Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials
Abstract
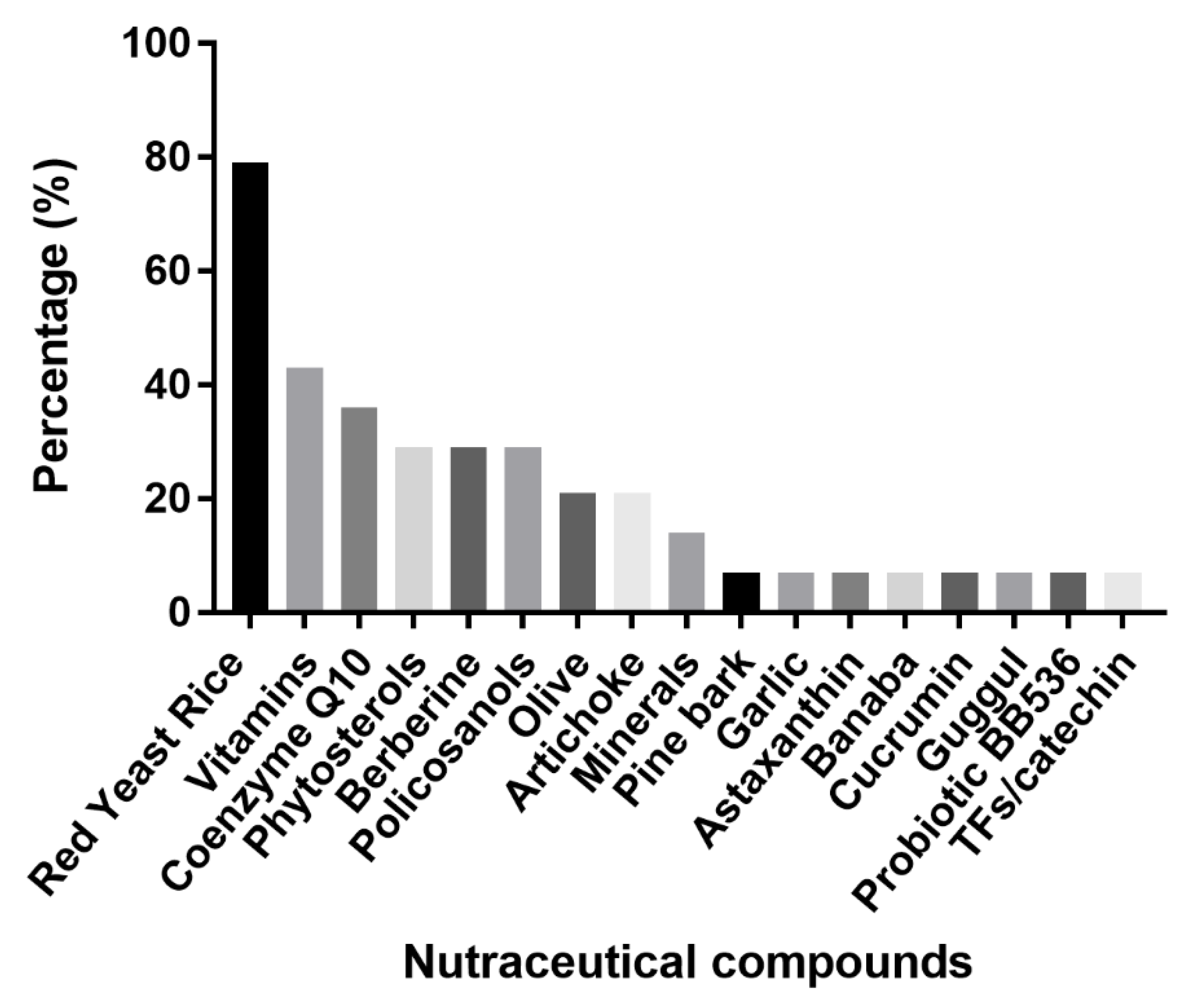
:1. Introduction
2. Methodology
3. Lipid-Lowering Molecular Mechanism of Action and Efficacy of Single Compound Present in the NC
4. Study Design in the Selected Clinical Trials
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [Green Version]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 75, 965–1005. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [Green Version]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.R.; Bernini, F.; Rivellese, A.A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. 2017, 27, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall′Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical approach for the management of cardiovascular risk—A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: Results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Domenech, M.; Casas, R.; Ruiz-León, A.M.; Sobrino, J.; Ros, E.; Estruch, R. Effects of a Novel Nutraceutical Combination (Aquilea Colesterol®) on the Lipid Profile and Inflammatory Biomarkers: A Randomized Control Trial. Nutrients 2019, 11, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.; Colletti, A.; Fogacci, F.; Bove, M.; Rosticci, M.; Borghi, C. Effects of a Combined Nutraceutical on Lipid Pattern, Glucose Metabolism and Inflammatory Parameters in Moderately Hypercholesterolemic Subjects: A Double-blind, Cross-over, Randomized Clinical Trial. High Blood Press. Cardiovasc. Prev. 2017, 24, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Veronesi, M.; Rizzo, M.; Giovannini, M.; Borghi, C. Short-Term Effects of a Combined Nutraceutical on Lipid Level, Fatty Liver Biomarkers, Hemodynamic Parameters, and Estimated Cardiovascular Disease Risk: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. Adv. Ther. 2017, 34, 1966–1975. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Rosticci, M.; D’Addato, S.; Grandi, E.; Borghi, C. Middle-Term Dietary Supplementation with Red Yeast Rice Plus Coenzyme Q10 Improves Lipid Pattern, Endothelial Reactivity and Arterial Stiffness in Moderately Hypercholesterolemic Subjects. Ann. Nutr. Metab. 2016, 68, 213–219. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 2018, 82, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Solà, R.; Valls, R.M.; Puzo, J.; Calabuig, J.R.; Brea, A.; Pedret, A.; Moriña, D.; Villar, J.; Millán, J.; Anguera, A. Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: A multicenter randomized trial. PLoS ONE 2014, 9, e101978. [Google Scholar] [CrossRef] [Green Version]
- Ogier, N.; Amiot, M.J.; Georgé, S.; Maillot, M.; Mallmann, C.; Maraninchi, M.; Morange, S.; Lescuyer, J.F.; Peltier, S.L.; Cardinault, N. LDL-cholesterol-lowering effect of a dietary supplement with plant extracts in subjects with moderate hypercholesterolemia. Eur. J. Nutr. 2013, 52, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Du, Y.; Meynen, E.; Yan, X.; Wen, Y.; Wang, H.; Molhuizen, H.O. Purified black tea theaflavins and theaflavins/catechin supplements did not affect serum lipids in healthy individuals with mildly to moderately elevated cholesterol concentrations. Eur. J. Nutr. 2010, 49, 27–35. [Google Scholar] [CrossRef] [PubMed]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; Di Loreto, G.; Comandini, A.; Maffioli, P.; Derosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q10 on lipid levels: A randomized, double-blind, placebo controlled study. Drug Des. Dev. Ther. 2017, 11, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; D′Addato, S.; Borghi, C. A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients 2020, 12, 3127. [Google Scholar] [CrossRef]
- Iskandar, I.; Harahap, Y.; Wijayanti, T.R.; Sandra, M.; Prasaja, B.; Cahyaningsih, P. Efficacy and tolerability of a nutraceutical combination of red yeast rice, guggulipid, and chromium picolinate evaluated in a randomized, placebo-controlled, double-blind study. Complement. Ther. Med. 2020, 48, 102282. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Veronesi, M.; Borghi, C. Short-Term Effects of Dry Extracts of Artichokeand Berberis in Hypercholesterolemic Patients Without Cardiovascular Disease. Am. J. Cardiol. 2019, 123, 588–591. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist DeBakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniades, C.; Antonopoulos, A.S.; Tousoulis, D.; Marinou, K.; Stefanadis, C. Homocysteine and coronary atherosclerosis: From folate fortification to the recent clinical trials. Eur. Heart J. 2009, 30, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.S.; Huang, Y.; Lu, Z.Y.; Jian, W.; Blair, I.A.; Whitehead, A.S. Mild folate deficiency induces a proatherosclerotic phenotype in endothelial cells. Atherosclerosis 2006, 189, 133–141. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin. Epigenet. 2015, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Kamanna, V.S.; Kashyap, M.L. Mechanism of action of niacin. Am. J. Cardiol. 2008, 101, 20B–26B. [Google Scholar] [CrossRef]
- Sorci, L.; Kurnasov, O.; Rodionov, D.; Osterman, A. Genomics and Enzymology of NAD Biosynthesis. Compr. Nat. Prod. II Chem. Biol. 2010, 7, 213–257. [Google Scholar] [CrossRef]
- Zeb Shah, T.; Ali, A.B.; Ahmad Jafri, S.; Qazi, M.H. Effect of Nicotinic Acid (Vitamin B3 or Niacin) on the lipid profile of diabetic and non–diabetic rats. Pak. J. Med. Sci. 2013, 29, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Gao, W.; Wang, Y.; Yao, Z.; Xu, Q.; Guo, C.; Li, B. Riboflavin deficiency affects lipid metabolism partly by reducing apolipoprotein B100 synthesis in rats. J. Nutr. Biochem. 2019, 70, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Ma, A.; Li, Y.; Han, X.; Liang, H. Effects of vitamin E on plasma lipid status and oxidative stress in Chinese women with metabolic syndrome. Int. J. Vitam. Nutr. Res. 2010, 80, 178–187. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Satapathy, S.; Bandyopadhyay, D.; Patro, B.K.; Khan, S.; Naik, S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial. Complement. Ther. Med. 2020, 53, 102526. [Google Scholar] [CrossRef]
- El Borolossy, R.; El Wakeel, L.M.; El Hakim, I.; Sabri, N. Efficacy and safety of nicotinamide in the management of hyperphosphatemia in pediatric patients on regular hemodialysis. Pediatr. Nephrol. 2016, 31, 289–296. [Google Scholar] [CrossRef]
- Ajuluchukwu, J.N.; Okubadejo, N.U.; Mabayoje, M.; Ojini, F.I.; Okwudiafor, R.N.; Mbakwem, A.C.; Fasanmade, O.A.; Oke, D.A. Comparative study of the effect of tocotrienols and -tocopherol on fasting serum lipid profiles in patients with mild hypercholesterolaemia: A preliminary report. Niger. Postgrad. Med. J. 2007, 14, 30–33. [Google Scholar]
- Rasool, A.H.; Rahman, A.R.; Yuen, K.H.; Wong, A.R. Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch. Pharm. Res. 2008, 31, 1212–1217. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical applications of coenzyme Q10. Front. Biosci. (Landmark Ed.) 2014, 19, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kaur, H.; Devi, P.; Mohan, V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol. Ther. 2009, 124, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Orlando, P.; Galeazzi, R.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.V.; Giuliani, A.; Bonfigli, A.R.; Mazzanti, L.; et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients 2020, 12, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorat, M.V.; Tabrizi, R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Mottaghi, R.; Asemi, Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusq, J.M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of lipid synthesis through activation of AMP kinase: An additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lim, H.J.; Park, J.H.; Lee, K.S.; Jang, Y.; Park, H.Y. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem. Biophys. Res. Commun. 2007, 362, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, Y.; Zhao, L.; Lu, F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013, 79, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Li, L.; Porter, T.D. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther. 2006, 318, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, R.; Amor, A.M.; Rodeiro, I.; González, R.M.; González, P.C.; Alfonso, J.L.; Más, R. Policosanol modulates HMG-CoA reductase activity in cultured fibroblasts. Arch. Med. Res. 2001, 32, 8–12. [Google Scholar] [CrossRef]
- Berthold, H.K.; Unverdorben, S.; Degenhardt, R.; Bulitta, M.; Gouni-Berthold, I. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: A randomized controlled trial. JAMA 2006, 295, 2262–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backes, J.M.; Gibson, C.A.; Ruisinger, J.F.; Moriarty, P.M. Modified-policosanol does not reduce plasma lipoproteins in hyperlipidemic patients when used alone or in combination with statin therapy. Lipids 2011, 46, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, J.; Fernandez-Travieso, J.C.; Illnait-Ferrer, J.; Fernandez-Dorta, L.; Mendoza-Castano, S.; Mas-Ferreiro, R.; Mesa-Angarica, M.; Reyes-Suarez, P. Effects of policosanol in the functional recovery of non-cardioembolic ischemic stroke hypertensive patients. Rev. Neurol. 2018, 67, 331–338. [Google Scholar]
- Farhangi, M.A.; Ostadrahimi, A.; Mahboob, S. Serum calcium, magnesium, phosphorous and lipid profile in healthy Iranian premenopausal women. Biochem. Med. (Zagreb) 2011, 21, 312–320. [Google Scholar] [CrossRef] [PubMed]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Calandra, S.; Tarugi, P.; Speedy, H.E.; Dean, A.F.; Bertolini, S.; Shoulders, C.C. Mechanisms and genetic determinants regulating sterol absorption, circulating LDL levels, and sterol elimination: Implications for classification and disease risk. J. Lipid Res. 2011, 52, 1885–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talati, R.; Sobieraj, D.M.; Makanji, S.S.; Phung, O.J.; Coleman, C.I. The comparative efficacy of plant sterols and stanols on serum lipids: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2010, 110, 719–726. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- Rietjens, S.J.; Bast, A.; Haenen, G.R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Inoue, J.; Choi, J.M.; Nakamura, S.; Yan, Z.; Fushinobu, S.; Kamada, H.; Kato, H.; Hashidume, T.; Shimizu, M.; et al. Identification of the Flavonoid Luteolin as a Repressor of the Transcription Factor Hepatocyte Nuclear Factor 4α. J. Biol. Chem. 2015, 290, 24021–24035. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Lin, S.M.; Leung, L.K. The Flavone Luteolin Suppresses SREBP-2 Expression and Post-Translational Activation in Hepatic Cells. PLoS ONE 2015, 10, e0135637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Pirro, M.; Banach, M.; Mikhailidis, D.P.; Atkin, S.L.; Cicero, A.F.G. Lipid-lowering activity of artichoke extracts: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2549–2556. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.Y. S-alk(en)yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 2002, 132, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.K.; Sharma, M.; Tiwari, M. Inhibitory effect of novel diallyldisulfide analogs on HMG-CoA reductase expression in hypercholesterolemic rats: CREB as a potential upstream target. Life Sci. 2009, 85, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Andrianova, I.V.; Demidova, O.N.; Gorchakova, T.; Orekhov, A.N. Lipid-lowering effects of time-released garlic powder tablets in double-blinded placebo-controlled randomized study. J. Atheroscler. Thromb. 2008, 15, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Ursoniu, S.; Sahebkar, A.; Serban, M.C.; Banach, M. Lipid profile and glucose changes after supplementation with astaxanthin: A systematic review and meta-analysis of randomized controlled trials. Arch. Med. Sci. 2015, 11, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and Anti-obesity Activity of Lagerstroemia speciosa. Evid.-Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Miller, H.; Kaats, G.R. A review of the efficacy and safety of banaba (Lagerstroemia speciosa L.) and corosolic acid. Phytother. Res. 2012, 26, 317–324. [Google Scholar] [CrossRef]
- Saumya, S.M.; Basha, P.M. Antioxidant effect of Lagerstroemia speciosa Pers (banaba) leaf extract in streptozotocin-induced diabetic mice. Indian J. Exp. Biol. 2011, 49, 125–131. [Google Scholar] [PubMed]
- Fukushima, M.; Matsuyama, F.; Ueda, N.; Egawa, K.; Takemoto, J.; Kajimoto, Y.; Yonaha, N.; Miura, T.; Kaneko, T.; Nishi, Y.; et al. Effect of corosolic acid on postchallenge plasma glucose levels. Diabetes Res. Clin. Pract. 2006, 73, 174–177. [Google Scholar] [CrossRef]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Prakash, U.N.; Srinivasan, K. Fat digestion and absorption in spice-pretreated rats. J. Sci. Food Agric. 2012, 92, 503–510. [Google Scholar] [CrossRef]
- Sahebkar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef]
- Yang, Y.S.; Su, Y.F.; Yang, H.W.; Lee, Y.H.; Chou, J.I.; Ueng, K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef]
- Cui, J.; Huang, L.; Zhao, A.; Lew, J.L.; Yu, J.; Sahoo, S.; Meinke, P.T.; Royo, I.; Pelaez, F.; Wright, S.D. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 2003, 278, 10214–10220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szapary, P.O.; Wolfe, M.L.; Bloedon, L.T.; Cucchiara, A.J.; DerMarderosian, A.H.; Cirigliano, M.D.; Rader, D.J. Guggulipid for the treatment of hypercholesterolemia: A randomized controlled trial. JAMA 2003, 290, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohr, L.A.; Rasmussen, L.B.; Straand, J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement. Ther. Med. 2009, 17, 16–22. [Google Scholar] [CrossRef]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.O.; Mochizuki, M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Andrade, S.; Borges, N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J. Dairy Res. 2009, 76, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; O′Sullivan, D.J. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeer, M.A.; Mulder, T.P.; Molhuizen, H.O. Theaflavins from black tea, especially theaflavin-3-gallate, reduce the incorporation of cholesterol into mixed micelles. J. Agric. Food Chem. 2008, 56, 12031–12036. [Google Scholar] [CrossRef]
- Erba, D.; Riso, P.; Bordoni, A.; Foti, P.; Biagi, P.L.; Testolin, G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005, 16, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Lu, G.P.; Cai, N.S.; Wu, Z.G.; Li, Y.H.; Chen, H.; Zhu, J.Q.; Jin, X.J.; Wouters, B.C.; Zhao, J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Vega-López, S.; Kaul, N.; Schönlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.M.; Llauradó, E.; Fernández-Castillejo, S.; Puiggrós, F.; Solà, R.; Arola, L.; Pedret, A. Effects of low molecular weight procyanidin rich extract from french maritime pine bark on cardiovascular disease risk factors in stage-1 hypertensive subjects: Randomized, double-blind, crossover, placebo-controlled intervention trial. Phytomedicine 2016, 23, 1451–1461. [Google Scholar] [CrossRef]
- Bonfigli, A.R.; Protic, O.; Olivieri, F.; Montesanto, A.; Malatesta, G.; Di Pillo, R.; Antonicelli, R. Effects of a novel nutraceutical combination (BruMeChol™) in subjects with mild hypercholesterolemia: Study protocol of a randomized, double-blind, controlled trial. Trials 2020, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Nair, B. Clinical Trial Designs. Indian Dermatol. Online J. 2019, 10, 193–201. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and Authenticity Control of Functional Red Yeast Rice—A Review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef] [Green Version]
- Heinz, T.; Schuchardt, J.P.; Möller, K.; Hadji, P.; Hahn, A. Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention. Nutr. Res. 2016, 36, 1162–1170. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Li, T.C.; Lai, M.M. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur. J. Endocrinol. 2005, 153, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Farkouh, A.; Baumgärtel, C. Mini-review: Medication safety of red yeast rice products. Int. J. Gen. Med. 2019, 12, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Philibert, C.; Bres, V.; Jean-Pastor, M.J.; Guy, C.; Lebrun-Vignes, B.; Robin, P.; Pinzani, V.; Hillaire-Buys, D. Red yeast-rice-induced muscular injuries: Analysis of French pharmacovigilance database and literature review. Therapie 2016. [Google Scholar] [CrossRef]
- Ong, Y.C.; Aziz, Z. Systematic review of red yeast rice compared with simvastatin in dyslipidaemia. J. Clin. Pharm. Ther. 2016, 41, 170–179. [Google Scholar] [CrossRef] [PubMed]
| Refs | Natural Active Components | Type of RCT | Study Intervention Period (Weeks) | Total Subjects (n) | Male (n) | Female (n) | Age Range (Years) | LDL-Cholesterol (LDL-C) | Total Cholesterol (TC) | Adverse Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Group | Placebo Group | Difference of Changes between Arms | Intervention Group | Placebo Group | Difference of Changes between Arms | |||||||||||||
| Baseline vs. Final Mean LDL-C ± SD, (mg/dL) | LDL-C Reduction | Baseline vs.Final Mean LDL-C ± SD (mg/dL) | LDL-C Reduction | p Value | Baseline vs. Final Mean TC ± SD (mg/dL) | TC Reduction | Baseline vs. Final Mean TC ± SD (mg/dL) | TC Reduction | p Value | |||||||||
| [10] | Bifidobacterium longum BB536 (1 bn UFC) Coenzyme Q10 (20 mg) Niacin (16 mg) RYR extract (10 mg monacolin K) | parallel | 12 | 33 | 16 | 17 | 18–70 | 177 (167, 193) 1 vs. 136.5 (118,151.5) 1 | −25.7% | 189 (174, 198) 1 vs. 183 (171, 202) 1 | NA | <0.0001 | 271 (239, 285) 1 vs. 208 (201, 263) 1 | −16.7% | 271 (256, 289) 1 vs. 267 (259, 293) | NA | <0.0001 | No |
| [11] | Phytosterols 1.5 g Vitamin E (12 mg) RYR extract (10 mg monacolin K) Olive leaf extract (5 mg hydroxytyrosol) | parallel | 12 | 40 | 13 | 27 | 35–75 | 150.1 (142, 159.8) 1 vs. 120 (112.7, 129.3) 1 | −19.7% | 143.2 (133.9, 152.1) 1 vs. 138.6 (130.5, 145.9) 1 | −6.7% | <0.001 | 234.3 (225.1, 244.0) 1 vs. 201.5 (215.4, 234.7) 1 | −13.9% | 225.1 (215.4, 234.7) 1 vs. 218.5 (207.7, 229.3) 1 | −3.0% | <0.001 | No |
| [12] | Artichoke extract (500 mg) Banaba extract (75 mg) Coenzyme Q10 (50 mg) RYR 200 (10 mg monacolin K) Vitamin B3 (9 mg) Vitamin B6 (1.4 mg) Vitamin B12 (0.83 mcg) Folic acid (110 mcg) | crossover | 6-2-6 | 30 | NA | NA | 25–75 | NA | −30.3 mg/dL | NA | −8.4 mg/dL | <0.05 | NA | −34.1 mg/dL | NA | −10.1 mg/dL | <0.05 | No |
| [13] | Phytosterols (400 mg) RYR extract (5 mg monacolin K) L-tyrosol (2.5 mg) | parallel | 8 | 50 | 30 | 20 | 35–69 | 162.3 ± 21.9 vs. NA | −38.0 mg/dL | 151.7 ± 24.3 vs. NA | −20 mg/dL | <0.05 | 234.5 ± 24.7 vs. NA | −38.2 mg/dL | 225.1 ± 25.3 vs. NA | −22.2 mg/dL | <0.05 | No |
| [14] | Coenzyme Q10 (30 mg) RYR extract (10 mg monacolin K) | parallel | 24 | 40 | 18 | 22 | 18–70 | 158.5 ± 15.4 vs. 116.8 ± 9.9 | −26.3% | 161.6 ± 13.8 vs. 167.1 ± 14.2 | +3.4% | <0.05 | 229.1 ± 14.1 vs. 180.7 ± 15.3 | −21.1% | 233.4 ± 12.8 vs. 238.4 ± 14.6 | +2.1% | <0.05 | No |
| [15] | Berberine (500 mg) RYR extract (10 mg monacolin K) Policosanols (10 mg) | parallel | 6 | 50 | 26 | 24 | 18–70 | 175.6 ± 25.1 vs. NA | −40.9 mg/dL | 170.6 ± 22.0 vs. NA | −1.5 mg/dL | <0.001 | 254.8 ± 28.9 vs. NA | −44.0 mg/dL | 250.9 ± 30.8 vs. NA | −1.16 mg/dL | <0.001 | No |
| [16] | Phytosterols (2 g) Curcumin (200 mg) | 2 × 2 fractional | 4 | 37 2 | NA | NA | 18–70 | 166.8 ± 5.8 3 vs. 142.4 ± 6.2 3 | −14.42% | 175.6 ± 6.9 3 vs. 172.9 ± 7.3 3 | −0.89% | 0.006 | 251.3 ± 7.3 3 vs. 222.3 ± 6.2 3 | −11.01% | 255.9 ± 6.9 3 vs. 252.9 ± 8.1 3 | −1.23% | 0.005 | No |
| [17] | Berberine (500 mg) RYR 200 mg (10 mg monacolin K) Policosanol (10 mg) Coenzyme Q10 (2 mg) Astaxanthin (0.5 mg) Folic acid (0.2 mg) | parallel | 12 | 104 | 32 | 72 | ˃18 | 155.7 ± 14.6 vs. 135.3 ± 28.7 | −14.93% | 159.3 ± 15.6 vs. 147.5 ± 26.0 | −8.02% | 0.029 | 243.6 ± 24.3 vs. 221.8 ± 34.4 | −10.46% | 243.4 ± 19.5 vs. 232.8 ± 28.8 | −5.5% | 0.01 | No |
| [18] | Artichoke leaf dry extract (200 mg) RYR 166.67 mg (0.4% monacolin K) Dicalcium phosphate (199 mg) Microcrystalline cellulose (87.36 mg) Calcium citrate (63.22 mg) Tricalcium phosphate (34 mg) Magnesium stearate (22 mg) Vitamin E (12.86 mg) Garlic dry extract (10 mg) Pine bark extract (6.67 mg) Sugar cane extract (3.70 mg) Vitamin B2 (1.60 mg) Vitamin B3 (2.92 mg) | parallel | 16 | 39 | 11 | 28 | 21–55 | 170.0 ± 20.0 vs. NA | −19.1% p < 0.001 vs. baseline | 170.0 ± 30.0 vs. NA | +2.8% p = 0.37 vs. baseline | NA | 250.0 ± 30.0 vs. NA | −14.1% p < 0.001 vs. baseline | 250.0 ± 30.0 vs. NA | +1.3 p = 0.53 vs. baseline | NA | No |
| [19] | Catechins (149.4 mg) Purified black tea theaflavins (75 mg) | parallel | 11 | 66 2 | 44 | 22 | 18–65 | 150.5 ± 25.8 vs. 151.3 ± 27.4 | NA | 154.0 ± 18.9 vs. 151.3 ± 17.8 | NA | 0.106 | 223.1 ± 32.0 vs. 221.6 ± 32.8 | NA | 217.3 ± 21.6 vs. 215.8 ± 21.62 | NA | 0.118 | Yes |
| [20] | Berberine (500 mg) RYR (10 mg monacolin K) Hydroxytyrosol (5 mg) Coenzyme Q10 (2 mg) | parallel | 4 | 106 2 | NA | NA | 18–75 | 147.5 ± 16.3 vs. 120.4 ± 18.8 | −39.1 mg/dL | 143.6 ± 15.0 vs. 149.3 ± 19.5 | +5.7 mg/dL | <0.001 | 234.6 ± 18.0 vs. 204.9 ± 22.2 | −45.9 mg/dL | 235.6 ± 17.9 vs. 238.0 ± 21.5 | +2.4 mg/dL | <0.001 | Yes |
| [21] | Phytosterols (800 mg) Niacin (27 mg) Policosanols (10 mg) RYR extract (5 mg monacolin K) | parallel | 8 | 88 | 38 | 47 | 30–75 | 155.1 ± 19.9 vs. 122.6 ± 24.7 | −32.5 mg/dL | 161.5 ± 21.3 vs. 164.0 ± 20.4 | +2.5 mg/dL | <0.01 | 229.1 ± 27.9 vs. 197.1 ± 28.3 | −33.0 mg/dL | 232.6 ± 21.6 vs. 239.8 ± 23.6 | +8.2 mg/dL | <0.01 | No |
| [22] | RYR extract 375 mg (3.75 mg monacolin K) Guggul extract (110 mg) Chromium picolinate (50 μg) | parallel | 8 | 80 | 38 | 42 | 18–65 | 137.6 ± 20.2 vs. 115.5 ± 22.2 | −16.1% | 134.4 ± 20.5 vs. 145.1 ± 23.7 | +8.0% | <0.05 | 205.0 ± 17.6 vs. 173.5 ± 21.7 | −15.4% | 200.8 ± 22.9 vs. 204.5 ± 22.8 | +1.8% | <0.05 | Yes |
| [23] | Artichoke leaf extract 4 Berberine 4 | parallel | 8 | 40 | NA | NA | ˃18 | 156.7 ± 10.9 vs. 131.9 ± 9.1 | −16% | 157.4 ± 9.8 vs. 136.7 ± 10.8 | NA | <0.01 | 247.1 ± 11.7 vs. 199.9 ± 10.2 | −19.0% | 245.8 ± 13.6 vs. 223.6 ± 11.9 | NA | <0.01 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protic, O.; Bonfigli, A.R.; Antonicelli, R. Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials. Nutrients 2021, 13, 3128. https://doi.org/10.3390/nu13093128
Protic O, Bonfigli AR, Antonicelli R. Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials. Nutrients. 2021; 13(9):3128. https://doi.org/10.3390/nu13093128
Chicago/Turabian StyleProtic, Olga, Anna Rita Bonfigli, and Roberto Antonicelli. 2021. "Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials" Nutrients 13, no. 9: 3128. https://doi.org/10.3390/nu13093128
APA StyleProtic, O., Bonfigli, A. R., & Antonicelli, R. (2021). Nutraceutical Combinations in Hypercholesterolemia: Evidence from Randomized, Placebo-Controlled Clinical Trials. Nutrients, 13(9), 3128. https://doi.org/10.3390/nu13093128






