The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
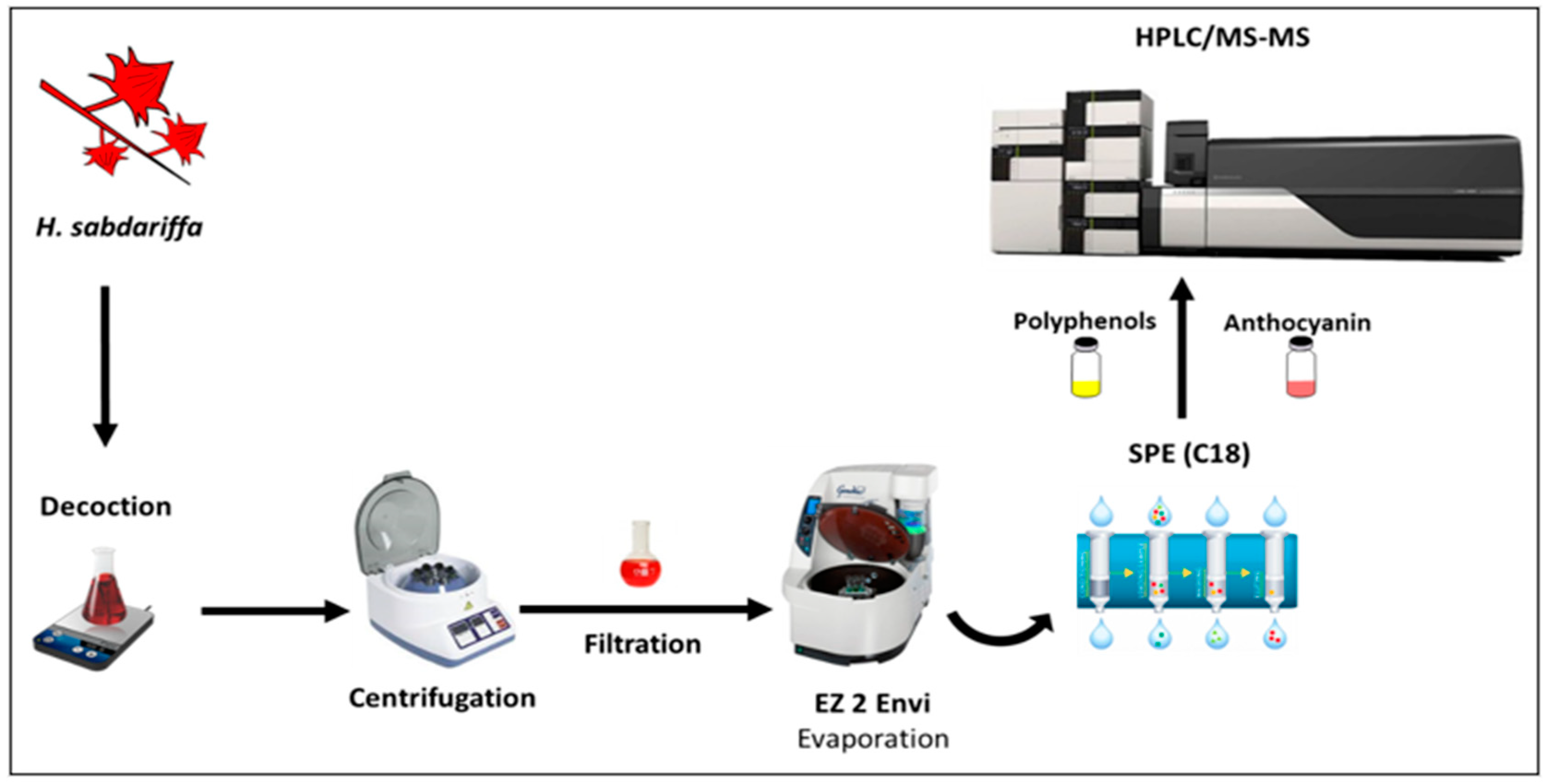
2.2. Sample and Sample Preparation
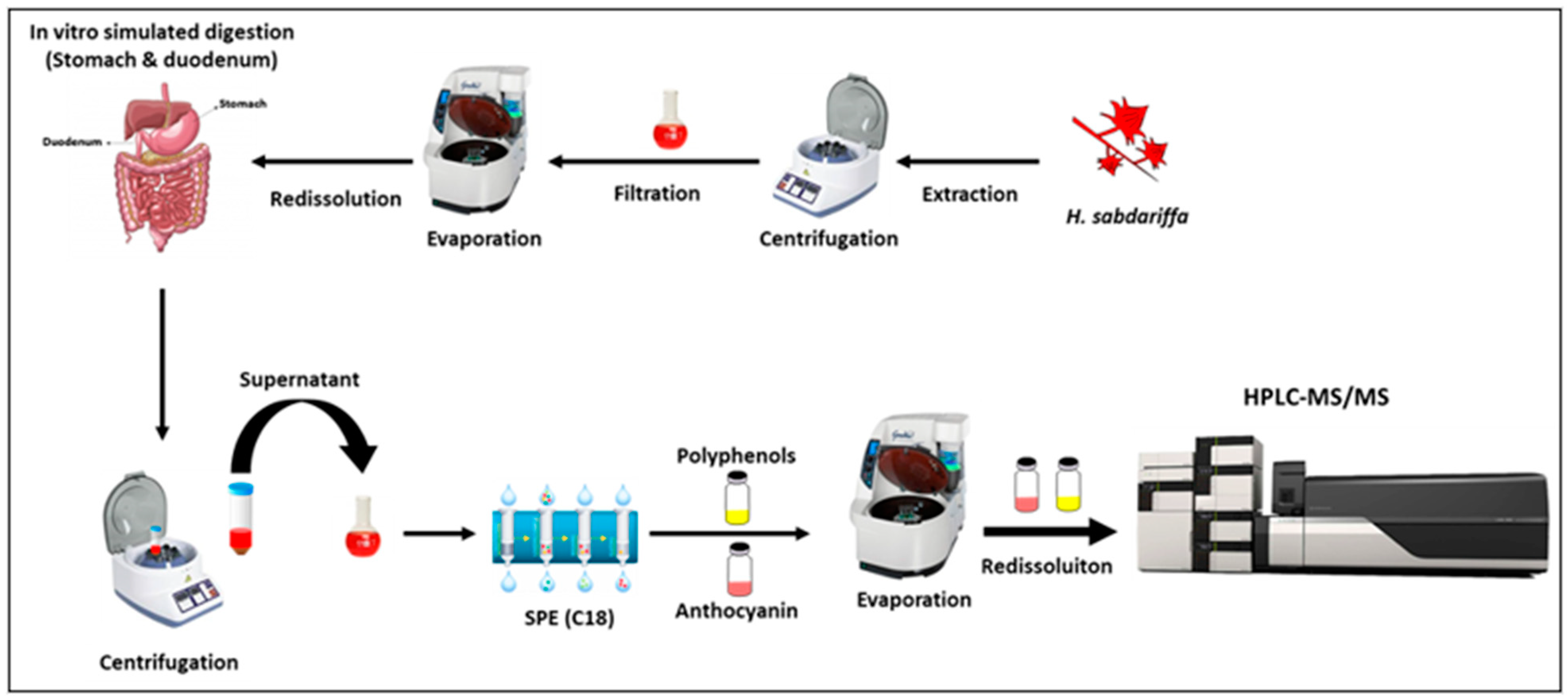
2.3. Simulated Human Digestion
2.3.1. Gastric Digestion
2.3.2. Duodenal Digestion
2.3.3. Polyphenols Extraction after Simulated Digestion
2.4. Polyphenolic Compounds Analysis by HPLC-PDA-MS/MS
2.4.1. Instrumentation and Software
2.4.2. Calibration Curves and Limits of Detection (LoD) and Quantification (LoQ)
2.4.3. Analysis of H.s. Anthocyanins
2.4.4. Analysis of H.s. Polyphenols
2.5. Antimicrobial Assays
2.5.1. Microbial Strains and Culture Conditions
2.5.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Identification of Polyphenols in the Extract of H.s.
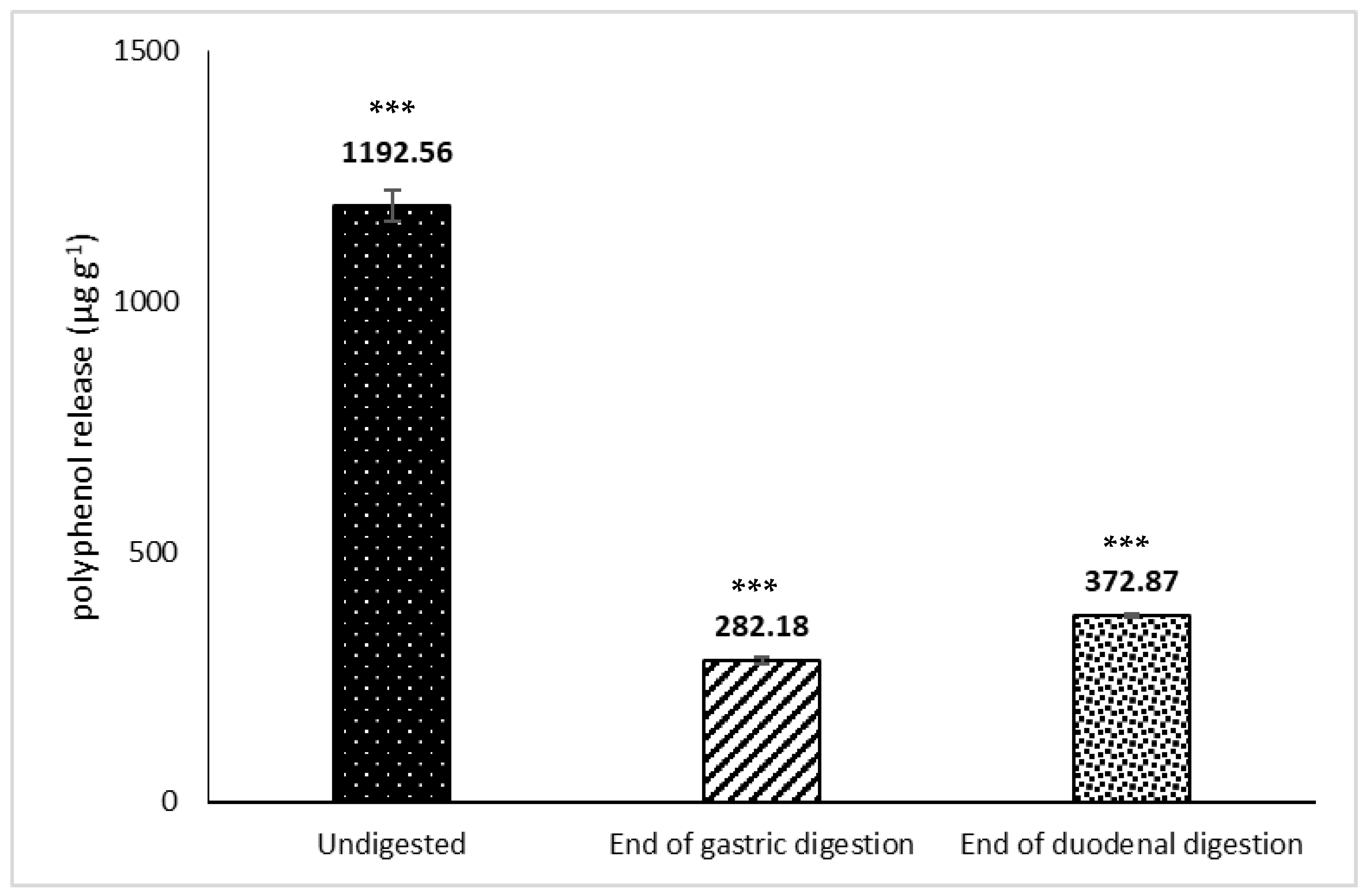
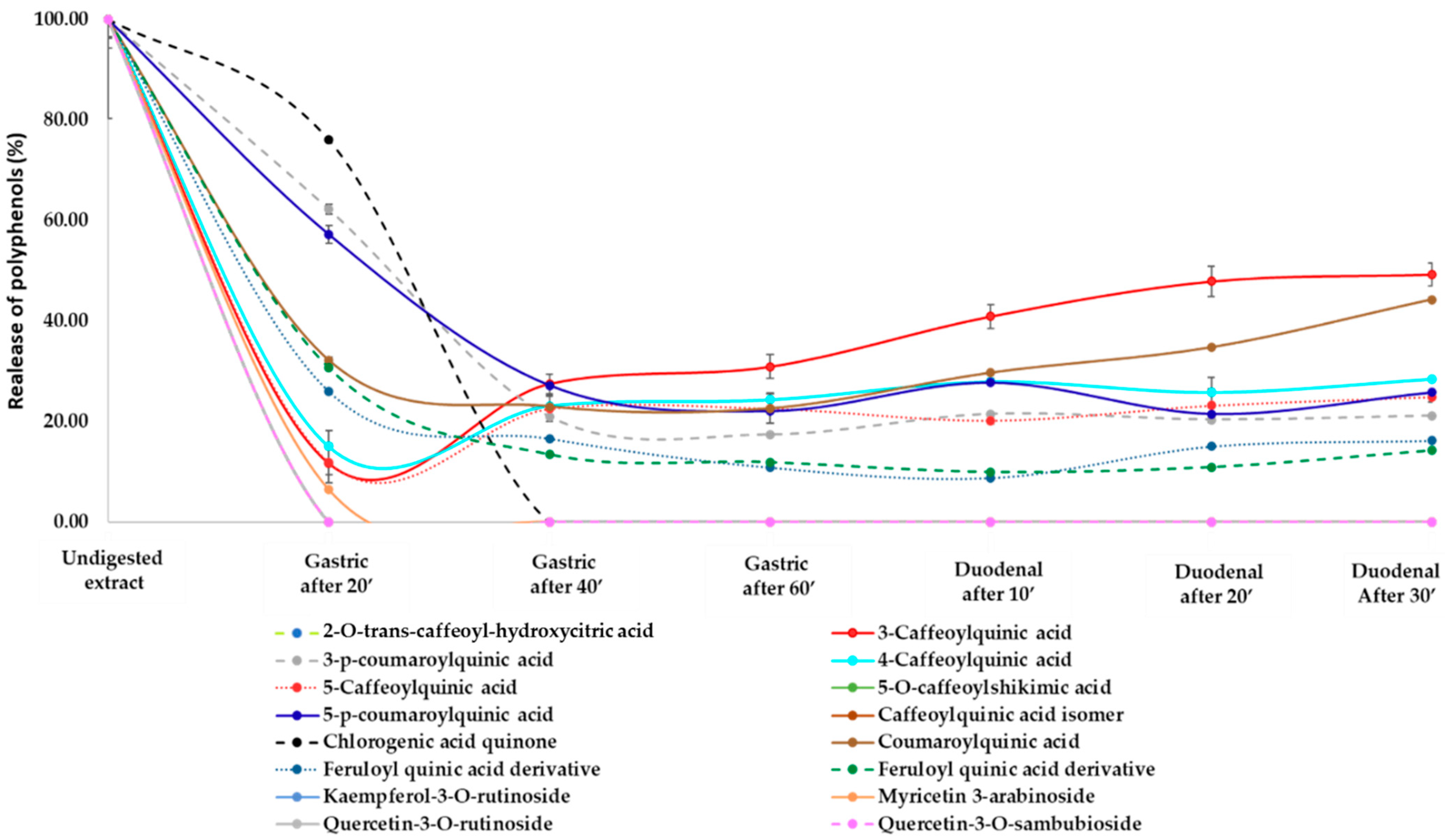
3.2. Release of Phenolic Compounds and Flavonoids from H.s. during In Vitro Digestion
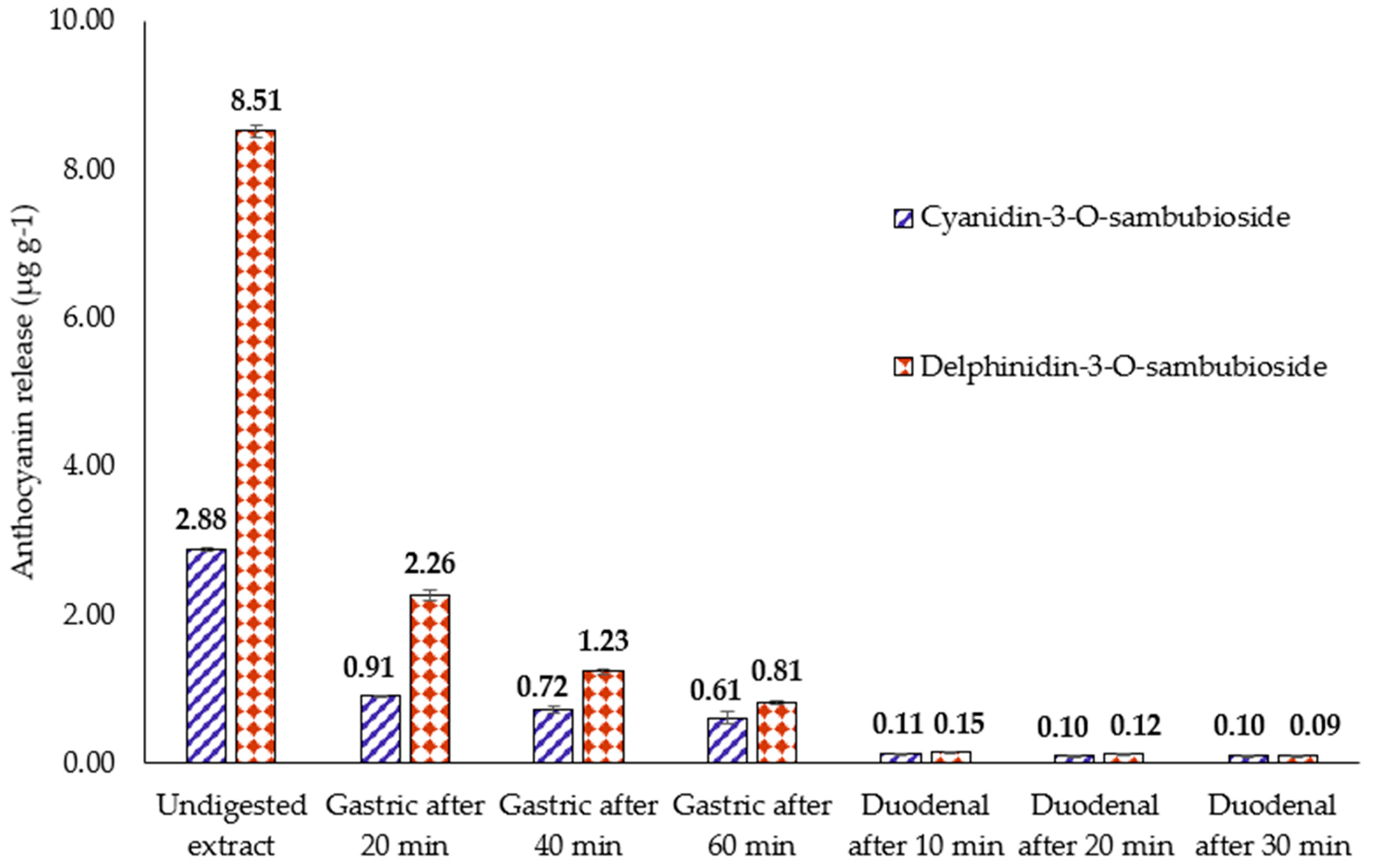
3.3. Release of Anthocyanins from H.s. during In Vitro Digestion
3.4. Antimicrobial Studies
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2007, 73, 637–670. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Morohashi, K.; Casas, M.I.; Ferreyra, M.L.F.; Mejia-Guerra, M.K.; Pourcel, L.; Yilmaz, A.; Feller, A.; Carvalho, B.; Emiliani, J.; Rodriguez, E.; et al. A genome-wide regulatory framework identifies maize pericarp Color1 controlled genes. Plant Cell 2012, 24, 2427–2745. [Google Scholar] [CrossRef] [Green Version]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [Green Version]
- Majdoub, Y.O.E.; Diouri, M.; Arena, P.; Arigò, A.; Cacciola, F.; Rigano, F.; Dugo, P.; Mondello, L. Evaluation of the availability of delphinidin and cyanidin-3-O-sambubioside from Hibiscus sabdariffa and 6-gingerol from Zingiber officinale in colon using liquid chromatography and mass spectrometry detection. Eur. Food Res. Technol. 2019, 245, 2425–2433. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 15, 568. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Faulks, R.M.; Rich, G.T.; Lo Turco, V.; Picout, D.R.; Lo Curto, R.B.; Bisignano, G.; Dugo, P.; Dugo, G.; Waldron, K.W.; et al. Release of protein, lipid, and vitamin E from almond seeds during digestion. J. Agric. Food Chem. 2008, 14, 3409–3416. [Google Scholar] [CrossRef]
- Trombetta, D.; Smeriglio, A.; Denaro, M.; Zagamai, R.; Tomassetti, M.; Pililli, R.; De Angelis, E.; Monaci, L.; Mandalari, G. Understanding the Fate of Almond (Prunus dulcis (Mill.) D.A. Webb) Oleosomes during Simulated Digestion. Nutrients 2020, 12, 3397. [Google Scholar] [CrossRef] [PubMed]
- Kallam, K.; Appelhagen, I.; Luo, J.; Albert, N.; Zhang, H.; Deroles, S.; Hill, L.; Findlay, K.; Andersen, Ø.M.; Davies, K.; et al. Aromatic Decoration Determines the Formation of Anthocyanic Vacuolar Inclusions. Curr. Biol. 2017, 27, 945–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M100-S22. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- Beltrán-Debón, R.; Alonso-Villaverde, C.; Aragonès, G.; Rodríguez-Medina, I.; Rull, A.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Camps, J.; Joven, J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Permeability Study of Polyphenols Derived from a Phenolic-Enriched Hibiscus sabdariffa Extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef]
- Peng, C.-H.; Chyau, C.-C.; Chan, K.-C.; Chan, T.-H.; Wang, C.-J.; Huang, C.-N. Hibiscus sabdariffa Polyphenolic Extract Inhibits Hyperglycemia, Hyperlipidemia, and Glycation-Oxidative Stress while Improving Insulin Resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Piovesana, A.; Rodrigues, E.; Noreña, C.P.Z. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC-DAD-MS/MS. Phytochem. Anal. 2019, 30, 208–217. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Balaban, M.O.; Marshall, M.R.; Rouseff, R.L. Hot and Cold Water Infusion Aroma Profiles of Hibiscus sabdariffa: Fresh Compared with Dried. J. Food Sci. 2011, 76, C212–C217. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus L. subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vicente, A.; Gil-Izquierdo, A.; Garcia-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and Bioaccessibility of Total Polyphenols in a Whole Diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- De Moura, S.C.S.R.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of anthocyanins from the hibiscus extract encapsulated by ionic gelation and application of microparticles in jelly candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Baena-Santillán, E.S.; Piloni-Martini, J.; Santos-López, E.M.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Castro-Rosas, J. Comparison of the Antimicrobial Activity of Hibiscus sabdariffa Calyx Extracts, Six Commercial Types of Mouthwashes, and Chlorhexidine on Oral Pathogenic Bacteria, and the Effect of Hibiscus sabdariffa Extracts and Chlorhexidine on Permeability of the Bacterial Membrane. J. Med. Food. 2021, 24, 67–76. [Google Scholar]
- Dwivedi, M.; Muralidhar, S.; Saluja, D. Hibiscus sabdariffa Extract Inhibits Adhesion, Biofilm Initiation and Formation in Candida albicans. Indian J. Microbiol. 2020, 60, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Mandalari, G.; Navarra, M. In Vitro Antimicrobial Activity and Effect on Biofilm Production of a White Grape Juice (Vitisvinifera) Extract. Evid. Based Complement. Alternat. Med. 2015, 2015, 856243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscarà, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; La Camera, E.; Occhiuto, C.; Grassi, G.; Circosta, C. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytother Res. 2021, 35, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
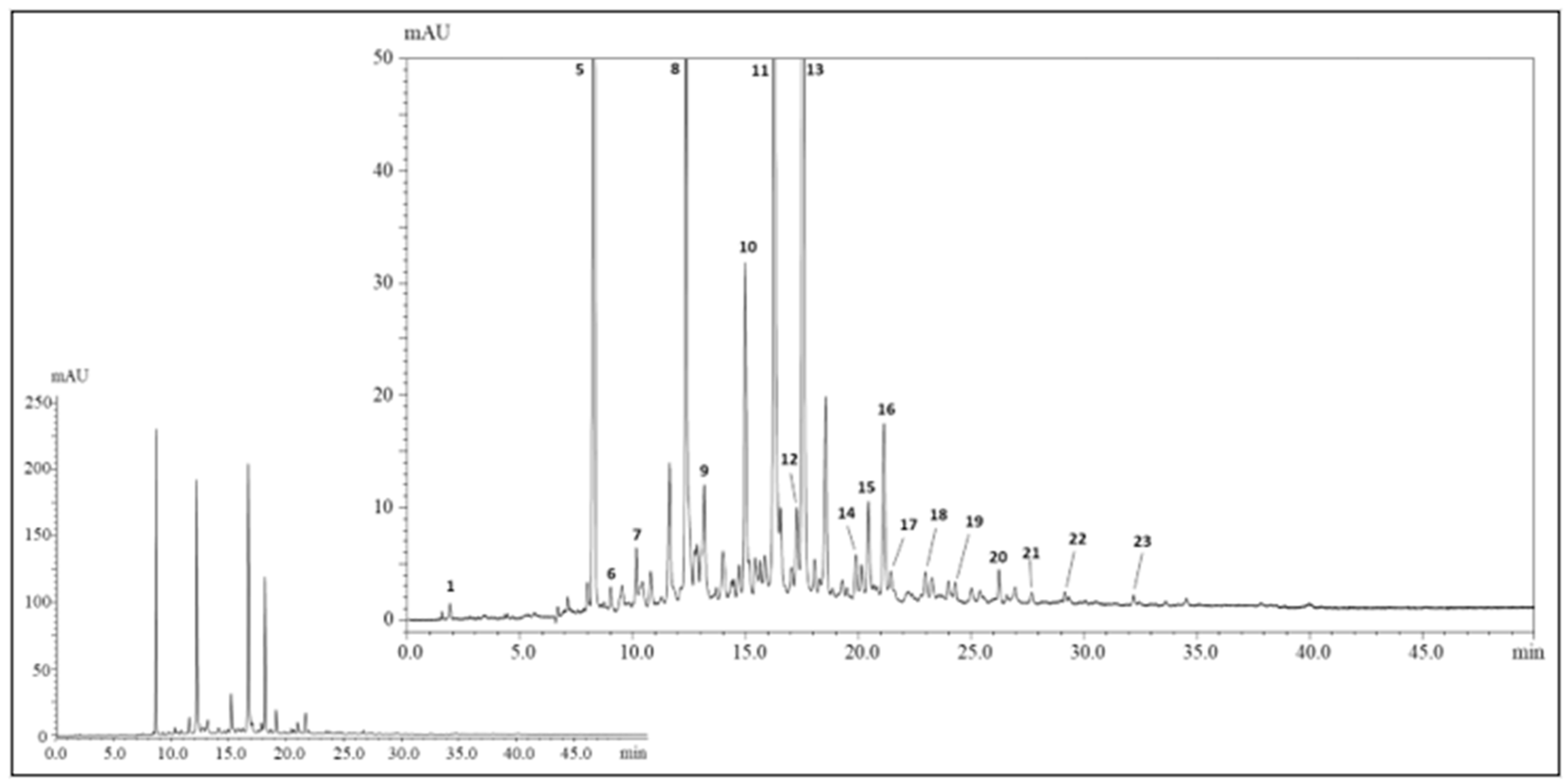
| Peak | tR (min) | λmax (nm) | [M-H]−; MS/MS | Tentative Identification | Undigested Extract | Gastric Digestion | Duodenal Digestion | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 min | 40 min | 60 min | 10 min | 20 min | 30 min | |||||||
| 1 | 1.74 | 260 | 189,127 | Hibiscus acid | × | × | × | × | × | × | × | [16,17,18,19,20,21,22,23] |
| 2 | 3.53 | 280 | 387 | Quinic acid | - | - | - | - | × | × | × | |
| 3 | 4.03 | 520 | 597,303 * | Delphinidin-sambubioside | × | × | × | × | × | × | × | [16,17,18,19,20,21,23] |
| 4 | 6.35 | 520 | 579,287 * | Cyanidin-sambubioside | × | × | × | × | × | × | × | [16,17,18,19,20,21,23] |
| 5 | 8.11 | 286 | 235 | Hibiscus acid hydroxyethylester | × | × | - | - | - | - | - | [17] |
| 6 | 8.89 | 314 | 315 | Chlorogenic acid quinone | × | × | - | - | - | - | - | [17] |
| 7 | 10.15 | 317 | 369,191 | Caffeoyl-hydroxycitric acid | × | - | - | - | - | - | - | [17,19] |
| 8 | 12.34 | 325 | 353,191,179 | Caffeoylquinic acid | × | × | × | × | × | × | × | [21,22,23] |
| 9 | 13.13 | 287 | 297 | Unknown | × | - | - | - | - | - | - | - |
| 10 | 15.06 | 310 | 337, 191 | Coumaroylquinic acid | × | × | × | × | × | × | × | [17,19] |
| 11 | 16.28 | 325 | 353,191,179 | Caffeoylquinic acid isomer | × | × | × | × | × | × | × | [20,21,22,23] |
| 12 | 17.23 | 326 | 367 | Unknown | × | × | × | × | × | × | × | - |
| 13 | 17.61 | 325 | 353,191,179 | Caffeoylquinic acid isomer | × | × | × | × | × | × | × | [20,21,22,23] |
| 14 | 19.74 | 325 | 353,191,179 | Caffeoylquinic acid isomer | × | - | - | - | - | - | - | [22] |
| 15 | 20.58 | 309 | 337,191 | Coumaroylquinic acid isomer | × | × | × | × | × | × | × | [19,21] |
| 16 | 21.16 | 309 | 337,191 | Coumaroylquinic acid isomer | × | × | × | × | × | × | × | [19,21] |
| 17 | 21.38 | 352 | 449,317 | Myricetin-arabinoside | × | × | - | - | - | - | - | [16,18,19] |
| 18 | 22.97 | 329 | 367,193 | Feruloylquinic acid derivative | × | × | × | × | × | × | × | [20] |
| 19 | 24.33 | 326 | 335,179 | Caffeoylshikimic acid | × | - | - | - | - | - | - | [16,17,18,19,21] |
| 20 | 26.29 | 329 | 367,193 | Feruloylquinic acid derivative | × | × | × | × | × | × | × | [20] |
| 21 | 27.88 | 345 | 595,301 | Quercetin-sambubioside | × | - | - | - | - | - | - | [16,17,18,19,21] |
| 22 | 29.18 | 353 | 609,301 | Quercetin-rutinoside | × | - | - | - | - | - | - | [16,17,18,19,21,22,23] |
| 23 | 32.27 | 350 | 593,285 | Kaempferol-rutinoside | × | - | - | - | - | - | - | [16,17,18,19,21,23] |
| Strain | MIC | MBC |
|---|---|---|
| Staphylococcus aureus ATCC 6538 | 2.5 | >2.5 |
| Escherichia coli ATCC 10536 | >2.5 | >2.5 |
| Salmonella typhimurim ATCC 14028 | >2.5 | >2.5 |
| Bacillus subtilis ATCC 6633 | >2.5 | >2.5 |
| Enterococcus hirae ATCC 10541 | >2.5 | >2.5 |
| Listeria monocytogenes ATCC 7644 | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | 2.5 | 2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
| Listeria monocytogenes (food isolate) | >2.5 | >2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majdoub, Y.O.E.; Ginestra, G.; Mandalari, G.; Dugo, P.; Mondello, L.; Cacciola, F. The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients 2021, 13, 2360. https://doi.org/10.3390/nu13072360
Majdoub YOE, Ginestra G, Mandalari G, Dugo P, Mondello L, Cacciola F. The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients. 2021; 13(7):2360. https://doi.org/10.3390/nu13072360
Chicago/Turabian StyleMajdoub, Yassine Oulad El, Giovanna Ginestra, Giuseppina Mandalari, Paola Dugo, Luigi Mondello, and Francesco Cacciola. 2021. "The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity" Nutrients 13, no. 7: 2360. https://doi.org/10.3390/nu13072360
APA StyleMajdoub, Y. O. E., Ginestra, G., Mandalari, G., Dugo, P., Mondello, L., & Cacciola, F. (2021). The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients, 13(7), 2360. https://doi.org/10.3390/nu13072360









