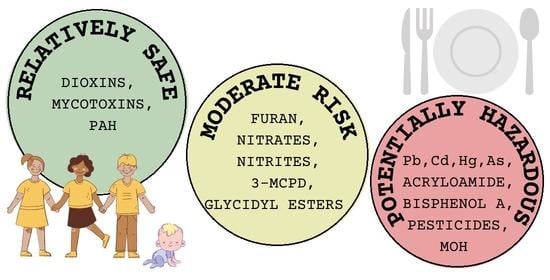
Assessment of the Risk of Contamination of Food for Infants and Toddlers
Abstract
1. Introduction
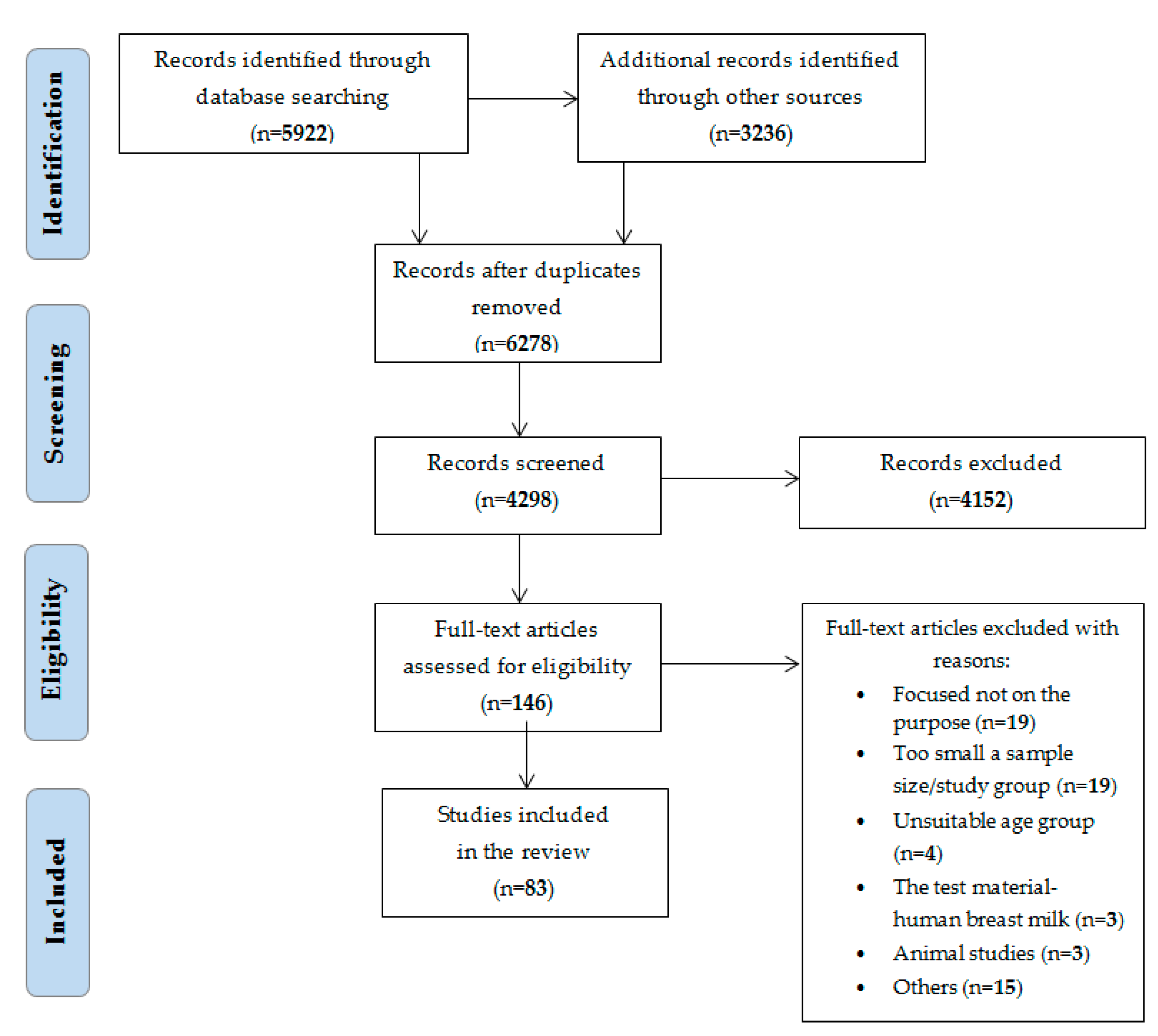
2. Materials and Methods
3. Results
3.1. Toxic Elements
3.2. Acrylamide
3.3. Bisphenol
3.4. Dioxins
3.5. Furan
3.6. Mycotoxins
3.7. Nitrates and Nitrites
3.8. Pesticide Residues
3.9. Polycyclic Aromatic Hydrocarbon (PAH)
3.10. 3-Monochloropropane-1,2-Diol (3-MCPD) and Glycidyl Esters
3.11. Mineral Oil Hydrocarbons (MOHs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patriarca, M.; Menditto, A.; Rossi, B.; Lyon, T.D.; Fell, G.S. Environmental exposure to metals of newborns, infants and young children. Microchem. J. 2000, 67, 351–361. [Google Scholar] [CrossRef]
- Makri, A.; Goveia, M.; Balbus, J.; Parkin, R. Children’s susceptibility to chemicalas: A review by developmental stage. J. Toxicol. Env. Health B 2004, 7, 417–435. [Google Scholar] [CrossRef]
- WHO. Assessment of the Health Risk of Dioxins: Re-Evaluation of the Tolerable Daily Intake (TDI); WHO European Centre for Environment and Health International Programme on Chemical Safety. Available online: https://www.who.int/publications/m/item/assessment-of-the-health-risk-of-dioxins-re-evaluation-of-the-tolerable-daily-intake-(tdi) (accessed on 25 April 2021).
- FAO. Safety Evaluation of Certain Contaminants in Food: Furan. World Health Organ. Tech. Rep. Ser. 2011, 8, 487–603. [Google Scholar]
- JEFCA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives, Nitrate; JEFCA: Geneva, Switzerland, 2002. [Google Scholar]
- Schecter, A.; Wallace, D.; Pavuk, M.; Piskac, A.; Päpke, O. Dioxins in commercial United States baby food. J. Toxicol. Environ. Health A 2002, 65, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Feeley, M.; Brouwer, A. Health risks to infants from exposure to PCBs, PCDDs and PCDFs. Food Addit. Contam. 2000, 17, 325–333. [Google Scholar] [CrossRef]
- Mondello, L.; Zoccali, M.; Purcaro, G.; Franchnina, F.A.; Sciarrone, D.; Moret, S.; Conte, L.; Tranchida, P.Q. Determination of saturated-hydrocarbon contamination in baby foods by using on-line liquid-gas chromatography and off-line liquid chromatography-comprehensive gas chromatography combined with mass spectrometry. J. Chromatogr. A 2012, 1259, 221–226. [Google Scholar] [CrossRef]
- Igweze, Z.N.; Ekhator, O.C.; Orisakwe, O.E. Lead and cadmium in infant milk and cereal based formulae marketed in Nigieria: A probabilistic non-carcinogenic human health risk assessment. Rocz. Panstw. Zakl. Hig. 2020, 71, 303–311. [Google Scholar]
- Gardener, H.; Bowen, J.; Callan, S.P. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci. Total. Environ. 2019, 651 Pt 1, 822–827. [Google Scholar] [CrossRef]
- Škrbić, B.; Živančev, J.; Jovanović, G.; Farre, M. Essential and toxic elements in commercial baby food on the Spanish and Serbian market. Food Addit. Contam. Part B 2016, 10, 27–38. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kiczorowska, B. Determining the content of lead and cadmium in infant food from the Polish market. Int. J. Food Sci. Nutr. 2012, 63, 708–712. [Google Scholar] [CrossRef] [PubMed]
- De Castro, C.S.; Arruda, A.F.; Da Cunha, L.R.; SouzaDe, J.R.; Braga, J.W.; Dórea, J.G. Toxic metals (Pb and Cd) and their respective antagonists (Ca and Zn) in infant formulas and milk marketed in Brasilia, Brazil. Int. J. Environ. Res. Public Health. 2010, 7, 4062–4077. [Google Scholar] [CrossRef]
- Iwegbue, C.M.; Nwozo, S.O.; Overah, L.C.; Nwajei, G.E. Survey of trace element composition of commercial infant formulas in the Nigerian market. Food Addit. Contam. Part B 2010, 3, 163–171. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Dong, J.; Cao, Y.; Li, T.; Mielke, H.W. Snack foods and lead ingestion risks for school aged children: A comparative evaluation of potentially toxic metals and children’s exposure response of blood lead, copper and zinc levels. Chemosphere 2020, 261, 127547. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Chafey, C.; Testu, C.; Hulin, M.; Noël, L. Mercury in foods from the first French total diet study on infants and toddlers. Food Chem. 2018, 239, 920–925. [Google Scholar] [CrossRef]
- Junqué, E.; Garí, M.; Arce, A.; Torrent, M.; Sunyer, J.; Grimalt, J. Integrated assessment of infant exposure to persistent organic pollutants and mercury via dietary intake in a central western Mediterranean site (Menorca Island). Environ. Res. 2017, 156, 714–724. [Google Scholar] [CrossRef]
- Kim, D.W.; Woo, H.D.; Joo, J.; Park, K.S.; Oh, S.Y.; Kwon, H.J.; Park, J.D.; Hong, Y.S.; Sohn, S.J.; Yoon, H.J.; et al. Estimated long-term dietary exposure to lead, cadmium, and mercury in young Korean children. Eur. J. Clin. Nutr. 2014, 68, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Dabeka, R.W.; McKenzie, A.D. Survey of total mercury in infant formulae and oral electrolytes sold in Canada. Food Addit. Contam. Part B 2012, 5, 65–69. [Google Scholar] [CrossRef]
- Martins, C.; Vasco, E.; Paixão, E.; Alvito, P. Total mercury in infant food, occurrence and exposure assessment in Portugal. Food Addit. Contam. Part B 2013, 6, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; de Silva, S.; Reichman, S.M. Arsenic concentrations and dietary exposure in rice-based infant food in Australia. Int. J. Environ. Res. Public Health 2020, 17, 415. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Jackson, B.P.; Carly, M.G.; Donohue, A.; Emmons, A.M. Co-exposure to methylmercury and inorganic arsenic in baby rice cereals and rice-containing teething biscuits. Environ. Res. 2017, 159, 639–647. [Google Scholar] [CrossRef]
- Ljung, K.; Palm, B.; Grandér, M.; Vahter, M. High concentrations of essential and toxic elements in infant formula and infant foods–A matter of concern. Food Chem. 2011, 127, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Melø, R.; Gellein, K.; Evje, L.; Syversen, T. Minerals and trace elements in commercial infant food. Food Chem. Toxicol. 2008, 46, 3339–3342. [Google Scholar] [CrossRef]
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011-2015 data. Food Addit. Contam. Part A Chem. Anal. Control. 2019, 36, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Sirot, V.; Rivière, G.; Leconte, S.; Vin, K.; Traore, T.; Jean, J.; Hulin, M.; Carne, G.; Gorecki, S.; Veyrand, B.; et al. French infant total diet study: Dietary exposure to heat-induced compounds (acrylamide, furan and polycyclic aromatic hydrocarbons) and associated health risks. Food Chem. Toxicol. 2019, 130, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Inthavong, C.; Hommet, F.; Leblanc, J.C.; Hulin, M.; Guérin, T. Levels of acrylamide in foods included in ‘the first French total diet study on infants and toddlers’. Food Chem. 2018, 240, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Roasto, M.; Reinik, M.; Nelis, K.; Nurk, E.; Elias, T. Acrylamide in commercial foods and intake by infants in Estonia. Food Addit. Contam. Part A Chem. Anal. Control. 2017, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Stoś, K. Determination of acrylamide level in commercial baby foods and an assessment of infant dietary exposure. Food Chem. Toxicol. 2012, 50, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Cao. X.L.; Corriveau. J.; Popovic. S.; Clement. G.; Beraldin. F.; Dufresne, G. Bisphenol a in baby food products in glass jars with metal lids from Canadian Markets. J. Agric. Food Chem. 2009, 57, 5345–5351. [Google Scholar] [CrossRef]
- Martínez, M.Á.; Castro, I.; Rovira, J.; Ares, S.; Rodríguez, J.M.; Cunha, S.C.; Casal, S.; Fernandes, J.O.; Schuhmacher, M.; Nadal, M. Early-life intake of major trace elements, bisphenol A, tetrabromobisphenol A and fatty acids: Comparing human milk and commercial infant formulas. Environ. Res. 2019, 169, 246–255. [Google Scholar] [CrossRef]
- Cirillo, T.; Latini, G.; Castaldi, M.A.; Dipaola, L.; Fasano, E.; Esposito, F.; Scognamiglio, G.; Di Francesco, F.; Cobellis, L. Exposure to di-2-ethylhexyl phthalate, di-N-butyl phthalate and bisphenol A through infant formulas. J. Agric. Food Chem. 2015, 63, 3303–3310. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, J.; Duan, H.; Wu, Y.; Shao, B. Bisphenol A and nonylphenol in foodstuffs: Chinese dietary exposure from the 2007 total diet study and infant health risk from formulas. Food Chem. 2015, 167, 320–325. [Google Scholar] [CrossRef]
- Karasauliya, K.; Bhateria, M.; Sonker, A.; Singh, S.P. Determination of bisphenol analogues in infant formula products from India and evaluating the health risk in infants associated with their exposure. J. Agric. Food Chem. 2021, 7, 3932–3941. [Google Scholar] [CrossRef]
- Sun, N.; Guo, Q.; Ou, J.B. Simultaneous determination of endogenous hormones and exogenous contaminants in infant formula powdered milk by salting-out assisted liquid–liquid extraction combined with solid-phase extraction purification and UPLC-MS/MS. Anal. Methods 2017, 9, 6177–6185. [Google Scholar] [CrossRef]
- Saito, K.; Ohmura, A.; Takekuma, M. Assessment of dioxin intake from commercial baby food in infant. Bull. Environ. Contam. Toxicol. 2008, 80, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Lorán, S.; Bayarri, S.; Conchello, P.; Herrera, A. Risk assessment of PCDD/PCDFs and indicator PCBs contamination in Spanish commercial baby food. Food Chem. Toxicol. 2010, 48, 145–151. [Google Scholar] [CrossRef]
- Sasamoto, T.; Tatebe, H.; Yamaki, Y.; Hashimoto, T.; Ushio, F.; Ibe, A. Estimation of daily intake of PCDDs, PCDFs and Co-PCBs from baby foods. Skokuhin Eiseigaku Zasshi 2006, 47, 157–163. [Google Scholar] [CrossRef][Green Version]
- Pandelova, M.; Piccinelli, R.; Lopez, W.L.; Henkelmann, B.; Molina-Molina, J.M.; Arrebola, J.P.; Olea, N.; Leclercq, C.; Schramm, K.W. Assessment of PCDD/F, PCB, OCP and BPA dietary exposure of non-breast-fed European infants. Food Addit. Contam. Part A Chem. Anal. Control. 2011, 28, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. Infant dietary exposure to dioxins and dioxin-like compounds in Greece. Food Chem. Toxicol. 2013, 59, 316–324. [Google Scholar] [CrossRef]
- Hulin, M.; Sirot, V.; Vasseur, P.; Mahe, A.; Leblanc, J.C.; Jean, J.; Marchand, P.; Venisseau, A.; Le Bizec, B.; Rivière, G. Health risk assessment to dioxins, furans and PCBs in young children: The first French evaluation. Food Chem. Toxicol. 2020, 139, 111292. [Google Scholar] [CrossRef]
- De Filippis, S.P.; Brambilla, G.; Dellatte, E.; Corrado, F.; Esposito, M. Exposure to polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), dioxin-like polychlorinated biphenyls (DL-PCBs) and polybrominated diphenyl ethers (PBDEs) through the consumption of prepared meals in Italy. Food Addit. Contam. Part A Chem. Anal. Control. 2014, 31, 1114–1126. [Google Scholar] [CrossRef]
- Lambert, M.; Inthavong, C.; Desbourdes, C.; Hommet, F.; Sirot, V.; Leblanc, J.C.; Hulin, M.; Guérin, T. Levels of furan in foods from the first French Total Diet Study on infants and toddlers. Food Chem. 2018, 266, 381–388. [Google Scholar] [CrossRef]
- Altaki, M.S.; Santos, F.J.; Puignou, L.; Galceran, M.T. Furan in commercial baby foods from the Spanish market: Estimation of daily intake and risk assessment. Food Addit. Contam. Part A Chem. Anal. Control 2017, 34, 728–739. [Google Scholar] [CrossRef]
- Sijia, W.; Enting, W.; Yuan, Y. Detection of furan levels in select Chinese foods by solid phase microextraction–gas chromatography/mass spectrometry method and dietary exposure estimation of furan in the Chinese population. Food Chem. Toxicol. 2014, 64, 34–40. [Google Scholar] [CrossRef]
- Scholl, G.; Humblet, M.F.; Scippo, M.L.; De Pauw, E.; Eppe, G.; Saegerman, C. Preliminary assessment of the risk linked to furan ingestion by babies consuming only ready-to-eat food. Food. Addit. Contam. Part A Chem. Anal. Control. 2013, 30, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Minorczyk, M.; Góralczyk, K.; Struciński, P.; Hernik, A.; Czaja, K.; Łyczewska, M.; Korcz, W.; Starski, W.; Ludwicki, J. Risk assessment for infants exposed to furan from ready-to-eat thermally processed food products in Poland. Rocz. Panstw. Zakl. Hig. 2012, 63, 403–410. [Google Scholar] [PubMed]
- Liu, Y.T.; Tsai, S.W. Assessment of dietary furan exposures from heat processed foods in Taiwan. Chemosphere 2010, 79, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Reusch, H.; Kuballa, T. Risk assessment of furan in commercially jarred baby foods, including insights into its occurrence and formation in freshly home-cooked foods for infants and young children. Food Addit. Contam. Part A 2009, 26, 776–785. [Google Scholar] [CrossRef]
- Mallmann, C.A.; Tyska, D.; Almeida, C.A.; Oliveira, M.S.; Gressler, L.T. Mycotoxicological monitoring of breakfast and infant cereals marketed in Brazil. Int. J. Food Microbiol. 2020, 331, 108628. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. Health risk assessment of Patulin intake through apples and apple-based foods sold in Qatar. Heliyon 2019, 5, e02754. [Google Scholar] [CrossRef]
- Herrera, M.; Bervis, N.; Carramiñana, J.; Juan, T.; Herrera, A.A.; Ariño, A.; Lorán, S. Occurrence and exposure assessment of aflatoxins and deoxynivalenol in cereal-based baby foods for infants. Toxins 2019, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Postupolski, J.; Starski, A.; Ledzion, E.; Kurpińska-Jaworska, J.; Szczęsna, M. Exposure assessment of infants and young children on selected Fusarium toxins. Rocz. Panstw. Zakl. Hig. 2019, 70, 5–14. [Google Scholar] [CrossRef]
- Khoshnamvand, Z.; Nazari, F.; Mehrasebi, M.R.; Hosseini, M.J. Occurrence and safety evaluation of ochratoxin A in cereal-based baby foods collected from Iranian retail market. J. Food Sci. 2019, 84, 695–700. [Google Scholar] [CrossRef]
- Ojuri, O.T.; Ezekiel, C.N.; Eskola, M.K.; Šarkanj, B.; Babalola, A.D.; Sulyok, M.; Hajšlová, J.; Elliott, C.T.; Krska, R. Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice. Food Control 2019, 98, 312–322. [Google Scholar] [CrossRef]
- Omar, S.S. Aflatoxin M1 levels in raw milk, pasteurized milk and infant formula. Ital. J. Food Saf. 2016, 5, 5788. [Google Scholar] [CrossRef]
- Sundheim, L.; Lillegaard, I.T.; Fæste, C.K.; Brantsæter, A.L.; Brodal, G.; Eriksen, G.S. Deoxynivalenol exposure in Norway, risk assessments for different human age groups. Toxins 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Er, B.; Demirhan, B.; Yentür, G. Short communication: Investigation of aflatoxin M1 levels in infant follow-on milks and infant formulas sold in the markets of Ankara, Turkey. J. Dairy Sci. 2014, 97, 3328–3331. [Google Scholar] [CrossRef]
- Torović, L. Aflatoxin M1 in processed milk and infant formulae and corresponding exposure of adult population in Serbia in 2013–2014. Food Addit. Contam. Part B 2015, 8, 235–244. [Google Scholar]
- Li, S.; Min, L.; Wang, G.; Li, D.; Zheng, N.; Wang, J. Occurrence of Aflatoxin M1 in raw milk from manufacturers of infant milk powder in China. Int. J. Environ. Res. Public Health 2018, 15, 879. [Google Scholar] [CrossRef]
- Vasco, E.R.; Alvito, P.C. Occurrence and infant exposure assessment of nitrates in baby foods marketed in the region of Lisbon, Portugal. Food Addit. Contam. Part B 2011, 4, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Marín, O.; Yusà-Pelechà, V.; Villalba-Martín, P.; Perez-Dasí, J.A. Monitoring programme on nitrates in vegetables and vegetable-based baby foods marketed in the Region of Valencia, Spain: Levels and estimated daily intake. Food Addit. Contam. Part A Chem. Anal. Control. 2010, 27, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.L.; Vollano, L.; Peruzy, M.F.; Marrone, R.; Mercogliano, R. Determination of nitrate and nitrite levels in infant foods marketed in Southern Italy. CyTA J. Food 2015, 13, 629–634. [Google Scholar] [CrossRef]
- Rebelo, J.S.; Almeida, M.D.; Vales, L.; Almeida, C.M. Presence of nitrates in baby foods marketed in Portugal. Cogent. Food Agric. 2015, 1, 1010414. [Google Scholar] [CrossRef]
- Chetty, A.A.; Prasad, S. Flow injection analysis of nitrate and nitrite in commercial baby foods. Food Chem. 2016, 197 Pt A, 503–508. [Google Scholar] [CrossRef]
- Elias, A.; Jalakas, S.; Roasto, M.; Reinik, M.; Nurk, E.; Kaart, T.; Tuvike, A.; Meramäe, K.; Nelis, K.; Elias, T. Nitrite and nitrate content in meat products and estimated nitrite intake by the Estonian children. Food Addit. Contam. Part A Chem. Anal. Control. 2020, 37, 1229–1237. [Google Scholar] [CrossRef]
- Mancini, F.R.; Paul, D.; Gauvreau, J.; Volatier, J.L.; Vin, K.; Hulin, M. Dietary exposure to benzoates (E210–E213), parabens (E214–E219), nitrites (E249–E250), nitrates (E251–E252), BHA (E320), BHT (E321) and aspartame (E951) in children less than 3 years old in France. Food Addit. Contam. Part A Chem. Anal. Control. 2015, 32, 293–306. [Google Scholar] [CrossRef]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Jurtsenko, S.; Tenńo, T.; Kiis, A. Nitrites, nitrates and N-nitrosoamines in Estonian cured meat products: Intake by Estonian children and adolescents. Food Addit. Contam. 2005, 22, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Nougadère, A.; Sirot, V.; Cravedi, J.-P.; Vasseur, P.; Feidt, C.; Fussell, R.J.; Hulin, M. Dietary exposure to pesticide residues and associated health risks in infants and young children–results of the French infant total diet study. Environ. Int. 2020, 137, 105529. [Google Scholar] [CrossRef] [PubMed]
- Stepán, R.; Tichá, J.; Hajslová, J.; Kovalczuk, T.; Kocourek, V. Baby food production chain: Pesticide residues in fresh apples and products. Food Addit. Contam. 2005, 22, 1231–1242. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Kim, S.; Choi, S.D.; Park, J.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; et al. Occurrence and exposure assessment of polychlorinated biphenyls and organochlorine pesticides from homemade baby food in Korea. Sci. Total. Environ. 2014, 470–471, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Torović, L.; Vuković, G.; Dimitrov, N. Pesticide active substances in infant food in Serbia and risk assessment. Food Addit. Contam. Part B 2020, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, U.; Srivastava, M.K.; Srivastava, A.K.; Patel, D.K.; Garg, V.; Srivastava, L.P. Analysis of imidacloprid residues in fruits, vegetables, cereals, fruit juices, and baby foods, and daily intake estimation in and around Lucknow, India. Environ. Toxicol. Chem. 2013, 32, 723–727. [Google Scholar] [CrossRef]
- Gilbert-Lòpez, B.; García-Reyes, J.F.; Ortega-Barrales, P.; Molina-Díaz, A.; Fernández-Alba, A.R. Analyses of pesticide residues in fruit-based baby food by liquid chromatography/electrospray ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2059–2071. [Google Scholar] [CrossRef]
- Panseri, S.; Nobile, M.; Arioli, F.; Biolatti, C.; Pavlovic, R.; Chiesa, L.M. Occurrence of perchlorate, chlorate and polar herbicides in different baby food commodities. Food Chem. 2020, 320, 127205. [Google Scholar] [CrossRef]
- Santonicola, S.; Albrizio, S.; Murru, N.; Ferrante, M.C.; Mercogliano, R. Study on the occurrence of polycyclic aromatic hydrocarbons in milk and meat/fish based baby food available in Italy. Chemosphere 2017, 184, 467–472. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy metals and PAHs in meat, milk, and seafood from augusta area (Southern Italy): Contamination levels, dietary intake, and human exposure assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Tenno, T.; Kiis, A. Polycyclic aromatic hydrocarbons (PAHs) in meat products and estimated PAH intake by children and the general population in Estonia. Food Addit. Contam. 2007, 24, 429–437. [Google Scholar] [CrossRef]
- Badibostan, H.; Feizy, J.; Daraei, B.; Shoeibi, S.; Rajabnejad, S.H.; Asili, J.; Taghizadeh, S.F.; Giesy, J.P.; Karimi, G. Polycyclic aromatic hydrocarbons in infant formulae, follow-on formulae, and baby foods in Iran: An assessment of risk. Food Chem. Toxicol. 2019, 131, 110640. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.; Edeme, J.N.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S.; Nwajei, G.E. Polycyclic aromatic hydrocarbon concentrations in commercially available infant formulae in Nigeria: Estimation of dietary intakes and risk assessment. Food Chem. Toxicol. 2014, 72, 221–227. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, M.J.; Shin, H.S. Evaluation of polycyclic aromatic hydrocarbon contents and risk assessment for infant formula in Korea. J. Appl. Biol. Chem. 2016, 57, 173–179. [Google Scholar] [CrossRef]
- Beekman, J.K.; Grassi, K.; MacMahon, S. Updated occurrence of 3-monochloropropane-1,2-diol esters (3-MCPD) and glycidyl esters in infant formulas purchased in the United States between 2017 and 2019. Food Addit. Contam. Part A 2020, 37, 374–390. [Google Scholar] [CrossRef]
- Wöhrlin, F.; Fry, H.; Lahrssen-Wiederholt, M.; Preiß-Weigert, A. Occurrence of fatty acid esters of 3-MCPD, 2-MCPD and glycidol in infant formula. Food Addit. Contam. Part A 2015, 32, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Di Campi, E.; Di Pasquale, M.; Coni, E. Contamination of some foodstuffs marketed in Italy by fatty acid esters of monochloropropanediols and glycidol. Food Addit. Contam. Part A Chem. Anal. Control. 2020, 37, 753–762. [Google Scholar] [CrossRef]
- Jiang, L.; Jing, Z.; Yibaina, W.; Yan, S.; Lili, X.; Yanxu, Z.; Lei, Z. Dietary exposure to fatty acid esters of monochloropropanediols and glycidol of 2- to 3-year-old children attending nursery schools from two areas in China using the duplicate-diet collection method. Food Addit Contam. Part A 2020, 38, 70–80. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieślik, E. Analysis of acrylamide, 3-monochloropropane-1,2-diol, its esters and glycidyl esters in carbohydrate-rich products availale on the Polish market. Rocz. Panstw. Zakl. Hig. 2018, 69, 127–137. [Google Scholar]
- Leigh, J.; MacMahon, S. Occurrence of 3-monochloropropanediol esters and glycidyl esters in commercial infant formulas in the United States. Food Addit. Contam. Part A 2017, 34, 356–370. [Google Scholar] [CrossRef]
- Sui, H.; Gao, H.; Chen, Y.; Ke, R.; Zhong, H.; Zhong, Q.; Song, Y. Survey of mineral oil hydrocarbons in infant formula from the Chinese market. Food Addit. Contam. Part A 2020, 37, 1040–1048. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Li, B.; Xie, Y.; Ouyang, J.; Wu, Y. Concentrations of migrated mineral oil/polyolefin oligomeric saturated hydrocarbons (MOSH/POSH) in Chinese commercial milk powder products. Food Addit. Contam. Part A 2019, 36, 1261–1272. [Google Scholar] [CrossRef]
- Lei, Z.; Hong, Z.; Yan, F.C.; Jing, L.; Dong, A.; Feng, P.; Jian, B.Z.; Huai, N.Z. Risk Assessment of MOAH and MOSH in infants and young children. Biomed. Environ. Sci. 2019, 32, 130–133. [Google Scholar]
- Bodeau-Livinec, F.; Glorennec, P.; Cot, M.; Dumas, P.; Durand, S.; Massougbodji, A.; Ayotte, P.; Le Bot, B. Elevated blood lead levels in infants and mothers in Benin and potential sources of exposure. Int. J. Environ. Res. Public Health 2016, 13, 316. [Google Scholar] [CrossRef]
- Téllez-Rojo, M.M.; Bellinger, D.C.; Arroyo-Quiroz, C.; Lamadrid-Figueroa, H.; Mercado-García, A.; Schnaas-Arrieta, L.; Wright, R.O.; Hernández-Avila, M.; Hu, H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 2006, 118, 323–330. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Murcia, M.; Aguinagalde, X.; Vioque, J.; Rebagliato, M.; Cases, A.; Iňiguez, C.; Lopez-Espinosa, M.J.; Amurrio, A.; Navarrete-Muňoz, M.; et al. Exposure to mercury among Spanish preschool children: Trend from birth to age four. Environ. Res. 2014, 132, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Gundert-Remy, U.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Golka, K.; Röhl, C.; Schupp, T.; Wollin, K.M.; Hengstler, J.G. High exposure to inorganic arsenic by food: The need for risk reduction. Arch. Toxicol. 2015, 89, 2219–2227. [Google Scholar] [CrossRef]
- Bielecka, J.; Markiewicz-Żukowska, R.; Nowakowski, P.; Grabia, M.; Puścion-Jakubik, A.; Mielcarek, K.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Socha, K. Content of toxic elements in 12 groups of rice products available on Polish market: Human health risk assessment. Foods 2020, 9, 1906. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide: A common food toxin related to physiological functions and health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef]
- Schecter, A.; Malik, N.; Haffner, D.; Smith, S.; Harris, T.R.; Paepke, O.; Birnbaum, L. Bisphenol A (BPA) in U.S. food. Environ. Sci. Technol. 2010, 44, 9425–9430. [Google Scholar] [CrossRef]
- Hsu, J.F.; Guo, Y.L.; Liu, C.H.; Hu, S.C.; Wang, J.N.; Liao, P.C. A comparison of PCDD/PCDFs exposure in infants via formula milk or breast milk feeding. Chemosphere 2007, 66, 311–319. [Google Scholar] [CrossRef]
- Bakhiya, N.; Appel, K.E. Toxicity and carcinogenicity of furan in human diet. Arch. Toxicol. 2010, 84, 563–578. [Google Scholar] [CrossRef]
- Kadan, G.; Aral, N. Effects of mycotoxins on child development. Curr. Mol. Pharmacol. 2020, 14, 114. [Google Scholar]
- Jones, J.A.; Hopper, A.O.; Power, G.G.; Blood, A.B. Dietary intake and bio-activation of nitrite and nitrate in newborn infants. Pediatr. Res. 2015, 77, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Fate of Fusarium mycotoxins during processing of Nigerian traditional infant foods (ogi and soybean powder). Food Res. Int. 2018, 116, 408–418. [Google Scholar] [CrossRef] [PubMed]
- McMullen, S.E.; Casanova, J.A.; Gross, L.K.; Schenck, F.J. Ion chromatographic determination of nitrate and nitrite in vegetable and fruit baby foods. J. AOAC Int. 2005, 88, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.F. Methemoglobinemia: Infants at risk. Curr. Probl. Pediatr. Adolesc. Halth Care 2019, 49, 57–67. [Google Scholar] [CrossRef]
- Sosan, M.; Adeleye, A.O.; Oyekunle, J.A.; Udah, O.; Oloruntunbi, P.; Daramola, M.; Saka, W.T. Dietary risk assessment of organochlorine pesticide residues in maize-based complementary breakfast food products in Nigeria. Heliyon 2020, 6, e05803. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Chakraborty, P.; Ray, S. Human exposure to organochlorine, pyrethroid and neonicotinoid pesticides: Comparison between urban and semi-urban regions of India. Environ. Pollut. 2021, 270, 116156. [Google Scholar] [CrossRef]
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food- a review. J Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of commercial and homemade washing agents in removing pesticide residues on and in apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef]
- Grob, K. Mineral oil hydrocarbons in food: A review. Food Addit. Contam. Part A Chem. Anal. Control. 2018, 35, 1845–1860. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, W.; Ye, Z.; Zhang, C. Contamination and spatial variation of heavy metals in the soil-rice system in Nanxun County, Southeastern China. Int. J. Environ. Res. Public Health. 2015, 28, 1577–1594. [Google Scholar] [CrossRef]
- Majumder, S.; Banik, P. Geographical variation of arsenic distribution in paddy soil, rice and rice-based products: A meta-analytic approach and implications to human health. J. Environ. Manag. 2019, 233, 184–199. [Google Scholar] [CrossRef]
- Ratnavathi, C.V.; Komala, V.V.; Kumar, B.S.V.; Das, I.K.; Patil, J.V. Natural occurrence of aflatoxin B1 in sorghum grown in different geographical regions of India. J. Sci. Food Agric. 2012, 92, 2416–2420. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Mari, N.; Goto, T. The relationship between ergosterol and mycotoxin contamination in maize from various countries. Mycotoxin Res. 2015, 31, 91–99. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Venâncio, A.; Lima, N.; Guilloux-Bénatier, M.; Rousseaux, S. Predominant mycotoxins, mycotoxigenic fungi and climate change related to wine. Food Res. Int. 2018, 103, 478–491. [Google Scholar] [CrossRef]
| Type of Contamination | Safe Contamination Levels |
|---|---|
| Acrylamide | RfD = 2 μg/kg/bw |
| Arsenic | PTWI = 15 μg/kg/bw |
| Bisphenol A | TDI = 50 μg/kg/bw |
| Cadmium | MTD = 4.1 μg/day, PTWI = 7 μg/kg/bw |
| Dioxins | TDI = 4 μg/kg/bw, TWI = 2 pg/kg/bw |
| Furan | ADI = 2 μg/kg/bw, RfD = 1 μg/kg/bw |
| Lead | MTD = 0.5 μg/day, PTWI = 25 μg/kg/bw |
| Mercury | PTWI = 1.6 μg/kg/bw |
| Mycotoxins | MCLaflatoxins = 50 ng/kg/bw |
| Nitrates, nitrites | ADItotal = 0–3.7mg/kg/bw |
| Residue pesticides | MRL = 0.1 mg/kg/bw |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Pb, Cd As, Hg | 76 | c Infant formula and milk | No sample was contaminated | Norway [24] |
| Pb and Cd | 564 | a Infant food | Contamination of 37% of samples with Pb and 57% with Cd, RfD exceeded by 0–3%, Pb and Cd by 6–23% | Miami, USA [10] |
| Pb and Cd | 114 | b Baby desserts, juices, dinners | No sample exceeded the MCL | Poland [12] |
| Pb and Cd | 55 | c Infant formula and milk | The median concentration of Pb exceeded the MCL | Brazil [13] |
| Pb and Cd | 26 | a Infant food, b commercial food | Excess of Pb in 96% samples and Cd in 53% of samples | Nigeria [14] |
| Pb and As | 90 | a Infant food | Pb only in 2 samples, As in 3 samples, RfD exceeding of Cd and Pb | Spain [11] |
| Hg | 291 | a Infant food and b commercial food for toddlers | No sample exceeded the MCL | France [16] |
| Hg | 150 | c Infant formula | No sample exceeded the standards | Canada [19] |
| Hg | 45 | b Commercial food for toddlers | 66% of fish samples exceeded the MRL | Menorca, Spain [17] |
| As | 48 | b Commercial food for toddlers | 91% of samples contained inorganic As, 100% exceeded total As | Columbia [22] |
| As | 39 | b Commercial food for toddlers | Contamination of 75% of samples, exceeding the EU maximum levels | Australia [21] |
| Cd | 18 | c Infant food | Cd was exceeded in rice-based products | Sweden [23] |
| ESTIMATION OF INTAKE | ||||
| Pb, Cd and Hg | 119 | b Commercial food for toddlers | Cd and Pb concentrations exceeded TWI (35%, 42%), Hg did not exceed TWI | Korea [18] |
| Pb and Cd | 190 | c Infant formula | 1 sample exceeded the PTWI | Nigeria [9] |
| Cd and Hg | 43 | b Commercial food for toddlers | No sample exceeded the PTWI | Tanzania [21] |
| Hg | 87 | a Infant food | No sample exceeded the PTWI | Portugal [20] |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| 141 | a Infant food, c commercial food | 7% exceeded the RfD | France [26] | |
| Acrylamide | 141 | a Infant food, b commercial food for toddlers | Detected in 80% of samples, exceeded the RfD | France [27] |
| 70 | a Infant food, c commercial food | Exceedance of RfD in baby food | Estonia [28] | |
| ESTIMATION OF INTAKE | ||||
| Acrylamide | 2517 | a Infant food and c commercial food | Exposure of children twice as high as that of adults | USA [25] |
| 111 | a Infant food | Average intake by infants and children exceeded RfD | Poland [29] | |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Bisphenol A | 154 | b Commercial food for toddlers, d commercial food | Detected in 36% of infant food samples, no TDI exceeded | China [33] |
| 122 | a Jarred infant food | Detected in 81% of samples, no MDL exceeded | Canada [30] | |
| 103 | Human milk and c infant formula | Detected in 38% of infant food samples, no RfD exceeded | Spain [31] | |
| 76 | c Infant formula | None detected | China [35] | |
| 68 | c Infant formula | No bisphenol exceedances, no RfD exceeded | India [34] | |
| 50 | c Infant formula | Detected in 60% of infant formula samples | Italy [32] | |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Dioxins | 163 | Commercial food, human milk, infant formula | No exceedance of the upper limit of the standard | Greece [40] |
| ESTIMATION OF INTAKE | ||||
| Dioxins | 180 | a Infant food, b commercial food for toddlers | TDI exceeded in children of 7–12 months by 4.5%, in 13–36-month- olds by 5.1–7.4% | France [41] |
| 63 | a Infant food, b commercial food for toddlers | Consumption is below the TWI | Italy [42] | |
| 60 | a Infant food, c infant formula | No exceedance of the TDI in baby food | Germany [39] | |
| 16 | a Infant food | No exceedance of the TDI in baby food | Spain [37] | |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Furan | 134 | a Infant food, b commercial food for toddlers | Furan found in 84% of samples | France [43] |
| 101 | b Commercial food for toddlers | Contamination of 12% of samples | Taiwan [48] | |
| ESTIMATION OF INTAKE | ||||
| Furan | 301 | a Infant food | EDI exceeded reference dose | Poland [47] |
| 191 | b Commercial food for toddlers, c commercial food | EDI about 3 times higher among infants than adults | China [45] | |
| 78 | a Infant food, b commercial food for toddlers | EDI 3.8 times higher among infants than adults | Belgium [46] | |
| EXPOSURE | ||||
| 230 | a Infant food, b commercial food for toddlers | Medium exposure is not a health risk | Germany [49] | |
| Furan | 76 | a Infant food, b commercial food for toddlers | Meat- and fish-based products potential risk for children | Spain [44] |
| Type of Contamination (Number of Mycotoxins Tested) | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Mycotoxins | 1207 | c Infant formula, milk | Only 1% of samples exceeded the norm | China [60] |
| Mycotoxins (14) | 215 | a Infant food (cereal products) | Contamination of 31% of cereals, 19% of baby cereals; norms were exceeded | Brazil [51] |
| Mycotoxins (1: aflatoxin M1) | 185 | a Infant food (dairy products), c infant formula | 85% of infant formula samples exceeded the MCL | Jordan [54] |
| Mycotoxins (5) | 137 | a Infant food (cereal and nuts products) | Contamination of 42% of baby food samples | Nigeria [56] |
| Mycotoxins (1: aflatoxin M1) | 101 | a Infant food (dairy products), c infant formula | 1 sample of infant formula contaminated | Serbia [59] |
| Mycotoxins (1: aflatoxin M1) | 84 | c Infant formula | Contamination of 3% of samples, norms were not exceeded | Turkey [58] |
| Mycotoxins (1: ochratoxin A) | 64 | a Infant food (cereal products) | Contamination of 41% of cereal samples | Iran [55] |
| Mycotoxins (5) | 60 | a Infant food (cereal products) | Contamination of 20% of cereal samples, of which 10% exceeded maximum level | Spain [52] |
| ESTIMATION OF INTAKE | ||||
| Mycotoxin (1: Deoxynivalenol) | 3309 | a Infant food, c infant formula, milk | Average exposure twice as high as TDI | Norway [57] |
| Mycotoxins (1: patulin) | 610 | b Commercial food for toddlers (apple-based products) | No exceedances of PMTDI standards | Qatar [50] |
| Mycotoxins (5) | 302 | a Infant food and b commercial food for toddlers (cereal products) | No exceedances of TDI standards | Poland [53] |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Nitrites, nitrates | 1319 | b Commercial food for toddlers, c commercial food | ADI of nitrite was exceeded in 16% of infant and 58% of toddler food samples | France [67] |
| Nitrites, nitrates, N-nitrosoamines | 315 | b Commercial food for toddlers (meat) | ADI of nitrite was exceeded in 40% and 29% of child food samples at different times | Estonia [68] |
| Nitrites, nitrates | 157 | b Commercial food for toddlers (meat) | ADI of nitrite was exceeded in 3% of child food samples | Estonia [66] |
| Nitrates, nitrites | 108 | a Infant food, c commercial food (vegetable, fruit, cereals and milk based) | Average nitrite content in the upper limit of the standard | Fiji [65] |
| ESTIMATION OF INTAKE | ||||
| Nitrates | 1150 | b Commercial food for toddlers (vegetable based), vegetables | No exceedance of the maximum permissible dose | Spain [62] |
| Nitrites, nitrates | 104 | a Infant food, c commercial food | No exceedance of the maximum permissible dose | Italy [63] |
| Nitrates | 80 | a Infant food (vegetable based) | Only 1 sample exceeded the ADI | Portugal [61] |
| Nitrates | 39 | a Infant food (vegetable, meat- based) | No exceedance of the maximum permissible dose | Portugal [64] |
| Type of Contamination (Number of Pesticides Tested) | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Pesticides (86) | 522 | a Infant food (fruit based) | Detected in 60% of samples, 1.4% exceeded the MRL | Czech Republic [70] |
| Pesticides (516) | 309 | b Commercial food for toddlers, c commercial food | Detected in 67% of samples, exceeded the TRV | France [69] |
| Pesticides: (1: imidacloprid) | 250 | a Infant food, fruit, vegetables, cereal | Detected in 15% of samples, exceeded the MRL | India [72] |
| Pesticides (4: glyphosate, glufosinate, perchlorate, chlorate) | 105 | a Infant food, c commercial food | Perchlorate detected in 10.5% of samples | Italy [75] |
| Pesticides (18) | 100 | c Commercial food (homemade) | Detected in 100% of samples | Korea [71] |
| Pesticides (69) | 54 | a Infant food (juices, purees) | Detected in 56% of samples | Serbia [72] |
| Pesticides (12) | 33 | a Infant food (juices, multi-fruit jars) | Three pesticides detected in 60% of samples | Spain and United Kingdom [74] |
| Type of Contamination | Number of Samples | Type of food Samples | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| PAH | 126 | b Commercial food (meat, fish) | No exceedances in meat and fish, exceedances in dairy products | Italy [77] |
| 40 | a Infant food, b commercial food (dairy products) | Exceedance in dairy samples 18.2%, meat and fish 5.6% | Italy [76] | |
| ESTIMATION OF INTAKE | ||||
| PAH | 322 | b Commercial food (meat) | No exceedances in average PAH intake | Estonia [78] |
| 42 | a Infant food | One sample exceeding MCL | Iran [79] | |
| EXPOSURE | ||||
| PAH | 152 | c Infant formula | No exceedances (MOE > 10,000) | Korea [81] |
| 40 | c Infant formula | No exceedances (MOE > 10,000) | Nigeria [80] | |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| Glycidyl esters, 3-MCPD | 275 | a Infant food (homemade), c commercial food for toddlers | Over 71% of diet samples contaminated with 3-MCPD and glycidyl esters | China [85] |
| Glycidyl esters, 3-MCPD | 222 | a Infant food | Lower concentrations of 3-MCPD (7-fold) and glycidyl esters (3-fold) during 3 years | USA [82] |
| Glycidyl esters, 3-MCPD | 130 | a Infant food, c commercial food | Products containing palm oil had a higher concentration of 3-MCPD and glycidyl esters | Italy [84] |
| Glycidyl esters, 3-MCPD | 96 | a Infant food | Lowest concentrations in products containing palm oil | USA [87] |
| Glycidyl esters, 2-MCPD, 3-MCPD | 77 | a Infant food | 2-MCPD detected in all samples | Germany [83] |
| EXPOSURE | ||||
| 3-MCPD | 60 | c Commercial food for toddlers | Exceeded TDIs in potato chips, crackers, peanuts, muesli, and biscuits | Poland [86] |
| Type of Contamination | Number of Samples | Type of Food Sample | Results | Country [Reference] |
|---|---|---|---|---|
| CONTAMINANT CONTENT | ||||
| MOH | 51 | c Infant formula | MOH detected in 33% | China [88] |
| 50 | c Infant formula | MOH detected in 66% | China [89] | |
| 16 | a Infant food | MOH detected in all samples containing meat and fish | Italy [8] | |
| EXPOSURE | ||||
| MOH | 230 | a Infant food, b commercial food for toddlers, c infant formula | Highest risk of MOH exposure among infants | China [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielech, A.; Puścion-Jakubik, A.; Socha, K. Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients 2021, 13, 2358. https://doi.org/10.3390/nu13072358
Mielech A, Puścion-Jakubik A, Socha K. Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients. 2021; 13(7):2358. https://doi.org/10.3390/nu13072358
Chicago/Turabian StyleMielech, Anita, Anna Puścion-Jakubik, and Katarzyna Socha. 2021. "Assessment of the Risk of Contamination of Food for Infants and Toddlers" Nutrients 13, no. 7: 2358. https://doi.org/10.3390/nu13072358
APA StyleMielech, A., Puścion-Jakubik, A., & Socha, K. (2021). Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients, 13(7), 2358. https://doi.org/10.3390/nu13072358