Abstract
The aim of this systematic review was to provide comprehensive and available data on the possible role of phytoestrogens (PE) for the treatment of endometriosis. We conducted an advanced, systematic search of online medical databases PubMed and Medline. Only full-length manuscripts written in English up to September 2020 were considered. A total of 60 studies were included in the systematic review. According to in vitro findings, 19 out of 22 studies reported the ability of PE in inducing anti-proliferative, anti-inflammatory and proapoptotic effects on cultured cells. Various mechanisms have been proposed to explain this in vitro action including the alteration of cell cycle proteins, the activation/inactivation of regulatory pathways, and modification of radical oxidative species levels. Thirty-eight articles on the effects of phytoestrogens on the development of endometriotic lesions in in vivo experimental animal models of endometriosis have been included. In line with in vitro findings, results also derived from animal models of endometriosis generally supported a beneficial effect of the compounds in reducing lesion growth and development. Finally, only seven studies investigated the effects of phytoestrogens intake on endometriosis in humans. The huge amount of in vitro and in vivo animal findings did not correspond to a consistent literature in the women affected. Therefore, whether the experimental findings can be translated in women is currently unknown.
1. Introduction
Endometriosis is a common benign chronic disease affecting reproductive-age women [1]. It is defined as the presence of endometrial tissue and fibrosis located outside the uterus and is frequently associated with pelvic pain, infertility, urinary and bowel dysfunction [2,3,4]. As a hormonal disease characterized by features of a chronic inflammatory condition, various theories on its development based on an uncontrolled hormonal response and immune-mediated dysfunctions have been proposed [5,6]. Estrogens are key promoters of endometrial cellular growth. Any insult that affects estradiol biosynthesis and catabolism in women with endometriosis have been proposed to play a part in aberrant cell growth. Levels of peripheral estrogens do not seem however altered in women with endometriosis. On the other hand, estrogens are both endocrine and paracrine agents and one may speculate that even a modest variation of estrogen production may be somehow detrimental locally. Indeed, locally accumulated estradiol can create an estrogenic microenvironment around endometriotic lesions. High local concentrations of estradiol and alterations in estrogen receptor (ER) α and ERβ receptor expression may activate a network of genes regulating cell proliferation [7,8]. In line with these observations, medical treatment for endometriosis is still focused on pain and lesion size control with hormonal therapies able to establish either a hypo-estrogenic or a hyper-progestogenic milieu [9,10]. In this context, a role of diet has been postulated based on the idea that estrogen activity can be influenced by nutrition [11,12]. In other conditions in which hormones exert a specific role, such as breast and endometrial carcinogenesis, research has demonstrated that diet may strongly affect incidence [13].
Phytoestrogens (PE) have been identified in various types of food stuffs including fruits, vegetables, sprouts, beans, cabbage, soybean, grains, tea and oilseeds. Based on their structure, the main classes of PE consist of flavonoids (i.e., puerarin, genistein, coumestrol, epicatechin and naringenin), lignans (i.e., enterolactone), and stilbenes (i.e., resveratrol). Classified into three main classes, PE include flavonoids (i.e., puerarin, genistein, coumestrol, epigallocatechin gallate (EGCG), naringenin, quercetin), lignans (i.e., eneterolactone), and stilbenes (i.e., resveratrol). [14]. Their close structural similarity to estrogens, characterized by a phenolic ring and two hydroxyl groups, allows them to act as weak estrogenic factors and to interfere with hormonal and molecular signaling, having positive effects including the prevention of menopausal symptoms, type 2 diabetes, cardiovascular disease, obesity and cancer [15]. Moreover, PE may have poor estrogenic activity in low-estrogen environments such as in menopause and have antiestrogenic activity in high-estrogen environments such as those observed in endometriosis or endometrial cancer [16,17]. Several studies have evaluated the associations between PE and endometriosis risk in animal and human models but the data obtained are quite inconsistent or conflicting [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
The aim of this systematic review was to gain insight into the mechanisms of action of PE in endometriosis and to offer a general view of available data on their possible role for the treatment of endometriosis.
2. Materials and Methods
The study protocol was registered “a priori” and accepted for inclusion in PROSPERO (PROSPERO ID CRD42020220847). The methods for this systematic review were developed in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA) guidelines [78]. No Institutional Review Board Approval was needed. We performed an advanced, systematic search of online medical databases PubMed and Medline using the following keywords: “endometriosis” in combination with “phytoestrogen”, “flavonoid”, “non-flavonoid”, “isoflavone”, “coumestan”, “lignan” and “resveratrol”. To optimize search output, we used specific tools available in each database, such as Medical Subject Headings (MeSH) terms (PubMed/Medline). The EndNote software (available online: https://endnote.com, accessed on 19 September 2020) was used to remove duplicate articles. Only full-length manuscripts written in English up to September 2020 were considered. We checked all citations found by title and abstract to establish the eligibility of the source and obtained the full text of eligible articles. We also performed a manual scan of the references list of the review articles to identify any additional relevant citations. Three review authors (R.V., M.S. and L.B.) independently assessed the risk of bias for each study using the risk-of-bias tool for case–control studies developed by clarity group [79]. We assessed the risk of bias according to the following domains: (i) Can we be confident in the assessment of exposure?; (ii) Can we be confident that cases had developed the outcome of interest and controls had not?; (iii) Were the cases properly selected?; (iv) Were the controls properly selected?; (v) Were cases and controls matched according to important prognostic variables or was statistical adjustment carried out for those variables? We graded each potential source of bias as Definitely yes (low risk of bias), Probably yes (Moderate risk of bias), Probably no (Serious risk of bias), or Definitely no (Critical, high risk of bias). We summarized the risk of bias judgments across different studies for each of the domains listed.
3. Results
A total of 286 studies were initially identified by the search criteria. After applying the selection criteria, a total of 60 trials were included in the systematic review [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. A flow diagram of the systematic review is shown in Figure 1 (PRISMA template). The risks of bias of the included studies are summarized in Supplementary Figure S1. Findings derived from the studies are herein presented based on in vitro results, evidence in in vivo animal models and finally in humans.
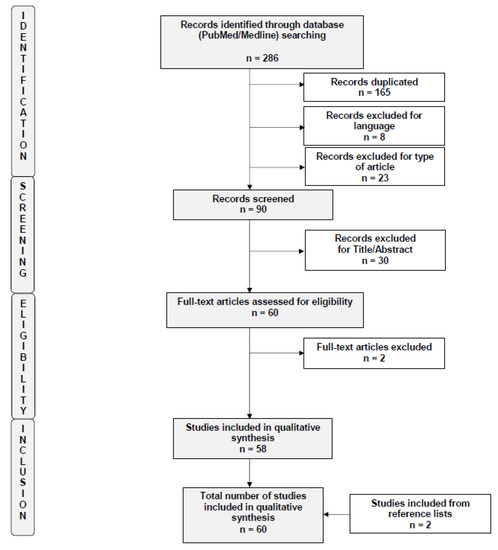
Figure 1.
Flow diagram of the search strategy, screening, eligibility and inclusion criteria.
3.1. Studies Included
3.1.1. Phytoestrogens and Endometriosis: In Vitro Experimental Human Models
Several studies tried to assess PE effect on human endometrial/endometriotic cells. The results from 22 studies are summarized in Table 1. What is surprising is the heterogeneity of the substances studied and their respective biological effects, although for some of them it is possible to designate common actions.

Table 1.
Studies investigating phytoestrogen effect on endometriosis in experimental in vitro in human models.
Resveratrol was the most studied substance whose effects have been investigated in 5 out of 22 studies with significant findings in all of them [35,41,47,59,74]. According to Ricci and coworkers [35], resveratrol could induce significant changes in cell proliferation and apoptosis of eutopic endometrial epithelial cell cultures although without significant differences between endometriosis patients and control women. In line, Khazei et al. have recently reported that the anti-proliferative, proapoptotic and anti-angiogenetic effect of this substance was not specific for ectopic endometrial cells [74].
They claimed a role for the treatment in reducing nitric oxide (NO) levels, found to be higher in endometriotic cells, and in increasing significantly the expression of apoptotic genes (P53, Bax, Bcl2 and caspase 3) and of sirtuin 1 (SIRT1) in both eutopic and ectopic cells. A relationship between activation of SIRT1 by resveratrol and interleukin (IL)-8 was investigated by Taguchi et al. [41] who, conversely, demonstrated that the anti-inflammatory effects of the compound were more prominent in endometriotic cells than in eutopic cells from controls. The same group, one year later, reported that, even if resveratrol alone was not capable of inducing apoptosis in endometriotic cells, it determined an altered expression of some key molecules involved in apoptosis such as survivin or TNF-α-related-apoptosis-inducing ligand (TRAIL), favoring cell death in ectopic lesions [47]. Finally, a higher insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) gene expression in ectopic endometrial cells has been demonstrated by Arablou and coworkers [59]. In this case, resveratrol biological effect in terms of decrease in IGF-1 and HGF protein production was reported for both eutopic and ectopic endometrial stromal cells from women with endometriosis but not for cells from controls. Resveratrol was also shown to inhibit IGF-1/ERK and HGF/MAPK signal transduction pathways in a dose-dependent manner, thus resulting in anti-inflammatory and anti-proliferative effects. Therefore, although the exact mechanism involved is still poorly defined, all the papers supported some in vitro benefit of resveratrol.
Three studies investigated the effects of puerarin (10−9 M), a major isoflavonoid compound extracted from the Chinese medicinal herb, Radix puerariae [28,30,34]. Studies were concordant in demonstrating that puerarin treatment in combination with ethinylestradiol (E2) significantly suppressed the E2-mediated proliferation of stromal cells from endometriotic lesions. Moreover, treating ectopic stromal cells with Puerarin abrogated ERK phosphorylation through a competition with estrogen for the binding to membrane receptors of MAPK signaling, thus significantly decreasing cell proliferation, as well as gene expression levels of cyclin D1, cyclo-oxygenase (COX) 2 and cyp19 involved in this process [30,34]. Finally, Ji and coworkers demonstrated that puerarin can partly suppress estrogen-stimulated proliferation by promoting the recruitment of corepressors to estrogen receptor, as well as limiting that of coactivators, in order to arrest ectopic stromal cells in the G1 phase [34]. Three studies out of 22 investigated the biological effect of chyrisin, a natural compound derived from honey, propolis, or passion flowers, on human endometrial cells [20,66,75]. Although shown to be potent inhibitor of aromatase activity in a free cell assay, chyrisin, daidzein or naringenin could not attenuate aromatase activity in endometrial stromal cells in women with and without endometriosis at any concentration tested. Only genistein (10−9–10−6 M) indirectly increased aromatase activity in endometrial stromal cells from controls. On the other hand, in both VK2/E6E7 and End1/E6E7 endometriotic cell lines, chyrisin was shown to suppress cell proliferation and induced the programmed cell death through changing the cell cycle proportion, increasing the cytosolic calcium level and generating reactive oxygen species (ROS) [66]. In addition, Chrysin activated endoplasmic reticulum (ER) stress by stimulating the unfolded protein response proteins, especially the 78-kDa glucose-regulated protein, GRP78, the PRKR-like ER kinase (PERK) and the eukaryotic translation initiation factor 2α (eIF2α). Finally, the compound was shown to inactivate the intracellular phosphatidylinositol 3-kinase (PI3K)/protein kinase B signaling pathway in a dose-dependent manner from 5 to 100 μM. Similar results and the same biological mechanisms were reported for chyrisin by Park et al. [75], actually testing 5,7-Dimethoxyflavone (DMF), a methylated form of chrysin extracted from Kaempferia parviflora (KP). The methylation of flavonoids has been demonstrated to greatly increase their absorption and bioavailability. A similar biological effect was demonstrated for naringenin by the same authors [50]. Indeed, naringenin (100 μM) decreased the proliferation and increased apoptosis of VK2/E6E7 and End1/E6E7 cells. In the same cells, it also increased the production of ROS 3-fold, induced mitochondrial pro-apoptotic proteins (Bax and Bak), in VK2/E6E7 cells by ~7-fold and in End1/E6E7 cells by 2-fold. Finally, naringenin significantly increased apoptosis through generation of ER stress regulatory genes, in particular G1 arrest and DNA damage 153 (GADD153), inositol-requiring protein 1α (IRE1α) and GRP78, and through activation of MAPK signaling and inactivation of PI3K pathway. It is interesting to note that the same group of authors investigated these same biological mechanisms highlighted for chirisin, narigenin for other substances, such as apigenin, delphinidin, luteolin, quercetin, silibinin and myricetin in VK2/E6E7 and End1/E6E7 endometriotic cells lines [50,55,61,67,68,69]. All these studies are summarized in Table 1. Overall, they demonstrated that the PE effect on endometriosis is always antiproliferative and proapoptic through the activation of intracellular signals of calcium, ER stress and ROS production and through the activation of the MAPK pathway and a decreased phosphorylation of ERK1/2 and PI3K/AKT signaling proteins.
Two studies out of 22 investigated the biological effect of EGCG in eutopic endometrial stromal cells (EuSC) from women with or without endometriosis [35] or in EuSC and ectopic endometrial stromal cells (EcSC) from women affected by endometriosis [40]. The results from these studies were contradictory: while Ricci and coworkers showed no significant difference in cell proliferation and apoptosis between cases and controls [35], Matsuzaki et al. demonstrated an inhibited cell proliferation, migration and invasion of both EuSC and EcSC after EGCG treatment. Moreover, EGCG significantly decreased the Tumor growth factor b-1 (TGF-b1)-dependent increase in the mRNA expression of fibrotic markers and significantly inhibited TGF-b1-stimulated activation of the MAPK and Smad signaling pathways in both cells [40].
Kim et al. [48] examined the effect of Pueraria flowers extract (PFE), a rich source of isoflavones such as genistein, daidzein, kakkalide, puerarin, and tectoridin, on immortalised human endometriotic cells, 11Z and 12Z. Mesothelial Met5A cells were used for adhesion assessment after PFE treatment. They concluded that PFE significantly inhibited adhesion and migration of endometriotic cells to mesothelial cells, suppressing the mRNA and protein expressions of matrix metalloproteinases (MMP)-2 and MMP-9 and increasing the phosphorylation of ERK1/2 in endometriotic cells. A decreased MMP expression was also reported for apigenin [55] and for quercetin [68].
Takaoka and coworkers showed that Daidzein-rich isoflavone aglycones (DRIAs) significantly inhibited the proliferation of ectopic cells in a concentration dependent manner [57]. It also decreased IL-6, IL-8, COX-2 and aromatase mRNA levels, prostaglandin E2 (PGE2) protein levels, and aromatase enzyme activity. DRIAs suppressed the Tumor necrosis factor-α (TNF-α) induced IκB expression, the nuclear factor-κB-IKB-inhibitory proteins (NF-kB-ikb) complex formation, and the uptake of p65 into the nucleus.
In contrast to all this evidence, Hernandes and colleagues have shown that rutin, a glycosylated flavonoid and extract of Uncaria guianensis, or a combination of both, was not able to reduce cellular viability, although ROS production did increase in both eutopic and ectopic cells [71]. In addition, significant increases levels of interleukin (IL)-15, IL-17A, IL-4, IL-6, TNF-α, and vascular endothelium growth factor (VEGF) were observed when eutopic endometrial cells were treated with aqueous bark extract of U. guianensis (ABE), while exposure to aqueous bark leaf of U. guianensis (ALE) induced significant increases in epidermal growth factor in lesion cells.
3.1.2. Phytoestrogens and Endometriosis: In Vivo Experimental Animal Models
Thirty-eight articles on the effects of PE on the development of endometriotic lesions in in vivo experimental animal models of endometriosis have been included in this systematic review. Among them, PE were administered non orally (18 studies, Table 2), orally (18 studies, Table 3) or both (2 studies, reported in both tables). Seven studies investigated the effects of EGCG on endometriosis development, five in mice and one in hamsters. ECGC was administered either orally [35] or through an intraperitoneal injection [23,24,29,35,37,40,45]. In all the studies in which endometriotic lesions were measured, ECGC induced the regression of lesions.

Table 2.
Studies investigating effects of non-oral intake of phytoestrogens on endometriosis in an animal in vivo model.

Table 3.
Studies investigating the effects of phytoestrogen oral intake on endometriosis in an animal in vivo model.
The compound could suppress E2-stimulated activation, proliferation and VEGF expression of endometrial cells in vitro isolated from hamsters. When evaluated by intravital fluorescence microscopy and histology, in vivo treatment with ECGC mediated a selective inhibition of angiogenesis and blood perfusion of endometriotic lesions without affecting blood vessel development in ovarian follicles [23,24,35,37]. Moreover, EGCG showed to increase total apoptotic cell numbers in the lesions [24,35,37]. Molecular mechanisms put forward to explain these phenomena in the lesions include: selective inhibition of VEGFC expression; down-regulation of MMP-9, chemokine (C-X-C motif) ligand 3 (CXCL3), c-JUN, and interferon-γ expression, decreased ROS generation and lipid peroxidation and reduced MMP-2 and MMP-9 activity [29,45]. Matsuzaki and colleagues observed significantly lower scores for both Sirius red and Masson trichrome staining in EGCG-treated mice suggesting that this treatment may prevent the progression of fibrosis [40].
Eight studies investigated the effects of resveratrol on endometriosis development, three in rats and five in mice. Resveratrol was administered either orally [26,36,44], or through an intramuscular [33,43], subcutaneous [38] or intraperitoneal [35,42] injection. The administration of resveratrol showed a marked reduction in endometriotic implants when they were measured. Histological evaluations of tissue sections revealed that both the dimensions and the vascularization of the implants were diminished in the resveratrol-treated animal model. Molecular mechanisms proposed to explain this finding include: decreased levels of VEGF, monocyte chemotactic protein 1 (MCP-1), IL-6, IL-8 and TNF-α in the peritoneal fluid and lower presence of lesional MMP-2, MMP-9 and VEGF [33,35,36,43,44]. Investigating the effects of resveratrol on the expression of estrogen receptor α (ER-α), the proliferative marker Ki-67, aryl hydrocarbon receptor (AhR) and members of the cytochrome P450 superfamily of enzymes, Amaya and colleagues found that mice treated with estradiol (E2) plus progesterone or E2 plus the highest dose (60 mg) of resveratrol exhibited a reduction in both ER-α and Ki-67 in eutopic endometrial epithelial cells. In stromal cells, ER-α levels were reduced by E2 plus P, but not by resveratrol while Ki-67 expression was reduced in presence of 60 mg/day of resveratrol suggesting the potential benefit of high doses of the compound in reducing the proliferation of human endometrium [38]. Bruner-Tran and colleagues have demonstrated that oral administration of resveratrol at dose of 6 mg either for 10–12 days or 18–20 days decreased number of endometrial implants per mouse by 60% and the total volume of lesions per mouse by 80% [26]. Moreover, the authors studied the effect of resveratrol on EcSC in vitro invasiveness, finding a concentration-dependent reduction up to 78%. Finally, Yavuz and coworkers demonstrated a reduced oxidative stress in cases compared to controls in a dose-dependent manner (I.P injection of resveratrol at low dose 1 mg/kg and high dose 10 mg/kg), confirming also reduced lesion size and reduced proliferative scores for the treatment group independent of the dose [42].
The potential role for the genistein to sustain endometriosis has been explored by Cotroneo et al. and Laschke et al. [19,25]. Totally in disagreement with the other studies in this context, the subcutaneous and intraperitoneal injections of genistein was shown to sustain the growth of the implanted tissue in a dose-responsive manner [19] and not to sustain the neoangiogenesis and blood perfusion of endometriotic lesions [25]. When measuring uterine receptor expression, the treatment resulted in a significantly uterine decreased expression of ER-α protein and in an increased progesterone receptor (PR) expression at all doses compared to controls [19]. When administered orally, the same group of authors found that genistein determined an increase of uterine PR type B (PRB) at higher dietary dose. By contrast, in his previous research Yavuz et al. demonstrated that administered orally genistein resulted in smaller areas of endometriotic lesions and lower histological scores if compared with control animals [22].
Subcutaneous administration of silymarin [52] and intraperitoneal injection of silibinin, scutellarin, nobiletin, quercetin and myricetin have all been shown to reduce lesion size in mice and rats [58,60,61,68,76]. Ham et al. also found that the expression of TNF-α, IL-1β, and IL-6 mRNA decreased to 80.4%, 73.8%, and 96.5% respectively in the endometriotic lesions upon intraperitoneal silibinin treatment in mice [61]. Since scutellarin is traditionally used as a potent antiplatelet agent, Ding et al. evaluated its potential therapeutic effect showing also improved hyperalgesia in both low-dose and high-dose and changes consistent with reduced proliferation, angiogenesis, and fibrogenesis of the lesions. Moreover, this flavonoid also significantly reduced the platelet activation rate in peripheral blood when administered intraperitoneally in mice [60]. Intraperitoneal-injected nobiletin was shown to be effective on the activation of NF-κB in endometriotic cells, mainly targeting on the activity of IκB kinases (IKKs) and reducing p65 phosphorylation level [58]. A potential anti-proliferative role on endometriosis through cell cycle regulation has been demonstrated by Park et al. upon intraperitoneal administration of myricetin, quercetinin or luteolin in a mouse model [68,69,76].
In a rat model of endometriosis, oral administration of Puerarin inhibited the growth of ectopic implants and reduced estrogens levels in endometriotic tissue even when administered at low dose and without systemic adverse effects [27].
The potential therapeutic action of Xanthohumol, a flavonoid belonging to the same family of resveratrol, has been investigated by Rudzitis [32]. Similarly to resveratrol, oral Xanthohumol was able to reduce lesion growth by decreasing cell proliferation. Similar results were obtained with the oral administration of Sylimarin, Naringenin and Wogonin, plant-derived flavonoids [51,54,64]. Melekoglu et al. have evaluated the effect of hesperidin, a flavanone glycoside found in citrus fruit, on endometriosis development in a rat model observing lower lesion volumes and increased levels of antioxidant parameters when administered orally at dose of 50 mg/kg for 14 days [53].
Oral isoliquiriteginin (ISL), a flavonoid found in liquorice, has been found not only to decrease lesion volume but also to reduce serum and tissue VEGF, IL1β and IL-6 and to increase Bax, Bcl-2 and E-cadherin [72]. Other authors have investigated the effect of a daidzein-rich isoflavone aglycones diet [57] or of extract of different plants known to contain several PE such as Achileea bierbersteinii [39], Urtica dioica and Anthemis austriaca [62,63], Melilotus officinalis [73] and Achillea critica [70] on endometriosis lesions. All of them have been found to decrease the volume of lesion and adhesion scores. Also, decreased concentration TNF-α were observed both in peritoneal [62,63] and in serum and tissue samples [70]. Moreover, Urtica dioica, Anthemis austriaca, Melilotus officinalis and Achillea critica (AC) extract were able to reduce peritoneal VEGF and IL-6 compared to controls. The anti-inflammatory properties of AC were observed in the ability to reduce serum TNF-α, VEGF and IL-6 as well.
3.1.3. Phytoestrogen Dietary Intake and the Risk of Endometriosis in Humans
Table 4 shows the results of the seven studies that have investigated the effects of PE intake on endometriosis in humans. The first study that evaluated the effects of intaking soy products such as genistein and daidzein found an inverse association between the isoflavone intake and the risk of undergoing premenopausal hysterectomy for benign gynecological conditions, including endometriosis [18]. Similar results have been obtained in a case-control study evaluating urinary levels of genistein and daidzein in 138 women. Levels of isoflavones were found to be inversely correlated to stage III-IV of the disease. Frequency of ER-2 gene RsaI polymorphism was also assessed. A significant association was noted between specific genotypes of ER-2 RsaI polymorphism and genistein levels in risk of advanced endometriosis. Since altered estrogen or soy isoflavone signal transduction thanks to ER-2 gene polymorphisms may be directly responsible for susceptibility to severe endometriosis, the authors suggested that isoflavones may play a more effective role among the ER-2 RsaI R/r + R/R genotype than the r/r genotype, although the latter itself is likely to be protective for endometriosis [21]. Three studies have evaluated the effects of resveratrol on endometriosis women [31,49,65].

Table 4.
Studies investigating the effect of phytoestrogen oral intake on humans.
4. Discussion
Most of the available therapies for endometriosis are hormonal-based therapies able to establish either a hypo-estrogenic or a hyper-progestogenic milieu [80,81,82]. Phytoestrogens are a heterogeneous group of naturally occurring compounds in plants structurally similar to estrogens [15]. They are characterized by a phenolic ring, which determines their agonist or antagonist properties, and two hydroxyl groups which are crucial for the binding to ER [15]. Classified into three main classes, PE include flavonoids (i.e., puerarin, genistein, coumestrol, EGCG, naringenin, quercetin), lignans (i.e., eneterolactone), and stilbenes (i.e., resveratrol) [14,83]. Flavonoids are characterized by a typical structure C6–C3–C6 with two rings of benzene A and B linked by a chain of 3 carbons cycled through an atom of oxygen [84]. Based on the connection, the position, the degree of saturation, oxidation, and hydroxylation of the B and C rings, they are commonly divided into isoflavones and coumestans [15,84,85,86]. Genistein and daidzein (up to 90% of isoflavones) are present in soybeans [87]. Among coumestans, coumestrol is one of the most studied and considered as an endocrine disruptor due to the high affinity in binding ERs [88], with an estrogenic activity greater than that of other isoflavones due to the position of its two hydroxyl groups [89]. It is present in a variety of plants including soybeans, clover, alfalfa sprouts, sunflower seeds, spinach, and legumes. Flavones, a subgroup of flavonoids whose main compound is apigenin, are characterized by a double bond between C2 and C3 that may induce cell cycle arrest and DNA damage in some cell types [90,91]. The skeleton and the position of phenolic group are the main characteristics of another flavonoid subgroup, named flavonols, of which quercetin and kaempferol are the most predominant components in plants [86]. Epicatechin, thought to be responsible for the main health effects of cocoa is another flavonoid compound found in unfermented cocoa beans. Epigallocatechin gallate (EGCG), formed by the ester of epigallocatechin and gallic acid, is present in green tea. Both of them have been associated with antioxidant and chemopreventive effects in several cell types [92,93]. Another flavonoid, narigenin, found in all citric fruits, seems to increase antioxidant defenses by limiting lipid peroxidation and protein carbonylation [85,94]. Lignans are non-flavonoid PE commonly found in grains, nuts, coffee and tea, cocoa, flaxseed, and some fruits [95]. According to some evidence, these PE are capable of mimicking the antioxidant effects of some hormones [96]. Finally, stilbenes are non-flavonoid PE of which the most studied is resveratrol, a compound with two phenolic rings connected by a styrene double bond, found in a wide variety of dietary foods, including grapes, wine, nuts, and berries [97,98,99]. Several in vitro and in vivo studies reported anti-cancer, antioxidant, anti-aging, anti-inflammatory and anti-pathogen properties of resveratrol [97,100,101].
Based on the results presented herein, these compounds may have some effects on the disease establishment. According to in vitro findings, 19 out of 22 studies reported the ability of PE to induce anti-proliferative, anti-inflammatory and proapoptotic effects on endometriotic cells. Only three studies did not find any positive effect exerted by PE in vitro [20,35,71]. Various mechanisms have been proposed to explain this in vitro action including the alteration of cell cycle proteins, the activation/inactivation of regulatory pathways, modification of ROS levels. Two considerations should be done in relation to the in vitro results obtained: 1. among the 22 published studies, nine were written by the same Chinese group [50,55,61,66,67,68,69,75,76]. Therefore, confirmatory findings by independent groups need to be obtained. 2. many studies have used cell lines as a model for endometriotic lesions. A number of immortalized cell lines deriving from endometriosis have been established by either forcing cells to survive through a cell crisis or by the introduction of one or more oncogene(s). However, genetic authentication and biological validation of these lines was disregarded by most authors. For instance, no STR profile was publicly available. Moreover, we have recently demonstrated that some of these endometriotic cell lines express ER-α but are PR-negative [8]. Since signaling initiated by both ER-α and PR is necessary for endometrial physiology, it is of foremost importance that cells are thoroughly characterized prior to each experiment for the maintenance of the proper phenotype and for their receptor status. This concept should be applied also to PE treatment of cells.
In line with in vitro findings, also results derived from animal models of endometriosis generally supported a beneficial effect of the compounds in reducing lesion growth and development. Indeed, a role of PE in limiting ectopic implants has been shown in 36 out of 38 studies independent of the specific drug used. Only two studies did not find any positive effect exerted by PE in in vivo experimental models [19,25] and both studies investigated the possible role of genistein in the treatment of induced models of endometriosis. Mechanisms proposed to explain this effect include decreased angiogenesis and microvessel density, enhanced fibrosis and apoptosis and alteration in MMP activity. Rats and mice offer attractive preclinical models of reproductive disorders because they are easily bred, they can be genetically manipulated, their reproductive system is well understood, and their small size means large quantities of drugs are not required for testing allowing for multiple replicates. In the context of endometriosis, these advantages apply but laboratory rats and mice do not exhibit spontaneous cyclical decidualization and menstruation. Therefore, although uterine tissue has been used to generate endometriosis-like lesions, the lesions are not formed from tissue undergoing active breakdown and remodeling as might be the case in women or menstruating primates. Therefore, similar to cell lines, experimental animal models of endometriosis are not devoid of limits. Due to their divergence from humans in key aspects of reproductive physiology, current experimental systems for the study of endometriosis are a very imperfect model [102]. As a matter of fact, most of the treatment for endometriosis used in experimental models provided satisfactory results while being of poor efficacy in humans [18,21,31,49,56,65,77].
As a matter of fact, the huge amount of in vitro and in vivo animal findings did not correspond to consistent literature in women affected. Randomized trials were only two using resveratrol and outcomes evaluated included pain score and MMP activity [49,65]. Quercitin was also shown to be able to reduce pain in a prospective cohort study [56]. Reasons for a limited reporting of PE effects in endometriosis patients is unclear. We cannot exclude that negative results have not been published. Alternatively, being natural compounds, they are viewed as dietary supplements and regularly prescribed with poor controls on outcomes. Certainly, based on results of experimental models, PE effect deserves to be investigated in more depth in future or ongoing clinical trials.
5. Conclusions
Phytoestrogens are naturally-occurring plant compounds that share a similar chemical structure and function to the estrogens found in the human body. Foods rich in phytoestrogens include soy, fruits, vegetables, spinach, sprouts, beans, cabbages, and grains. The effect of diet on hormonal activity, inflammatory markers, and the immune system means that the food choices women make might play a key role in the development of endometriosis. Furthermore, endometriosis has been shown to be related to prolonged exposure to the hormone estrogen in the absence of progesterone and to a prolonged inflammatory environment in the pelvis. Although there is consistent evidence, deriving from in vitro or in vivo animal model studies, for phytoestrogens’ biological properties in endometriosis, only a few studies have been published regarding their use in patients with endometriosis, with inconsistent results. Phytoestrogens have many favorable characteristics, such as anti-proliferative, anti-angiogenic, anti-inflammatory, pro-apoptotic and anti-oxidant properties, which could make them a viable alternative in the future for the control and prevention of endometriosis. More powered and well-designed trials are needed to better investigate PE effects in women affected by endometriosis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13082532/s1, Figure S1: Risk of bias assessment according to the risk of bias tool by Clarity Group [79].
Author Contributions
Conceptualization, L.B., M.S., R.V. and P.V.; Methodology, P.V.; Validation, P.V., M.C. and J.O.; Investigation, L.B., M.S., R.V., C.D. and N.S.; Resources, L.B., M.S., R.V., C.D. and N.S.; Data Curation, L.B., M.S. and R.V.; Writing—Original Draft Preparation, L.B., M.S., R.V., C.D. and N.S.; Writing—Review and Editing, P.V., J.O. and M.C.; Visualization, P.V., J.O. and M.C.; Supervision, P.V., J.O. and M.C.; Project Administration, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest with respect to the research, authorship and/or publication of this article.
References
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Vercellini, P.P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2013, 10, 261–275. [Google Scholar] [CrossRef]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef]
- Vigano, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Huhtinen, K.; Stahle, M.; Perheentupa, A.; Poutanen, M. Estrogen biosynthesis and signaling in endometriosis. Mol. Cell Endocrinol. 2012, 358, 146–154. [Google Scholar] [CrossRef]
- Kyama, C.M.; Debrock, S.; Mwenda, J.M.; D’Hooghe, T.M. Potential involvement of the immune system in the development of endometriosis. Reprod. Biol. Endocrinol. 2003, 1, 123. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat Rev Dis Primers 2018, 19, 4–9. [Google Scholar] [CrossRef]
- Romano, A.; Xanthoulea, S.; Giacomini, E.; Delvoux, B.; Alleva, E.; Vigano, P. Endometriotic cell culture contamination and authenticity: A source of bias in in vitro research? Hum. Reprod. 2020, 35, 364–376. [Google Scholar] [CrossRef]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Update 2011, 17, 327–346. [Google Scholar] [CrossRef]
- Goncalves, G.A. p27(kip1) as a key regulator of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 1–4. [Google Scholar] [CrossRef]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Eke, A.C.; Chavarro, J.E.; Missmer, S.A. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- Roca, P.; Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Blanquer-Rossello, M.D.; Oliver, J. Phytoestrogens and mitochondrial biogenesis in breast cancer. Influence of estrogen receptors ratio. Curr. Pharm. Des. 2014, 20, 5594–5618. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Myasoedova, V.A.; Orekhova, V.A.; Ravani, A.L.; Nikitina, N.A.; Grechko, A.V.; Sobenin, I.A.; Orekhov, A.N. Phytoestrogen-rich natural preparation for treatment of climacteric syndrome and atherosclerosis prevention in Perimenopausal women. Phytother. Res. 2017, 31, 1209–1214. [Google Scholar] [CrossRef]
- Shukla, V.; Chandra, V.; Sankhwar, P.; Popli, P.; Kaushal, J.B.; Sirohi, V.K.; Dwivedi, A. Phytoestrogen genistein inhibits EGFR/PI3K/NF-kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Adv. 2015, 5, 56075–56085. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Soy product intake and premenopausal hysterectomy in a follow-up study of Japanese women. Eur. J. Clin. Nutr. 2001, 55, 773–777. [Google Scholar] [CrossRef]
- Cotroneo, M.S.; Lamartiniere, C.A. Pharmacologic, but not dietary, genistein supports endometriosis in a rat model. Toxicol. Sci. 2001, 61, 68–75. [Google Scholar] [CrossRef]
- Edmunds, K.M.; Holloway, A.C.; Crankshaw, D.J.; Agarwal, S.K.; Foster, W.G. The effects of dietary phytoestrogens on aromatase activity in human endometrial stromal cells. Reprod. Nutr. Dev. 2005, 45, 709–720. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Miura, T.; Hanaoka, T.; Iwasaki, M.; Sasaki, H.; Tanaka, T.; Nakao, H.; Katoh, T.; Ikenoue, T.; Kabuto, M.; et al. Effect of soy isoflavones on endometriosis: Interaction with estrogen receptor 2 gene polymorphism. Epidemiology 2007, 18, 402–408. [Google Scholar] [CrossRef]
- Yavuz, E.; Oktem, M.; Esinler, I.; Toru, S.A.; Zeyneloglu, H.B. Genistein causes regression of endometriotic implants in the rat model. Fertil. Steril. 2007, 88, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef]
- Xu, H.; Lui, W.T.; Chu, C.Y.; Ng, P.S.; Wang, C.C.; Rogers, M.S. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009, 24, 608–618. [Google Scholar] [CrossRef]
- Laschke, M.W.; Schwender, C.; Vollmar, B.; Menger, M.D. Genistein does not affect vascularization and blood perfusion of endometriotic lesions and ovarian follicles in dorsal skinfold chambers of Syrian golden hamsters. Reprod. Sci. 2010, 17, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Shi, S.; Han, J.; Wang, J.; Hu, J.; Liu, Y.; Cai, Z.; Yu, C. Endometriotic implants regress in rat models treated with puerarin by decreasing estradiol level. Reprod. Sci. 2011, 18, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Han, J.; Zai, D.; Ji, M.; Cheng, W.; Xu, L.; Yang, L.; He, M.; Ni, J.; et al. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17β-estradiol. PLoS ONE 2011, 6, e25011. [Google Scholar] [CrossRef]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, L.; Yang, S.; Han, J.; Zhai, D.; Ni, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17ß-estradiol-BSA. PLoS ONE 2012, 7, e45529. [Google Scholar] [CrossRef]
- Maia, H.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Womens Health 2012, 4, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Rudzitis-Auth, J.; Körbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef]
- Ergenoglu, A.M.; Yeniel, A.O.; Erbas, O.; Aktug, H.; Yildirim, N.; Ulukus, M.; Taskiran, D. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod. Sci. 2013, 20, 1230–1236. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Yang, S.; Zhai, D.; Zhang, D.; Bai, L.; Wang, Z.; Yu, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells in part via differential recruitment of nuclear receptor coregulators to estrogen receptor-α. J. Steroid Biochem. Mol. Biol. 2013, 138, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Rudzitis-Auth, J.; Menger, M.D.; Laschke, M.W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 2013, 28, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Amaya, S.C.; Savaris, R.F.; Filipovic, C.J.; Wise, J.D.; Hestermann, E.; Young, S.L.; Lessey, B.A. Resveratrol and endometrium: A closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod. Sci. 2014, 21, 1362–1369. [Google Scholar] [CrossRef]
- Demirel, M.A.; Suntar, I.; Ilhan, M.; Keles, H.; Kupeli Akkol, E. Experimental endometriosis remission in rats treated with Achillea biebersteinii Afan: Histopathological evaluation and determination of cytokine levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 172–177. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef]
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778. [Google Scholar] [CrossRef]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef]
- Bayoglu Tekin, Y.; Guven, S.; Kirbas, A.; Kalkan, Y.; Tumkaya, L.; Guvendag Guven, E.S. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 1–6. [Google Scholar] [CrossRef]
- Cenksoy, O.P.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A potential novel treatment strategy: Inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol. Endocrinol. 2015, 31, 219–224. [Google Scholar] [CrossRef]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-assisted combinatorial therapy for effective treatment of endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol enhances apoptosis in endometriotic stromal cells. Am. J. Reprod. Immunol. 2016, 75, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-endometriotic effects of pueraria flower extract in human endometriotic cells and mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef]
- Mendes da Silva, D.; Gross, L.A.; Neto, E.P.G.; Lessey, B.A.; Savaris, R.F. The use of resveratrol as an adjuvant treatment of pain in endometriosis: A randomized clinical trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Ferella, L.; Bastón, J.I.; Bilotas, M.A.; Singla, J.J.; González, A.M.; Olivares, C.N.; Meresman, G.F. Active compounds present inRosmarinus officinalis leaves andScutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod. Biomed. Online 2018, 37, 769–782. [Google Scholar] [CrossRef]
- Jouhari, S.; Mohammadzadeh, A.; Soltanghoraee, H.; Mohammadi, Z.; Khazali, S.; Mirzadegan, E.; Lakpour, N.; Fatemi, F.; Zafardoust, S.; Mohazzab, A.; et al. Effects of silymarin, cabergoline and letrozole on rat model of endometriosis. Taiwan J. Obstet. Gynecol. 2018, 57, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. The beneficial effects of nerolidol and hesperidin on surgically induced endometriosis in a rat model. Gynecol. Endocrinol. 2018, 34, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Nahari, E.; Razi, M. Silymarin amplifies apoptosis in ectopic endometrial tissue in rats with endometriosis; implication on growth factor GDNF, ERK1/2 and Bcl-6b expression. Acta Histochem. 2018, 120, 757–767. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. Novel dietary supplement association reduces symptoms in endometriosis patients. J. Cell Physiol. 2018, 233, 5920–5925. [Google Scholar] [CrossRef]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T.; et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid. Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-κB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef]
- Arablou, T.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef]
- Ding, D.; Cai, X.; Zheng, H.; Guo, S.W.; Liu, X. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod. Sci. 2019, 26, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Kim, J.; Bazer, F.W.; Lim, W.; Song, G. Silibinin-induced endoplasmic reticulum stress and mitochondrial dysfunction suppress growth of endometriotic lesions. J. Cell Physiol. 2019, 234, 4327–4341. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Tastan, H.; Kupeli Akkol, E. Promising activity of Anthemis austriaca Jacq. on the endometriosis rat model and isolation of its active constituents. Saudi Pharm. J. 2019, 27, 889–899. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Ryu, S.; Bazer, F.W.; Lim, W.; Song, G. Chrysin leads to cell death in endometriosis by regulation of endoplasmic reticulum stress and cytosolic calcium level. J. Cell Physiol. 2019, 234, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Song, G. Delphinidin induces antiproliferation and apoptosis of endometrial cells by regulating cytosolic calcium levels and mitochondrial membrane potential depolarization. J. Cell Biochem. 2019, 120, 5072–5084. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 2019, 67, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bina, F.; Daglia, M.; Santarcangelo, C.; Baeeri, M.; Abdollahi, M.; Nabavi, S.M.; Tabarrai, M.; Rahimi, R. Phytochemical profiling and ameliorative effects of Achillea cretica L. on rat model of endometriosis. J. Ethnopharmacol. 2020, 254, 112747. [Google Scholar] [CrossRef]
- Hernandes, C.; de Oliveira, R.N.; de Souza Santos, A.H.; Malvezzi, H.; de Azevedo, B.C.; Gueuvoghlanian-Silva, B.Y.; Pereira, A.M.S.; Podgaec, S. The Effect of rutin and extracts of uncaria guianensis (Aubl.) J. F. gmeland on primary endometriotic cells: A 2D and 3D study. Molecules 2020, 13, 1325. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Chen, H.Y.; Chiang, Y.F.; Chang, L.C.; Lin, P.H.; Hsia, S.M. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020, 77, 153214. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. The regression of endometriosis with glycosylated flavonoids isolated from Melilotus officinalis (L.) Pall. in an endometriosis rat model. Taiwan J Obstet. Gynecol. 2020, 59, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Niromand, E.; Khazaei, M. Inhibitory effect of resveratrol on the growth and angiogenesis of human endometrial tissue in an In Vitro three-dimensional model of endometriosis. Reprod. Biol. 2020, 20, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, M.Y.; Song, G.; Lim, W. 5,7-Dimethoxyflavone induces apoptotic cell death in human endometriosis cell lines by activating the endoplasmic reticulum stress pathway. Phytother. Res. 2020, 34, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef]
- Youseflu, S.; Jahanian Sadatmahalleh, S.H.; Mottaghi, A.; Kazemnejad, A. Dietary phytoestrogen intake and the risk of endometriosis in iranian women: A case-control study. Int. J. Fertil. Steril. 2020, 13, 296–300. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- EFSA Scientific Committee; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. The principles and methods behind EFSA’s guidance on uncertainty analysis in scientific assessment. EFSA J. 2018, 16, 5122. [Google Scholar]
- Agarwal, S.K.; Daniels, A.; Drosman, S.R.; Udoff, L.; Foster, W.G.; Pike, M.C.; Spicer, D.V.; Daniels, J.R. Treatment of endometriosis with the GnRHa deslorelin and add-back estradiol and supplementary testosterone. Biomed. Res. Int. 2015, 2015, 934164. [Google Scholar] [CrossRef] [PubMed]
- Abu Hashim, H. Potential role of aromatase inhibitors in the treatment of endometriosis. Int. J. Womens Health 2014, 6, 671–680. [Google Scholar] [CrossRef][Green Version]
- Nawathe, A.; Patwardhan, S.; Yates, D.; Harrison, G.R.; Khan, K.S. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG 2008, 115, 818–822. [Google Scholar] [PubMed]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Wang, T.-Y.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones and humanhealth: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Konar, N. Non-isoflavone phytoestrogenic compound contents of various legumes. Eur. Food Res. Technol. 2013, 236, 523–530. [Google Scholar] [CrossRef]
- Onzalez-Mejia, M.E.; Voss, O.H.; Murnan, E.J.; Dose, A.I. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein–27. Cell Death Dis. 2010, 1, e64. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.A.J.; Berghe, D.V. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (-) -epicatechin and other polyphenols in cancer, inflammation, diabetes and neurodegeneration. Oxidative Med. Cell. Longev. 2015, 181260, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic potential of naringenin: A Review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef]
- Cotterchio, M.; Boucher, B.; Kreiger, N.; Mills, C.A.; Thompson, L.U. Dietary phytoestrogen intake—Lignans and isoflavones—And breast cancer risk (Canada). Cancer Causes Control 2007, 19, 259–272. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxidative Med. Cell. Longev. 2015, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, N.; Nepomnaschy, P.; Crespi, B. The evolutionary biology of endometriosis. Evol. Med. Public Health 2021, 9, 174–191. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).