on infants and toddlers. Food Chem. 2018, 240, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Inthavong, C.; Desbourdes, C.; Hommet, F.; Sirot, V.; Leblanc, J.-C.; Hulin, M.; Guérin, T. Levels of furan in foods from the first French Total Diet Study on infants and toddlers. Food Chem. 2018, 266, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kacmaz, S.; Zelinkova, Z.; Wenzl, T. Rapid and sensitive method for the determination of four EU marker polycyclic aromatic hydrocarbons in cereal-based foods using isotope-dilution GC/MS. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 631–638. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Ranking of Low Moisture Foods in Support of Microbiological Risk Management—Preliminary Report; FAO: Rome, Italy, 2014. [Google Scholar]
- World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012; ISBN 9789241504836. [Google Scholar]
- World Health Organization (WHO). Guideline: Sugars Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2015; ISBN 9789241549028. [Google Scholar]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Alarcão, V.; Guiomar, S.; Mota, J.; Teixeira, P.; Rodrigues, S.; Lobato, L.; et al. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015–2016: Relatório de Resultados; University of Porto: Porto, Portugal, 2017; ISBN 978989746181. [Google Scholar]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Guiomar, S.; Alarcão, V.; Vilela, S.; Ramos, E.; Rodrigues, S.; Oliveira, L.; et al. National Food, Nutrition and Physical Activity Survey of the Portuguese general population. EFSA Support. Publ. 2017, 14. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Guiomar, S.; Alarcão, V.; Ramos, E.; Rodrigues, S.; Vilela, S.; Oliveira, L.; et al. National Food, Nutrition, and Physical Activity Survey of the Portuguese General Population (2015–2016): Protocol for Design and Development. JMIR Res. Protoc. 2018, 7, e42. [Google Scholar] [CrossRef]
- Alvito, P.C.; Sizoo, E.A.; Almeida, C.M.M.; van Egmond, H.P. Occurrence of aflatoxins and ochratoxin A in baby foods in Portugal. Food Anal. Methods 2010, 3, 22–30. [Google Scholar] [CrossRef]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: A contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016, 96, 205–214. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Energy and Protein Requirements; WHO: Geneva, Switzerland, 1985; ISBN 9241207248. [Google Scholar]
- Assunção, R.; Alvito, P.; Brazão, R.; Carmona, P.; Fernandes, P.; Jakobsen, L.S.; Lopes, C.; Martins, C.; Membré, J.M.; Monteiro, S.; et al. Building capacity in risk-benefit assessment of foods: Lessons learned from the RB4EU project. Trends Food Sci. Technol. 2019, 91, 541–548. [Google Scholar] [CrossRef]
- Nauta, M.J.; Andersen, R.; Pilegaard, K.; Pires, S.M.; Ravn-Haren, G.; Tetens, I.; Poulsen, M. Meeting the challenges in the development of risk-benefit assessment of foods. Trends Food Sci. Technol. 2018, 76, 90–100. [Google Scholar] [CrossRef]
- Pires, S.M.; Boué, G.; Boobis, A.; Eneroth, H.; Hoekstra, J.; Membré, J.M.; Persson, I.M.; Poulsen, M.; Ruzante, J.; van Klaveren, J.; et al. Risk Benefit Assessment of foods: Key findings from an international workshop. Food Res. Int. 2019, 116, 859–869. [Google Scholar] [CrossRef] [Green Version]
- EFSA Scientific Committee Guidance on human health risk-benefit assessment of foods. EFSA J. 2010, 8, 1673. [CrossRef]
- Verhagen, H.; Tijhuis, M.J.; Gunnlaugsdόttir, H.; Kalogeras, N.; Leino, O.; Luteijn, J.M.; Magnússon, S.H.; Odekerken, G.; Pohjola, M.V.; Tuomisto, J.T.; et al. State of the art in benefit–risk analysis: Introduction. Food Chem. Toxicol. 2012, 50, 2–4. [Google Scholar] [CrossRef]
- Alvito, P.; Brazão, R.; Carmona, P.; Carvalho, C.; Correia, D.; Fernandes, P.; Jakobsen, L.S.; Lopes, C.; Martins, C.; Membré, J.; et al. RiskBenefit4EU—Partnering to strengthen Risk-Benefit Assessment within the EU using a holistic approach. EFSA Support. Publ. 2019, 16, 1768E. [Google Scholar] [CrossRef]
- Tijhuis, M.J.; de Jong, N.; Pohjola, M.V.; Gunnlaugsdóttir, H.; Hendriksen, M.; Hoekstra, J.; Holm, F.; Kalogeras, N.; Leino, O.; van Leeuwen, F.X.R.; et al. State of the art in benefit—Risk analysis: Food and nutrition. Food Chem. Toxicol. 2012, 50, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). A Review of Human Carcinogens: Chemical Agents and Related Occupations; IARC Publications: Lyon, France, 2012; Volume 100F, ISBN 978 92 832 1323 9. [Google Scholar]
- International Agency for Research on Cancer (IARC). Cancer Fact Sheets: Liver Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 22 June 2020).
- Duc, L.H.; Dong, T.C.; Logan, N.A.; Sutherland, A.D.; Taylor, J.; Cutting, S.M. Cases of emesis associated with bacterial contamination of an infant breakfast cereal product. Int. J. Food Microbiol. 2005, 102, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.; Belo Correia, C.; Alvito, P. Contaminantes de Origem Microbiológica em Alimentação Infantil. Bol. EPIDEMIOLÓGICO Obs. 2013, Volume 2, pp. 6–7. Available online: http://www2.insa.pt/sites/INSA/Portugues/PublicacoesRepositorio/Documents/observacoesN62013_artigo2.pdf (accessed on 1 September 2021).
- Lesley, M.B.; Velnetti, L.; Yousr, A.N.; Kasing, A.; Samuel, L. Presence of Bacillus cereus s.l. From ready-to-eat cereals (RTE) products in Sarawak. Int. Food Res. J. 2013, 20, 1031–1034. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Panel on Dietetic Products Nutrition and Allergies Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef] [Green Version]
- Scientific Advisory Committee on Nutrition (SACN). Carbohydrates and Health; TSO: London, UK, 2015; ISBN 978 0 11 708284 7.
- World Cancer Research Fund and the American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Colorectal Cancer. Continuous Update Project Expert Report 2018. Available online: https://www.wcrf.org/diet-and-cancer/ (accessed on 22 June 2020).
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G. Evidence-Based Guideline of the German Nutrition Society: Carbohydrate Intake and Prevention of Nutrition-Related Diseases. Ann. Nutr. Metab. 2012, 60, 1–58. [Google Scholar] [CrossRef] [PubMed]
- WHO. Joint WHO/FAO Expert Consultation on Diet Nutrition and the Prevention of Chronic Diseases. In Diet, Nutrition and Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary reference values for sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Saúde Doutor Ricardo Jorge Tabela da Composição dos Alimentos (Foodcomposition Database). Available online: http://www2.insa.pt/sites/INSA/Portugues/AreasCientificas/AlimentNutricao/AplicacoesOnline/TabelaAlimentos/Paginas/TabelaAlimentos.aspx (accessed on 15 June 2020).
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- ASAE Plano Nacional de Colheita de Amostras. Available online: https://www.asae.gov.pt/ (accessed on 22 June 2020).
- Devleesschauwer, B.; Haagsma, J.A.; Angulo, F.; Bellinger, D.C.; Cole, D.; Dopfer, D.; Aamir, F.; Fevre, E.M.; Gibb, H.; Hald, T.; et al. Methodological Framework for World Health Organization Estimates of the GLobal Burden of Foodborne Disease. PLoS ONE 2015, 10, e0142498. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, S.T.; Pires, S.M.; Devleesschauwer, B.; Poulsen, M.; Fagt, S.; Ygil, K.H.; Andersen, R. Investigating the risk-benefit balance of substituting red and processed meat with fish in a Danish diet. Food Chem. Toxicol. 2018, 120, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute for Health Metrics and Evaluation (IHME). GBD Results Tool 2017. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 22 June 2020).
- FAO/WHO Expert Committee on Food Assitives. Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHOExpert Committee on Food Additives; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Dekkers, A.L.; Verkaik-Kloosterman, J.; van Rossum, C.T.; Ocké, M.C. SPADE, a New Statistical Program to Estimate Habitual Dietary Intake from Multiple Food Sources and Dietary Supplements. J. Nutr. 2014, 144, 2083–2091. [Google Scholar] [CrossRef] [Green Version]
- Berlin, J.A.; Longnecker, M.P.; Epidemiology, S.; May, N.; Berlin, J.A.; Longnecker, M.P.; Greenland, S. Meta-analysis of Epidemiologic Dose-Response Data. Epidemiology 1993, 4, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Veerman, J.L. Categorical versus continuous risk factors and the calculation of potential impact fractions. J. Epidemiol. Community Health 2010, 64, 209–212. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019; ISBN 9780309488341. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on contaminants in the food chain [CONTAM] related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived prod. EFSA J. 2007, 5, 446. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1–96. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Scientific opionion on the risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Farakos, S.S.; Pouillot, R.; Spungen, J.; Flannery, B.; Dolan, L.; Doren, J. Van Implementing a Framework to Evaluate the Impact of Food Intake Shifts on Risk of Illness Using a Case Study with Infant Cereal (P11-118-19). Curr. Dev. Nutr. 2019, 3, 1096. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Angelino, D.; Rosi, A.; Dall’Asta, M.; Pellegrini, N.; Martini, D. Evaluation of the Nutritional Quality of Breakfast Cereals Sold on the Italian Market: The Food Labelling of Italian Products (FLIP) Study. Nutrients 2019, 11, 2827. [Google Scholar] [CrossRef] [Green Version]
- Italian Ministry of Health. Common Objectives for the Improvement of the Nutritional Characteristics of Food Products with a Special Focus on Children (Ages 3–12) 2017. Monitoring Report. Available online: https://www.salute.gov.it/imgs/C_17_opuscoliPoster_376_0_alleg.pdf (accessed on 15 June 2020).
- Ministério das Finanças; Ministério da Administração Interna; Ministério da Educação; Ministério da Saúde; Ministério da Economia; Ministério da Agricultura Florestas e Desenvolvimento Rural; Ministério do Mar. Despacho N° 11418/2017 Estratégia integrada para a promoção da alimentação saudável. Diário da República 2017, 249, 29595–29598. [Google Scholar]
- Freitas-Silva, O.; Venâncio, A. Brazil nuts: Benefits and risks associated with contamination by fungi and mycotoxins. Food Res. Int. 2011, 44, 1434–1440. [Google Scholar] [CrossRef]
- Nugent, A.P.; Thielecke, F. Wholegrains and health: Many benefits but do contaminants pose any risk? Nutr. Bull. 2019, 44, 107–115. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Panel on Dietetic Products Nutrition and Allergies Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2016, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Abushelaibi, A.A.; Sofos, J.N.; Samelis, J.; Kendall, P.A. Survival and growth of Salmonella in reconstituted infant cereal hydrated with water, milk or apple juice and stored at 4 °C, 15 °C and 25 °C. Food Microbiol. 2003, 20, 17–25. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Enterobacter Sakazakii and Salmonella in Powdered Infant Formula: Meeting Report; WHO: Geneva, Switzerland, 2006; ISBN 9241563311. [Google Scholar]
- Lee, H.-Y.; Chai, L.-C.; Tang, S.-Y.; Jinap, S.; Ghazali, F.M.; Nakaguchi, Y.; Nishibuchi, M.; Son, R. Application of MPN-PCR in biosafety of Bacillus cereus s.l. for ready-to-eat cereals. Food Control 2009, 20, 1068–1071. [Google Scholar] [CrossRef]
- Young, I.; Waddell, L.; Cahill, S.; Kojima, M.; Clarke, R.; Rajic, A. Application of a Rapid Knowledge Synthesis and Transfer Approach To Assess the Microbial Safety of Low-Moisture Foods. J. Food Prot. 2015, 78, 2264–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirot, V.; Traore, T.; Guérin, T.; Noël, L.; Bachelot, M.; Cravedi, J.-P.; Mazur, A.; Glorennec, P.; Vasseur, P.; Jean, J.; et al. French infant total diet study: Exposure to selected trace elements and associated health risks. Food Chem. Toxicol. 2018, 120, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Cruz Munos, J.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marrero, N.M.; MacGregor, G.A. Salt and blood pressure in children and adolescents. J. Hum. Hypertens. 2008, 22, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Kranz, S.; Brauchla, M.; Slavin, J.L.; Miller, K.B. What Do We Know about Dietary Fiber Intake in Children and Health? The Effects of Fiber Intake on Constipation, Obesity, and Diabetes in Children. Adv. Nutr. 2012, 3, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; He, F.J.; Macgregor, G.A. High salt intake: Independent risk factor for obesity? Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef]
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, L.; Rossi, G.; Ippolito, R.; Cappuccio, F.P.; Strazzullo, P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin. Nutr. 2012, 31, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leirisalo-Repo, M.; Helenius, P.; Hannu, T.; Lehtinen, A.; Kreula, J.; Taavitsainen, M.; Koskimies, S. Long term prognosis of reactive salmonella arthritis. Ann. Rheum. Dis. 1997, 56, 516–520. [Google Scholar] [CrossRef] [Green Version]
- Ehling-Schulz, M.; Fricker, M.; Scherer, S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food Res. 2004, 48, 479–487. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [Green Version]
- Mahler, H.; Pasi, A.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Bär, W.; Krähenbühl, S. Fulminant Liver Failure in Association with the Emetic Toxin of Bacillus cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef]
| Food Components | Health Effects | Type of Analysis | |
|---|---|---|---|
| Nutrition | Total fiber | Type 2 Diabetes mellitus Cardiovascular diseases | Quantitative a |
| Sodium | Type 2 diabetes | Semi-quantitative b | |
| Free sugars | Cardiovascular disease Cancer (different organs) | ||
| Toxicology | Aflatoxins (AFB1) c | Hepatocellular carcinoma | Quantitative a |
| Microbiology | Bacillus cereus | Gastrointestinal disease | Quantitative a |
| Fiber (g/100 kcal) | Sodium (mg/100 kcal) | Free Sugar (g/100 kcal) | |
|---|---|---|---|
| Best BC | 3.9 | 1.4 | 0 |
| Worst IC | 0.2 | 51.4 | 4.2 |
| Dose–Response (Cases/100,000/year/ng/kg AFT bw/day) | Incidence 2 (Cases/100 k) (Mean, 95% CI) | Risk Ratios (Mean, 95% CI) | DALY Rate 2 (DALY/100 k) (Mean, 95% CI) | |
|---|---|---|---|---|
| Hepatocellular carcinoma (HCC) | 0.01 3 | NA | NA | 9.96 |
| (7.77–12.61) | ||||
| Cardiovascular disease (CVD) | NA | 25.07 | 0.78 1 | 60.78 |
| (21.36–29.12) | (0.68–0.90) | (49.67–73.44) | ||
| Diabetes mellitus 2 (DM2) | NA | 168.07 | 0.85 1 | 22.4 |
| (125.70–215.47) | (0.82–0.89) | (12.71–34.47) |
| Reference | 100% BC | 100% IC | Best BC | Worst IC | |
|---|---|---|---|---|---|
| BC (g/day) | 0.0 (0.0–20.8) | 13.4 (0.0–51.1) | - | 14.5 (0.0–55.5) | - |
| IC (g/day) | 8.7 (0.0–42.7) | - | 12.5 (0.0–50.1) | - | 13.7 (0.0–52.3) |
| Reference | 100% BC | 100% IC | Best BC | Worst IC | ||
|---|---|---|---|---|---|---|
| Fiber (g/day) | Mean | 9.3 | 9.5 | 9.2 | 11.3 | 8.9 |
| Median | 9.0 | 9.2 | 9.0 | 11.0 | 8.7 | |
| (P25–P75) | (7.2–11.1) | (7.3–11.4) | (7.1–11.0) | (8.8–13.5) | (6.8–10.7) | |
| Sodium (g/day) | Mean | 1.17 | 1.18 | 1.16 | 1.15 | 1.18 |
| Median | 1.15 | 1.17 | 1.14 | 1.13 | 1.16 | |
| (P25–P75) | (0.79–1.50) | (0.80–1.51) | (0.79–1.49) | (0.77–1.47) | (0.80–1.51) | |
| Free sugars (g/day) | Mean | 19.5 | 20.0 | 19.4 | 16.3 | 19.0 |
| Median | 17.8 | 18.4 | 17.7 | 14.2 | 17.4 | |
| (P25–P75) | (11.8–25.2) | (12.4–25.8) | (11.8–25.1) | (8.8–21.5) | (11.6–24.6) | |
| Aflatoxins (ng/kg bw/day) | Mean | 0.065 | 0.073 | 0.065 | 0.052 | 0.090 |
| Median | 0.049 | 0.054 | 0.047 | 0.038 | 0.066 | |
| (P25–P75) | (0.0–0.95) | (0.0–0.109) | (0.0–0.094) | (0.0–0.078) | (0.0–0.136) | |
| Bacillus cereus (log CFU/day) | 2.5 | 2.1 | 2.6 | <0 * | 3.3 |
| 100% BC | 100% IC | Best BC | Worst IC | |
|---|---|---|---|---|
| DM2 (fiber) | ||||
| Number of cases/100,000/year * (95% CI) | −0.714 | 0.211 | −6.618 | 1.272 |
| (−0.998; −0.482) | (0.143; 0.295) | (−9.229; −4.489) | (0.858; 1.782) | |
| CVD (fiber) | ||||
| Number of cases/100,000/year * (95% CI) | −0.160 | 0.047 | −1.469 | 0.288 |
| (−0.253; −0.072) | (0.021; 0.074) | (−2.288; −0.668) | (0.127; 0.458) | |
| HCC (AFB1) | ||||
| Number of cases/100 k/year ** (95% CI) | 0.00073 | 0.00065 | 0.00077 | 0.00090 |
| (0; 0.0027) | (0; 0.0023) | (0; 0.0028) | (0; 0.0033) | |
| Number of Gastrointestinal disease (Bacillus cereus) | 0 | 0 | 0 | 0 |
| 100% BC | 100% IC | Best BC | Worst IC | |
|---|---|---|---|---|
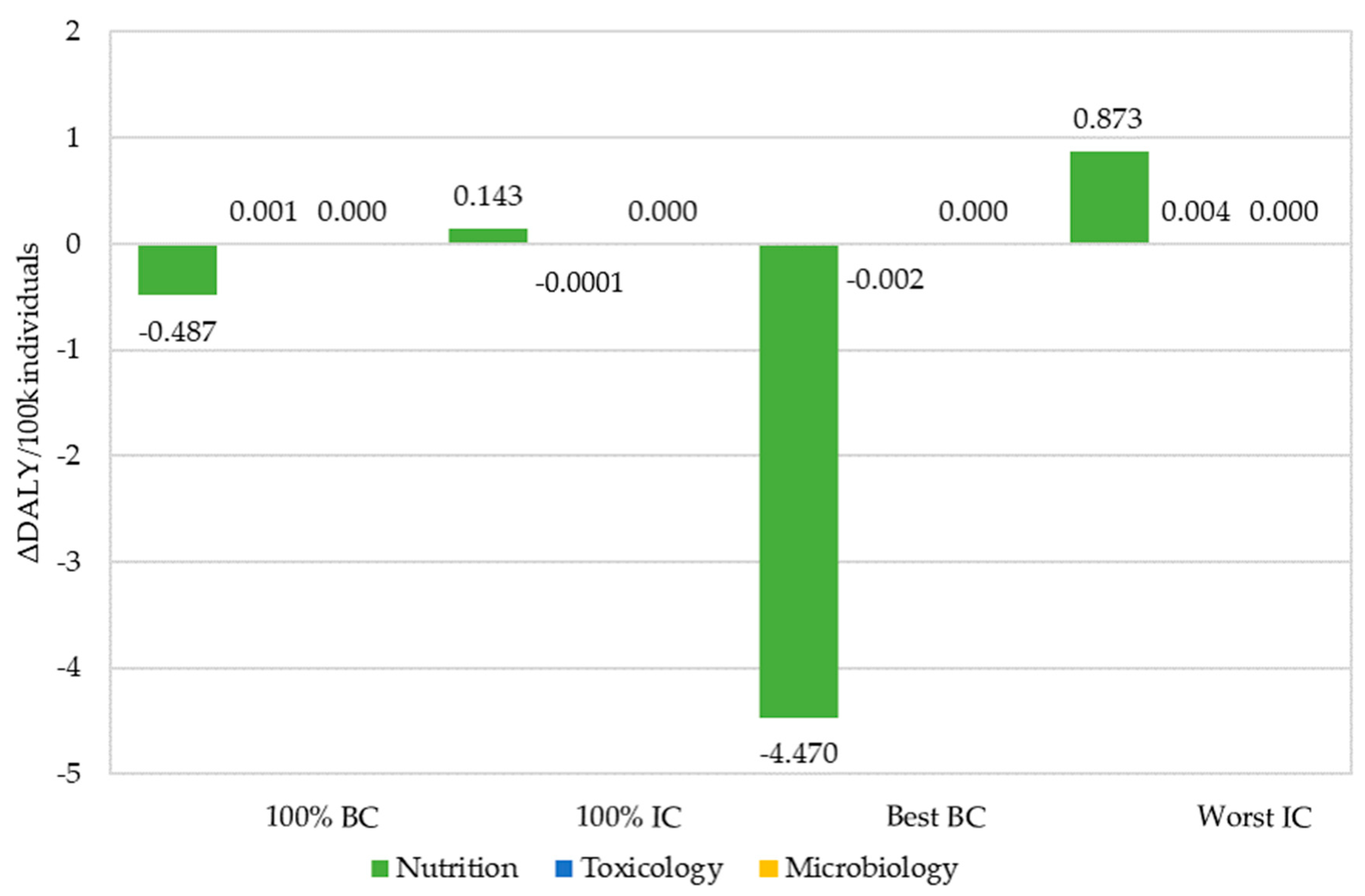
| Sum of ΔDALY (per 100 k individuals) (95% CI) | −0.486 | 0.143 | −4.473 | 0.877 |
| (−0.727;−0.262) | (0.078;0.213) | (−6.614;−2.449) | (0.471;1.317) |
| DRV | Reference | 100% BC | 100% IC | Best BC | Worst IC |
|---|---|---|---|---|---|
| Sodium (UL) | |||||
| 1500 mg/day | 24.8% | 25.7% | 24.3% | 23.4% | 25.4% |
| Free sugars (RI) | |||||
| 5% TEI | 80.0% | 81.9% | 79.8% | 69.7% | 79.0% |
| 10% TEI | 29.4% | 31.6% | 29.2% | 16.0% | 27.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, R.; Boué, G.; Alvito, P.; Brazão, R.; Carmona, P.; Carvalho, C.; Correia, D.; Fernandes, P.; Lopes, C.; Martins, C.; et al. Risk-Benefit Assessment of Cereal-Based Foods Consumed by Portuguese Children Aged 6 to 36 Months—A Case Study under the RiskBenefit4EU Project. Nutrients 2021, 13, 3127. https://doi.org/10.3390/nu13093127
Assunção R, Boué G, Alvito P, Brazão R, Carmona P, Carvalho C, Correia D, Fernandes P, Lopes C, Martins C, et al. Risk-Benefit Assessment of Cereal-Based Foods Consumed by Portuguese Children Aged 6 to 36 Months—A Case Study under the RiskBenefit4EU Project. Nutrients. 2021; 13(9):3127. https://doi.org/10.3390/nu13093127
Chicago/Turabian StyleAssunção, Ricardo, Géraldine Boué, Paula Alvito, Roberto Brazão, Paulo Carmona, Catarina Carvalho, Daniela Correia, Paulo Fernandes, Carla Lopes, Carla Martins, and et al. 2021. "Risk-Benefit Assessment of Cereal-Based Foods Consumed by Portuguese Children Aged 6 to 36 Months—A Case Study under the RiskBenefit4EU Project" Nutrients 13, no. 9: 3127. https://doi.org/10.3390/nu13093127
APA StyleAssunção, R., Boué, G., Alvito, P., Brazão, R., Carmona, P., Carvalho, C., Correia, D., Fernandes, P., Lopes, C., Martins, C., Membré, J.-M., Monteiro, S., Nabais, P., Thomsen, S. T., Torres, D., Pires, S. M., & Jakobsen, L. S. (2021). Risk-Benefit Assessment of Cereal-Based Foods Consumed by Portuguese Children Aged 6 to 36 Months—A Case Study under the RiskBenefit4EU Project. Nutrients, 13(9), 3127. https://doi.org/10.3390/nu13093127








