Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Sampling and Placental Tissue Collection
2.3. Analysis of Maternal and Umbilical Venous Blood Nutrient Contents
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Histopathological, Immunohistochemistry and TUNEL Assessment of CON and ICSI Placentae
2.6. Statistical Analysis
3. Results
3.1. Clinical Data
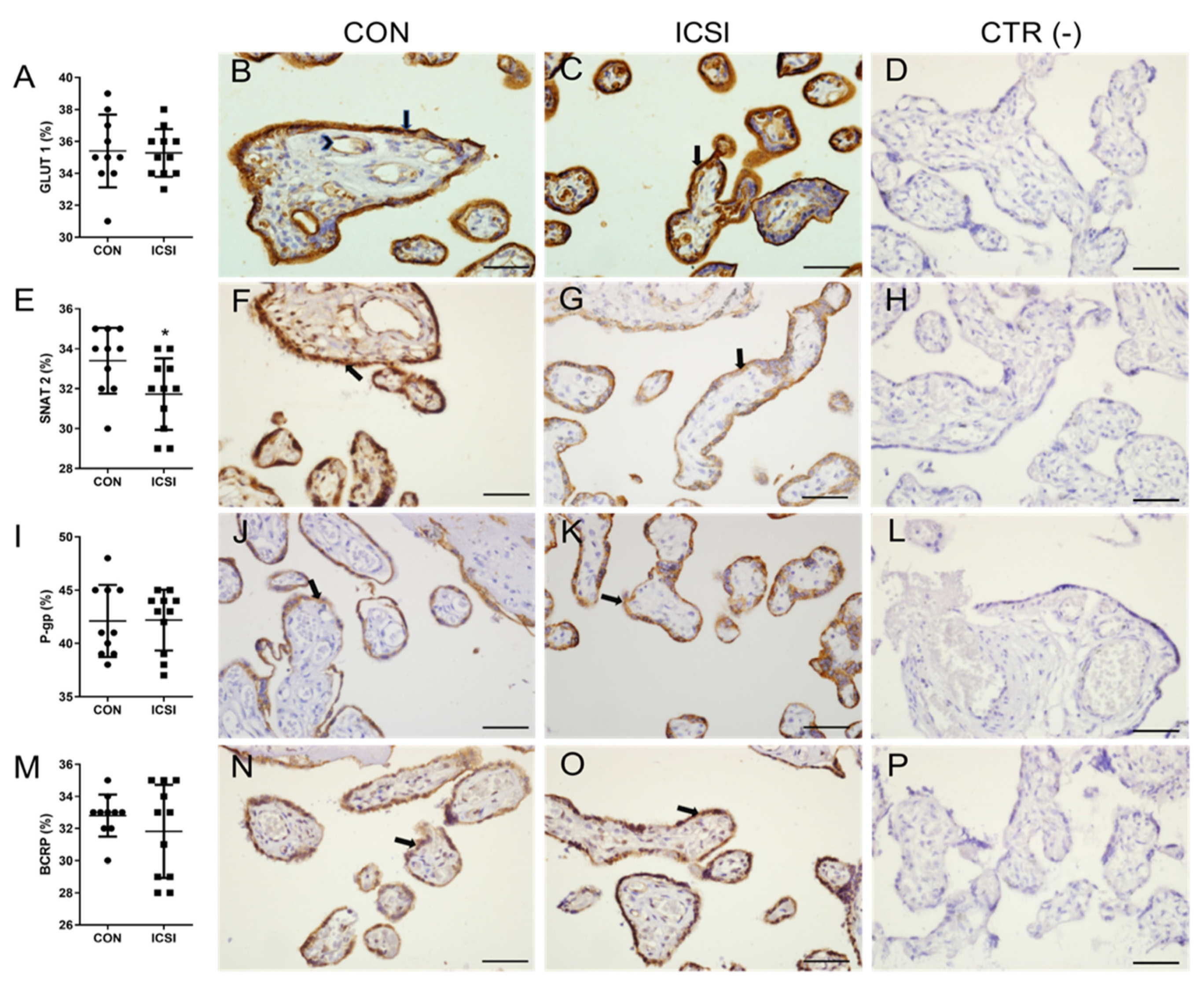
3.2. ICSI Impairs Placental SNAT 2 Immunostaining
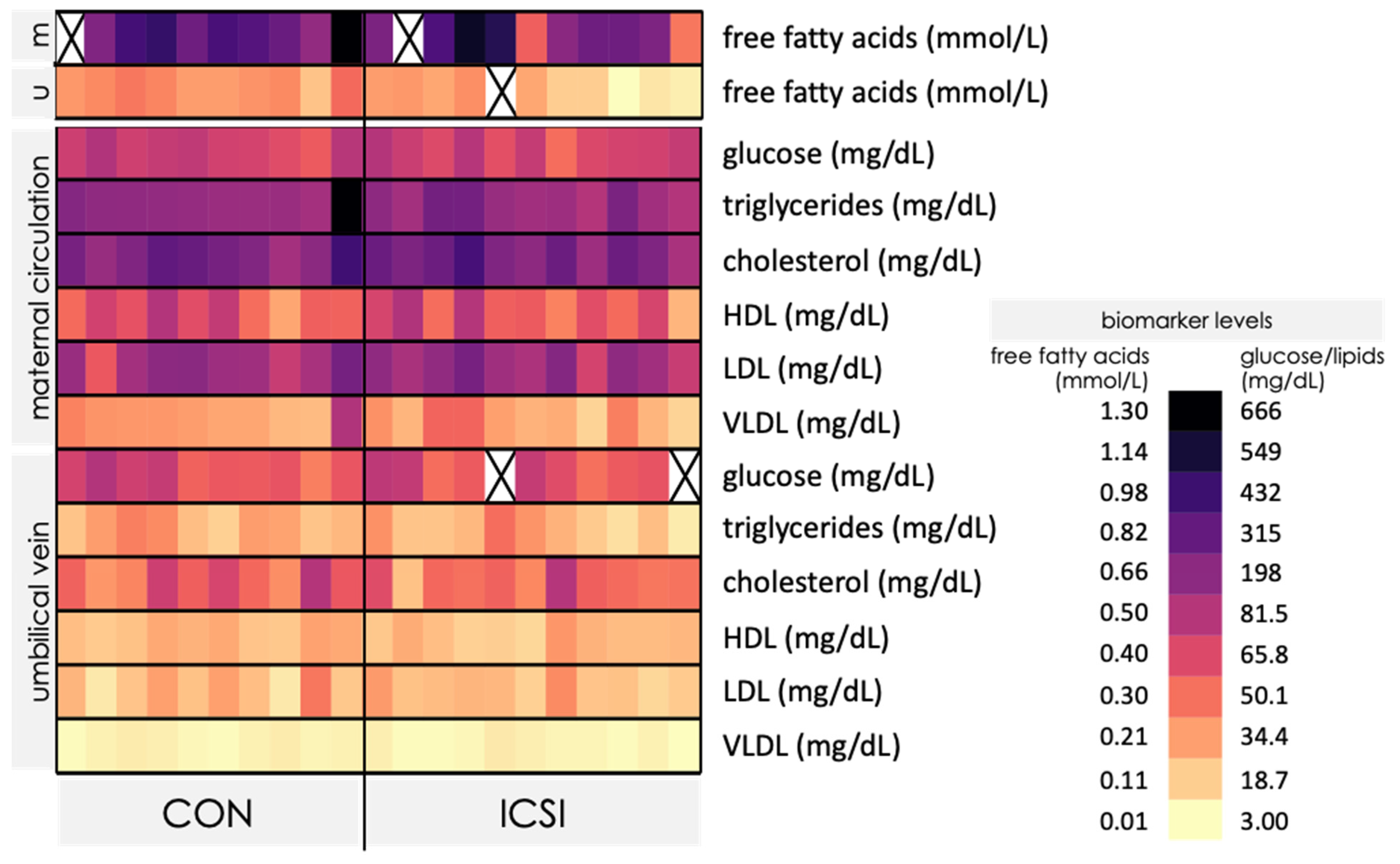
3.3. Venous umbilical Cord Blood from ICSI Term Pregnancies Exhibit Specific Changes in Nutrient Levels
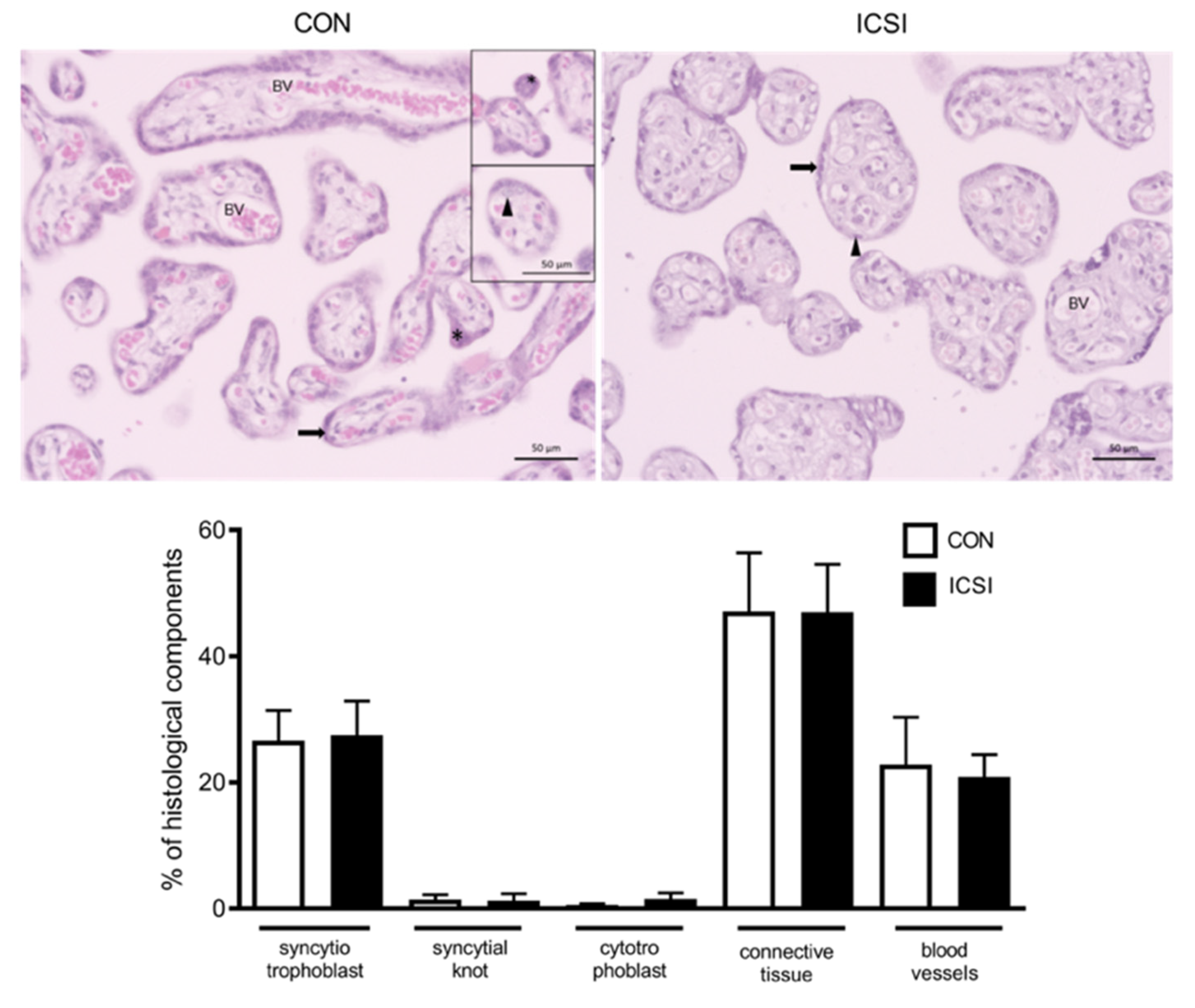
3.4. Placental Proliferation and Apoptosis Are Increased in the Human ICSI Placenta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef]
- Bloise, E.; Feuer, S.K.; Rinaudo, P.F. Comparative intrauterine development and placental function of ART concepti: Implications for human reproductive medicine and animal breeding. Hum. Reprod. Update 2014, 20, 822–839. [Google Scholar] [CrossRef]
- Vannuccini, S.; Ferrata, C.; Perelli, F.; Pinzauti, S.; Severi, F.M.; Reis, F.M.; Petraglia, F.; Di Tommaso, M. Peripartum and postpartum outcomes in uncomplicated term pregnancy following ART: A retrospective cohort study from two Italian obstetric units. Hum. Reprod. Open 2018, 2018, hoy012. [Google Scholar] [CrossRef]
- Feuer, S.K.; Camarano, L.; Rinaudo, P.F. ART and health: Clinical outcomes and insights on molecular mechanisms from rodent studies. Mol. Hum. Reprod. 2013, 19, 189–204. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Jiang, Y.; Zhang, R.; Wang, J.; Li, C.; Zhao, H.; Gao, L.; Cui, Y.; Zhou, Z.; et al. Ultrastructural Study on Human Placentae from Women Subjected to Assisted Reproductive Technology Treatments. Biol. Reprod. 2011, 85, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Sibley, C.P. Placental nutrient supply and fetal growth. Int. J. Dev. Biol. 2010, 54, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Lin, W.; Liu, X.; Simbulan, R.; Kolahi, K.S.; Petraglia, F.; Maltepe, E.; Donjacour, A.; Rinaudo, P. Impaired Placental Nutrient Transport in Mice Generated by in Vitro Fertilization. Endocrinology 2012, 153, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Delle Piane, L.; Lin, W.; Liu, X.; Donjacour, A.; Minasi, P.; Revelli, A.; Maltepe, E.; Rinaudo, P.F. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum. Reprod. 2010, 25, 2039–2046. [Google Scholar] [CrossRef]
- Haavaldsen, C.; Tanbo, T.; Eskild, A. Placental weight in singleton pregnancies with and without assisted reproductive technology: A population study of 536 567 pregnancies. Hum. Reprod. 2012, 27, 576–582. [Google Scholar] [CrossRef]
- Chen, S.; Sun, F.; Huang, X.; Wang, X.; Tang, N.; Zhu, B.; Li, B. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci. Rep. 2015, 5, 10596. [Google Scholar] [CrossRef]
- Risnes, K.R.; Romundstad, P.R.; Nilsen, T.I.L.; Eskild, A.; Vatten, L.J. Placental Weight Relative to Birth Weight and Long-term Cardiovascular Mortality: Findings From a Cohort of 31,307 Men and Women. Am. J. Epidemiol. 2009, 170, 622–631. [Google Scholar] [CrossRef]
- Sibley, C.P.; Turner, M.A.; Cetin, I.; Ayuk, P.; Boyd, C.A.R.; D’Souza, S.W.; Glazier, J.D.; Greenwood, S.L.; Jansson, T.; Powell, T. Placental Phenotypes of Intrauterine Growth. Pediatr. Res. 2005, 58, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Schmon, B.; Hartmann, M.; Jones, C.J.; Desoye, G. Insulin and Glucose Do not Affect the Glycogen Content in Isolated and Cultured Trophoblast Cells of Human Term Placenta. J. Clin. Endocrinol. Metab. 1991, 73, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Shafrir, E. Placental metabolism and its regulation in health and diabetes. Mol. Aspects Med. 1994, 15, 505–682. [Google Scholar] [CrossRef]
- Lager, S.; Powell, T.L. Regulation of Nutrient Transport across the Placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. SLC38 Family of Transporters for Neutral Amino Acids. In Handbook of Neurochemistry and Molecular Neurobiology; Lajtha, A., Reith, M.E.A., Eds.; Springer: Boston, MA, USA, 2007; pp. 327–338. ISBN 978-0-387-30347-5. [Google Scholar] [CrossRef]
- Coan, P.M.; Angiolini, E.; Sandovici, I.; Burton, G.J.; Constância, M.; Fowden, A.L. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice: Adaptations in placental nutrient transfer capacity. J. Physiol. 2008, 586, 4567–4576. [Google Scholar] [CrossRef]
- Bloise, E.; Bhuiyan, M.; Audette, M.C.; Petropoulos, S.; Javam, M.; Gibb, W.; Matthews, S.G. Prenatal Endotoxemia and Placental Drug Transport in The Mouse: Placental Size-Specific Effects. PLoS ONE 2013, 8, e65728. [Google Scholar] [CrossRef]
- Bloise, E.; Ortiga-Carvalho, T.M.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G. ATP-binding cassette transporters in reproduction: A new frontier. Hum. Reprod. Update 2016, 22, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Imperio, G.E.; Javam, M.; Lye, P.; Constantinof, A.; Dunk, C.E.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G.; Ortiga-Carvalho, T.M.; et al. Gestational age-dependent gene expression profiling of ATP-binding cassette transporters in the healthy human placenta. J. Cell. Mol. Med. 2019, 23, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, L.M.; Fontes, K.N.; Reginatto, M.W.; Andrade, C.B.V.; Monteiro, V.R.S.; Gomes, H.R.; Silva-Filho, J.L.; Pinheiro, A.A.S.; Vago, A.R.; Almeida, F.R.C.L.; et al. Malaria in pregnancy regulates P-glycoprotein (P-gp/Abcb1a) and ABCA1 efflux transporters in the Mouse Visceral Yolk Sac. J. Cell. Mol. Med. 2020, 24, 10636–10647. [Google Scholar] [CrossRef]
- Martinelli, L.M.; Reginatto, M.W.; Fontes, K.N.; Andrade, C.B.V.; Monteiro, V.R.S.; Gomes, H.R.; Almeida, F.R.C.L.; Bloise, F.F.; Matthews, S.G.; Ortiga-Carvalho, T.M.; et al. Breast cancer resistance protein (Bcrp/Abcg2) is selectively modulated by lipopolysaccharide (LPS) in the mouse yolk sac. Reprod. Toxicol. 2020, 98, 82–91. [Google Scholar] [CrossRef]
- Audette, M.C.; Greenwood, S.L.; Sibley, C.P.; Jones, C.J.P.; Challis, J.R.G.; Matthews, S.G.; Jones, R.L. Dexamethasone stimulates placental system A transport and trophoblast differentiation in term villous explants. Placenta 2010, 31, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lv, C.; Xu, C.; Li, Y.; Cui, X.; Gu, H.; Ni, X. Differential Regulation of Glucose Transporters Mediated by CRH Receptor Type 1 and Type 2 in Human Placental Trophoblasts. Endocrinology 2012, 153, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Laurentino, S.; Silva, J.; Sá, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2012, 1820, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lye, P.; Bloise, E.; Javam, M.; Gibb, W.; Lye, S.J.; Matthews, S.G. Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. Am. J. Pathol. 2015, 185, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Drewlo, S.; Levytska, K.; Kingdom, J. Revisiting the housekeeping genes of human placental development and insufficiency syndromes. Placenta 2012, 33, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Spinosa, J.C.; McGinniss, M.J.; Marchevsky, A.; Sanchez, J. Partial molar transformation of the placenta of presumably monozygotic twins. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2000, 3, 95–100. [Google Scholar] [CrossRef]
- Perni, S.C.; Predanik, M.; Cho, J.E.; Baergen, R.N. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J. Perinat. Med. 2005, 33. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Gannon, C.; McClure, N.; Cooke, I. Is Assisted Reproduction Associated with Abnormal Placentation? Pediatr. Dev. Pathol. 2012, 15, 306–314. [Google Scholar] [CrossRef]
- Fontes, K.N.; Reginatto, M.W.; Silva, N.L.; Andrade, C.B.V.; Bloise, F.F.; Monteiro, V.R.S.; Silva-Filho, J.L.; Imperio, G.E.; Pimentel-Coelho, P.M.; Pinheiro, A.A.S.; et al. Dysregulation of placental ABC transporters in a murine model of malaria-induced preterm labor. Sci. Rep. 2019, 9, 11488. [Google Scholar] [CrossRef]
- Reginatto, M.; Fontes, K.; Monteiro, V.; Silva, N.; Andrade, C.; Gomes, H.; Imperio, G.; Bloise, F.; Kluck, G.; Atella, G.; et al. Effect of sublethal prenatal endotoxaemia on murine placental transport systems and lipid homeostasis. Physiology 2020. [Google Scholar] [CrossRef]
- Andrade, C.B.V.; de Monteiro, V.R.S.; Coelho, S.V.A.; Gomes, H.R.; Sousa, R.P.C.; de Nascimento, V.M.O.; Bloise, F.F.; Matthews, S.G.; Bloise, E.; Arruda, L.B.; et al. ZIKV Disrupts Placental Ultrastructure and Drug Transporter Expression in Mice. Front. Immunol. 2021, 12, 680246. [Google Scholar] [CrossRef]
- Tschanz, S.A.; Burri, P.H.; Weibel, E.R. A simple tool for stereological assessment of digital images: The STEPanizer: TOOL FOR STEREOLOGICAL ASSESSMENT. J. Microsc. 2011, 243, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Raunig, J.M.; Yamauchi, Y.; Ward, M.A.; Collier, A.C. Placental inflammation and oxidative stress in the mouse model of assisted reproduction. Placenta 2011, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Clifton, V.L.; Fraser, I.S.; Taylor, H.S.; Critchley, H.; Giudice, L.C.; Petraglia, F. Infertility and reproductive disorders: Impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum. Reprod. Update 2016, 22, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Audette, M.C.; Challis, J.R.G.; Jones, R.L.; Sibley, C.P.; Matthews, S.G. Antenatal Dexamethasone Treatment in Midgestation Reduces System A-Mediated Transport in the Late-Gestation Murine Placenta. Endocrinology 2011, 152, 3561–3570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, H.N.; Ashworth, C.J.; Page, K.R.; McArdle, H.J. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo). Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E596–E603. [Google Scholar] [CrossRef]
- Nelson, D.M.; Smith, S.D.; Furesz, T.C.; Sadovsky, Y.; Ganapathy, V.; Parvin, C.A.; Smith, C.H. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am. J. Physiol. Cell Physiol. 2003, 284, C310–C315. [Google Scholar] [CrossRef]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef]
- Rosario, F.J.; Jansson, N.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Maternal Protein Restriction in the Rat Inhibits Placental Insulin, mTOR, and STAT3 Signaling and Down-Regulates Placental Amino Acid Transporters. Endocrinology 2011, 152, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ylvén, K.; Wennergren, M.; Powell, T.L. Glucose Transport and System A Activity in Syncytiotrophoblast Microvillous and Basal Plasma Membranes in Intrauterine Growth Restriction. Placenta 2002, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Tabano, S.; Pileri, P.; Colapietro, P.; Marino, M.A.; Avagliano, L.; Doi, P.; Bulfamante, G.; Miozzo, M.; Cetin, I. SNAT2 expression and regulation in human growth-restricted placentas. Pediatr. Res. 2013, 74, 104–110. [Google Scholar] [CrossRef]
- Pantham, P.; Rosario, F.J.; Weintraub, S.T.; Nathanielsz, P.W.; Powell, T.L.; Li, C.; Jansson, T. Down-Regulation of Placental Transport of Amino Acids Precedes the Development of Intrauterine Growth Restriction in Maternal Nutrient Restricted Baboons. Biol. Reprod. 2016, 95, 98. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.; Castermans, T.; Hommen, M.; Meesters, D.; Poeze, M. Arginine and Citrulline and the Immune Response in Sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef]
- Ginguay, A.; De Bandt, J.-P. Citrulline production and protein homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 371–376. [Google Scholar] [CrossRef]
- Bahri, S.; Curis, E.; El Wafi, F.-Z.; Aussel, C.; Chaumeil, J.-C.; Cynober, L.; Zerrouk, N. Mechanisms and kinetics of citrulline uptake in a model of human intestinal epithelial cells. Clin. Nutr. 2008, 27, 872–880. [Google Scholar] [CrossRef]
- Casanello, P.; Sobrevia, L. Intrauterine Growth Retardation Is Associated With Reduced Activity and Expression of the Cationic Amino Acid Transport Systems y+/hCAT-1 and y+/hCAT-2B and Lower Activity of Nitric Oxide Synthase in Human Umbilical Vein Endothelial Cells. Circ. Res. 2002, 91, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vadgama, J.V.; Evered, D.F. Characteristics of L-Citrulline Transport across Rat Small Intestine In Vitro. Pediatr. Res. 1992, 32, 472–478. [Google Scholar] [CrossRef]
- Mitsuoka, K.; Shirasaka, Y.; Fukushi, A.; Sato, M.; Nakamura, T.; Nakanishi, T.; Tamai, I. Transport characteristics of L-citrulline in renal apical membrane of proximal tubular cells. Biopharm. Drug Dispos. 2009, 30, 126–137. [Google Scholar] [CrossRef]
- Krause, B.J.; Carrasco-Wong, I.; Caniuguir, A.; Carvajal, J.; Faras, M.; Casanello, P. Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta 2013, 34, 20–28. [Google Scholar] [CrossRef]
- Connor, K.L.; Kibschull, M.; Matysiak-Zablocki, E.; Nguyen, T.T.-T.N.; Matthews, S.G.; Lye, S.J.; Bloise, E. Maternal malnutrition impacts placental morphology and transporter expression: An origin for poor offspring growth. J. Nutr. Biochem. 2020, 78, 108329. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Perazzolo, S.; Sengers, B.G.; Lewis, R.M.; Gruber, M.; Desoye, G.; Wadsack, C. Placental mobilization of free fatty acids contributes to altered materno-fetal transfer in obesity. Int. J. Obes. 2021, 45, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Parisi, F.; Berti, C.; Mandò, C.; Desoye, G. Placental fatty acid transport in maternal obesity. J. Dev. Orig. Health Dis. 2012, 3, 409–414. [Google Scholar] [CrossRef]
- Raunig, J.M.; Yamauchi, Y.; Ward, M.A.; Collier, A.C. Assisted reproduction technologies alter steroid delivery to the mouse fetus during pregnancy. J. Steroid Biochem. Mol. Biol. 2011, 126, 26–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaughan, O.; Fowden, A. Placental metabolism: Substrate requirements and the response to stress. Reprod. Domest. Anim. 2016, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.C.; Miyagi, S.J.; Yamauchi, Y.; Ward, M.A. Assisted reproduction technologies impair placental steroid metabolism. J. Steroid Biochem. Mol. Biol. 2009, 116, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Wang, E. Fetal Programming and Metabolic Syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wen, L.; Guo, X.; Xiao, X.; Jiang, F.; Li, B.; Jin, N.; Wang, J.; Wang, X.; Chen, S.; et al. The increased expression of glucose transporters in human full-term placentas from assisted reproductive technology without changes of mTOR signaling. Placenta 2019, 86, 4–10. [Google Scholar] [CrossRef]


| Gene | Primer Sequences | Reference |
|---|---|---|
| SLC38A1 (SNAT1) | F: 5′-GTGTATGCTTTACCCACCATTGC-3′ R: 5′-GCACGTTGTCATAGAATGTCAAGT-3″ | [24] |
| SLC38A2 (SNAT2) | F: 5′-AGATCAGAATTGGCACAGCATA-3′ R: 5′-ACGAAACAATAAACACCACCTTAA-3′ | [24] |
| SLC38A4 (SNAT4) | F: 5′-GAGGACAATGGGCACAGTTAGT-3′ R: 5′-TTGCCGCCCTCTTTGGTTAC-3′ | [24] |
| SLC2A1 (GLUT1) | F: 5′-ATCAACCGCAACGAGGAGAAC-3′ R: 5′-CACCACAAACAGCGACACGAC-3′ | [25] |
| SLC2A3 (GLUT3) | F: 5′-TCAGGCTCCACCCTTTGCGGA-3′ R: 5′-TGGGGTGACCTTCTGTGTCCCC-3′ | [26] |
| ABCB1 (P-gp) | F: 5′-AGCAGAGGCCGCTGTTCGTT-3′ R: 5′-CCATTCCGACCTCGCGCTCC-3′ | [27] |
| ABCG2 (BCRP) | F: 5′-TGGAATCCAGAACAGAGCTGGGGT-3′ R: 5′-AGAGTTCCACGGCTGAAACACTGC-3′ | [27] |
| PPIB | F: 5′ GAGACTTCACCAGGGG -3′ R: 5′- CTGTCTGTCTTGGTGCTCTCC-3′ | [28] |
| YWHAZ | F: 5′-ACTTTTGGTACATTGTGGCTTCAA-3′ R: 5′-CCGCCAGGACAAACCAGTAT-3′ | [28] |
| TBP | F: 5′-TGCACAGGAGCCAAGAGTGAA-3′ R: 5′-CACATCACAGCTCCCCACCA-3′ | [28] |
| Parameter | CON (n = 10) | ICSI (n = 11) | p-Value |
|---|---|---|---|
| Maternal age (years) | 32.50 ± 7.71 | 35.36 ± 6.61 | NS |
| Gestational age at delivery (days) | 271.6 ± 2.37 | 272.0 ± 3.79 | NS |
| Weight gain (kg; 12 weeks-birth) | 10.31 ± 4.44 | 14.30 ± 5.90 | NS |
| Initial maternal BMI (kg/m2) | 25.62 ± (19.0–29.4) | 23.58 ± (21.7–28.3) | NS |
| Final maternal BMI (kg/m2) | 29.57 ± 3.17 | 28.59 ± 2.53 | NS |
| Newborn sex | M (4); F (6) | M (6); F (5) | |
| Birthweight (g) | 3365 ± 318 | 3199 ± 194 | NS |
| Placental weight (g) | 592.6 ± (359–689) | 547.8 ± 22.21 (438–655) | NS |
| Newborn head circumference (cm) | 35.00 ± 0.97 | 34.41 ± 1.36 | NS |
| Newborn length (cm) | 48.20 ± 1.69 | 48.77 ± 1.65 | NS |
| Fetal/Placental Weight ratio | 5.81 ± 0.98 | 5.94 ± 0.91 | NS |
| Apgar 1′/Apgar 5′ | 9/10 | 9/10 | NS |
| Nutrient | CON | ICSI | p-Value |
|---|---|---|---|
| Maternal circulation | |||
| Citrulline (μmol/L) | 8.2 ± 3.0 (6) | 10.7 ± 3.1 (10) | NS |
| Free fatty acids (mmol/L) | 0.89 ± 0.19 (9) | 0.75 ± 0.28 (10) | NS |
| Umbilical Vein Circulation | |||
| Citrulline (μmol/L) | 6.5 ± 1.6 (6) | 8.5 ± 1.9 (10) | 0.0385 |
| Free fatty acids (mmol/L) | 0.24 ± 0.05 (10) | 0.14 ± 0.08 (10) | 0.0057 |
| Nutrient | Spearman’s Rho | p-Value |
|---|---|---|
| Free fatty acids (mmol/L) | 0.6757 | 0.002 |
| Glucose (mg/dL) | 0.5271 | 0.02 |
| Triglycerides (mg/dL) | 0.2182 | NS |
| Cholesterol (mg/dL) | 0.4717 | 0.03 |
| HDL (mg/dL) | 0.0095 | NS |
| LDL (mg/dL) | 0.5436 | 0.01 |
| VLDL (mg/dL) | −0.0250 | NS |
| Aspartic acid (μmol/L) | 0.6824 | 0.0007 |
| Glutamic acid (μmol/L) | 0.5143 | 0.02 |
| Alanine (μmol/L) | 0.5896 | 0.005 |
| Arginine (μmol/L) | 0.6998 | 0.0004 |
| Phenylalanine (μmol/L) | 0.5055 | 0.02 |
| Glycine (μmol/L) | 0.7351 | 0.0001 |
| Methionine (μmol/L) | 0.4297 | NS |
| Ornithine (μmol/L) | 0.6701 | 0.0009 |
| Serine (μmol/L) | 0.7165 | 0.0003 |
| Tyrosine (μmol/L) | 0.7545 | <0.0001 |
| Threonine (μmol/L) | 0.8078 | <0.0001 |
| Tryptophan (μmol/L) | 0.6359 | 0.002 |
| Valine (μmol/L) | 0.9299 | <0.0001 |
| Citrulline (μmol/L) | 0.7900 | 0.0003 |
| Histidine (μmol/L) | 0.6461 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, E.; Braga, J.R.S.; Andrade, C.B.V.; Imperio, G.E.; Martinelli, L.M.; Antunes, R.A.; Silva, K.R.; Nunes, C.B.; Cobellis, L.; Bloise, F.F.; et al. Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI). Nutrients 2021, 13, 2587. https://doi.org/10.3390/nu13082587
Bloise E, Braga JRS, Andrade CBV, Imperio GE, Martinelli LM, Antunes RA, Silva KR, Nunes CB, Cobellis L, Bloise FF, et al. Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI). Nutrients. 2021; 13(8):2587. https://doi.org/10.3390/nu13082587
Chicago/Turabian StyleBloise, Enrrico, Jair R. S. Braga, Cherley B. V. Andrade, Guinever E. Imperio, Lilian M. Martinelli, Roberto A. Antunes, Karina R. Silva, Cristiana B. Nunes, Luigi Cobellis, Flavia F. Bloise, and et al. 2021. "Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI)" Nutrients 13, no. 8: 2587. https://doi.org/10.3390/nu13082587
APA StyleBloise, E., Braga, J. R. S., Andrade, C. B. V., Imperio, G. E., Martinelli, L. M., Antunes, R. A., Silva, K. R., Nunes, C. B., Cobellis, L., Bloise, F. F., Matthews, S. G., Connor, K. L., & Ortiga-Carvalho, T. M. (2021). Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI). Nutrients, 13(8), 2587. https://doi.org/10.3390/nu13082587







