Abstract
Kidney transplant recipients are at high risk of progressive bone loss and low-energy fractures in the years following transplantation. Marine n-3 polyunsaturated fatty acids (n-3 PUFA) supplementation may have beneficial effects on bone strength. The Omega-3 fatty acids in Renal Transplantation (ORENTRA) trial was an investigator initiated, randomized, placebo-controlled trial investigating the effects of marine n-3 PUFA supplementation after kidney transplantation. Effects of supplementation on bone mineral density (BMD) and calcium metabolism were pre-defined secondary endpoints. Adult kidney transplant recipients (n = 132) were randomized to 2.6 g marine n-3 PUFA supplement or olive oil (control) from 8 to 52 weeks post-transplant. Dual energy X-ray absorptiometry was performed to assess changes in bone mineral density of hip, spine, and forearm, as well as trabecular bone score (TBS) of the lumbar spine. Student’s t test was used to assess between-group differences. There were no differences in ΔBMD between the two groups (intervention vs. control) at lumbar spine (−0.020 ± 0.08 vs. −0.007 ± 0.07 g/cm², p = 0.34), total hip (0.001 ± 0.03 vs. −0.005 ± 0.04, p = 0.38), or other skeletal sites in the intention-to-treat analyses. There was no difference in the change in TBS score (0.001 ± 0.096 vs. 0.009 ± 0.102, p = 0.62). Finally, no effect on biochemical parameters of mineral metabolism was seen. Results were similar when analyzed per protocol. In conclusion, we found no significant effect of 44 weeks of supplementation with 2.6 g of marine n-3 PUFA on BMD in kidney transplant recipients.
1. Introduction
Kidney transplant recipients are at high risk of fracture in the years following kidney transplantation [1,2,3]. Contributors include traditional risk factors such as age, gender [4], and ethnicity [5], pre-existing renal bone disease [6], ongoing disturbances of calcium- and phosphate-metabolism [7], pre-existing or new-onset diabetes mellitus, [8] and immunosuppressive therapy [5,9].
Marine n-3 polyunsaturated fatty acids (n-3 PUFA) are essential fatty acids with known anti-inflammatory properties [10]. n-3 PUFA may also have beneficial effects in bone [11], as in vivo studies have demonstrated inhibition of osteoclasts [12,13], and stimulation of osteoblasts [14], which could potentially translate into reduced bone resorption and increased bone mineralization. High levels of plasma marine n-3 PUFA was also associated with higher bone mineral density (BMD) [15,16] and a reduced risk of fractures [17]. Few studies have been published regarding the effect of n-3 PUFA supplementation on BMD and with inconsistent results reported [18,19]. Increased hip BMD was seen in elderly women treated for 18 months with 6 g of mixed oils, of which 420 mg were marine n-3 PUFA [18]. However, no effect was found of 12 months supplementation with 440 mg of marine fish oil on whole body BMD in pre- or post-menopausal women [19]. A positive association between plasma concentrations of marine n-3 PUFA and BMD was previously reported in kidney transplant recipients [20,21]; but no studies have yet investigated the effect of n-3 PUFA supplementation on bone metabolism in patients with chronic kidney disease.
The aim of this study was to investigate the effects of a moderate to high (≈2.6 g daily) supplement with marine n-3 PUFA on bone mass and mineral metabolism, as pre-defined secondary endpoints of a randomized, placebo-controlled trial in kidney transplant recipients.BMD determined at multiple sites, as well as trabecular bone score (TBS) of the lumbar spine, were included in the skeletal assessment.
2. Materials and Methods
2.1. Study Design and Cohort
This was a secondary endpoint analysis of the Omega-3 fatty acids in Renal Transplantation (ORENTRA) study, a randomized, double-blinded trial, conducted at the national transplant center at Oslo University Hospital Rikshospitalet in Norway between 2013 and 2015. A detailed description of inclusion and exclusion criteria has been published previously [22]. In brief, adult kidney transplant recipients with stable kidney function (>30 mL/min/1.73 m²) providing written, informed consent were included in the early post-transplant phase. Exclusion criteria were allergies to seafood or fish oil or kidney donor age >75 years. Participants (n = 132) were randomly assigned to receive either ≈2.6 g n-3 PUFA supplements (460 mg/g Eicosapentaenoic acid (EPA) + 380 mg/g Docosahexaenoic acid (DHA) (Omacor®, Pronova Biopharma, Oslo, Norway)), or extra virgin olive oil, given as 1 capsule of 1 g, three times daily. Treatment was initiated eight weeks post-transplant, and trial duration was 44 weeks. Thirty patients were excluded or withdrew from the study due to (treatment vs. control group): screening failure (1 vs. 1), gastrointestinal discomfort (9 vs. 8), serious adverse event (4 vs. 5), or dropout (2 vs. 0).
2.2. Immunosuppressive Protocol
Patients received induction therapy with basiliximab, followed by maintenance immunosuppressive therapy with prednisolone, mycophenolate, and the calcineurin inhibitor tacrolimus. One dose of methylprednisolone was given at the time of transplantation, followed by prednisolone 20 mg daily (day 0 to 14) tapered gradually to 5 mg per day at 6 months post-transplant. Tacrolimus dosage was adjusted according to trough concentrations, with a target of 3 to 7 μg/L. Prophylactic treatment with trimethoprim-sulfamethoxazole was used for 6 months, and valganciclovir was given to cytomegalovirus seronegative recipients with seropositive donors.
2.3. Biochemical Analyses
Fasting blood samples were drawn at the baseline visit and the last follow-up visit. Plasma intact parathyroid hormone (iPTH), phosphate, and total and ionized calcium were measured by Roche Modular E170 until 2016, then by the Roche Cobas e602 platform (Roche Diagnostics, Basel, Switzerland). All analyses were performed by an accredited laboratory (Department of Medical Biochemistry, Oslo University Hospital Rikshospitalet, Norway) according to standardized method protocols. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, adjusting for age, gender, ethnicity, height, and weight.
Samples for plasma n-3 PUFA measurements were centrifuged, frozen, and stored at 80 °C at the Laboratory of Renal Physiology Biobank at Oslo University Hospital, Rikshospitalet, Norway. Aliquots of bio-banked plasma were sent to The Lipid Research Center, Aalborg University Hospital, Denmark, for fatty acid (FA) analysis, which was performed in four steps: (1) extraction of total lipids, performed by a modified Folch method [23]; (2) isolation of the phospholipid fraction as described by Burdge [24]; (3) transmethylation of phospholipid FAs, and finally; (4) quantification of FAs using a Varian 3900 gas chromatograph with a CP-8400 autosampler, a flame ionization detector and a CP-Sil 88 60 m × 0.25 mm capillary column (Varian, Middleburg, The Netherlands). FAs were identified from their relative retention time, and quantitated as the weight percent of total fatty acids (wt%). Total marine n-3 PUFA level was defined as the sum of EPA and DHA. Coefficients of variation (CV) for the analyses of EPA and DHA were 1.1% and 1.8%, respectively.
2.4. Bone Density
A dual energy X-ray absorptiometry (DXA) scan (GE Medical Systems, Lunar Corp., Madison, WI, USA) was performed at baseline and end of study to determine BMD at whole body, lumbar spine, proximal femur (total hip and femoral neck), and the non-dominant forearm (proximal and ultra-distal radius). Bone density is reported as absolute BMD in g/cm², with the addition of T-, and Z-scores calculated from normative data provided by the manufacturer. The Lunar reference database has previously been validated for clinical use in this population [25]. Standard imaging and positioning protocols were used, and all scans and subsequent analyses were performed by Certified Densitometry Technologists (CDT, The International Society for Clinical Densitometry, Middletown, CT, USA). Quality assurance check was carried out twice weekly, using an aluminum spine phantom in water (Lunar 17810, GE Medical Systems, Madison, WI, USA). Short- and long-term CV were 0.8% and 1.4%, respectively. The TBS parameter was retrospectively extracted from the DXA images of lumbar spine L1–L4, by using the TBS iNsight software v2.1.2.0 (Medimaps Group SA, Geneva, Switzerland). TBS at L1–L4 has a short-term in vivo precision of 1.1% to 1.9%.
2.5. Ethics
The ORENTRA trial was approved by the Regional Committees for Medical and Health Research Ethics (identifier 2012/1419) and The Norwegian Medicines Agency, and was performed in accordance with Good Clinical Practice and the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (identifier NCT01744067), and the European Union Drug Regulating Authorities Clinical Trials Database (identifier 2012-004992-37; 5 February 2013).
2.6. Statistics
Data are expressed as mean ± standard deviation (SD), median with interquartile range (IQR), or n (%) as appropriate. Endpoints were analyzed in both the intention-to-treat (ITT) and per-protocol (PP) populations. Normality was assessed by qq-plots and the Shapiro-Wilk normality test. Differences in Δ values between groups were evaluated by Student’s t test, and associations between continuous variables were evaluated by Spearman’s correlations. Skewed data were transformed to their natural logarithm to enable parametrical testing. For all analyses, a two-sided p value < 0.05 was considered statistically significant. Statistical analysis was performed using software package Stata/IC 13.1 for Windows (StataCorp LP, College Station, TX, USA).
3. Results
Baseline demographic variables are presented in Table 1. Two-thirds of patients received dialysis therapy prior to transplantation, either in the form of chronic intermittent hemodialysis (n = 60, median duration 17 months, range 1 to 61), or peritoneal dialysis (n = 31, median duration of 10 months, range 1 to 34). Delayed graft function was seen in 11.4%.

Table 1.
Baseline data of participating adult kidney transplant recipients.
Baseline plasma levels of n-3 PUFA were positively correlated with age (Spearman’s rho = 0.42, p < 0.001), and negatively correlated with eGFR (rho = −0.18, p = 0.04). There were no associations with gender, body mass index, diabetes mellitus prior to transplantation, new onset diabetes mellitus, or biochemical measures of calcium metabolism. Baseline n-3 PUFA content in plasma phospholipids was positively correlated with Z-scores of L1-L4 lumbar spine (rho = 0.26, p = 0.003), total hip (rho = 0.24, p = 0.007) and femoral neck (rho = 0.19, p = 0.03), but not with Z-scores of whole body (rho = 0.08, p = 0.36), proximal radius (rho = 0.11, p = 0.23), or distal radius (rho = 0.14, p = 0.13). There was also no significant correlation between n-3 PUFA and the L1–L4 TBS (rho = −0.05, p = 0.57).
Table 2 shows changes in plasma marine n-3 PUFA levels in relation to biochemical markers of calcium metabolism, and BMD after 44 weeks. Supplementation resulted in a significant increase in plasma n-3 PUFA content, from 6.04 to 10.7 wt% (p < 0.001) in the intervention group, with no change in the control group (6.02 to 6.27 wt%, p = 0.43). Plasma levels of iPTH, calcium, and phosphate were unaffected by marine n-3 PUFA supplementation.

Table 2.
Effects of marine n-3 polyunsaturated fatty acid supplementation on bone mineral density in adult kidney transplantation recipients, by intention to treat and per protocol analyses.
There were no differences in ΔBMD at the whole body, lumbar spine, proximal femur, or forearm between the two groups (Figure 1), and the same was true for Δ-values of the lumbar spine TBS score.
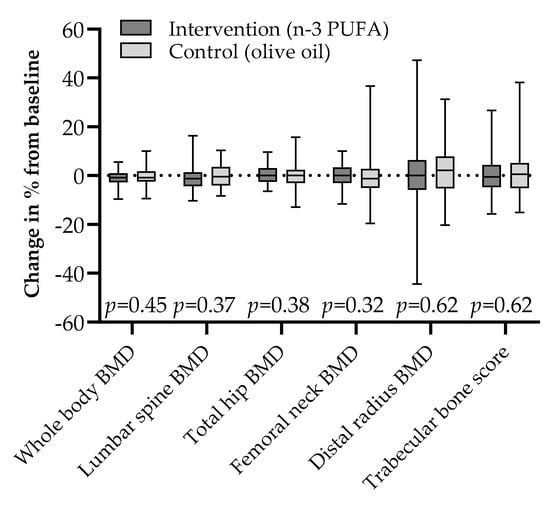
Figure 1.
Changes in bone mineral density and trabecular bone score by intention to treat after 44 weeks of 2.6 g daily supplementation with marine n-3 polyunsaturated fatty acids or olive oil in adult kidney transplant recipients; median (line) with interquartile range (box) and full range (whiskers).
Further, we found no significant correlations between the increase in plasma level of marine n-3 PUFA and ΔBMD, at the lumbar spine, the total hip, or the distal forearm (Figure 2). A significant inverse correlation was found between baseline plasma levels of marine n-3 PUFA and change in lumbar spine BMD (r = −0.25, p = 0.006; Supplementary Figure S1). However, no significant between-group differences were seen when restricting analyses to patients with below median levels of marine n-3 PUFA at baseline (Supplementary Table S1). ΔBMD of other skeletal sites measured were not associated with baseline n-3 PUFA levels.
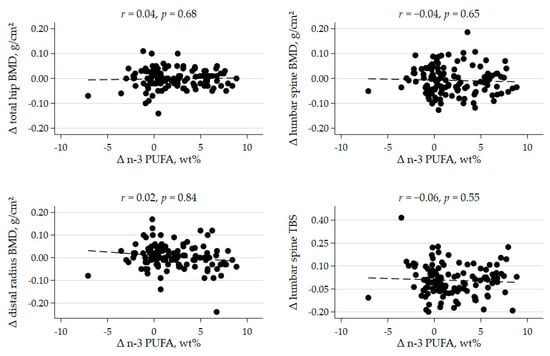
Figure 2.
Scatterplots of the correlation between change in plasma marine n-3 polyunsaturated fatty acid (n-3 PUFA) concentration and change in bone mineral density (BMD) and trabecular bone score (TBS) after 44 weeks, r = Pearson’s correlation coefficient, with corresponding p value.
4. Discussion
We found no effect of a 2.6 g daily combined EPA + DHA supplement on BMD or biochemical markers of calcium metabolism. These were pre-specified, secondary endpoints of the ORENTRA trial, and to our knowledge this is the first randomized, placebo-controlled trial to consider the effects of marine n-3 PUFA on bone disease and mineral metabolism after kidney transplantation.
A priori there were indications that marine n-3 PUFA might positively affect bone strength and reduce fracture risk in kidney transplant recipients. Direct effects of EPA and DHA on bone cell maturation, function, and apoptosis, favoring bone formation over resorption, have been demonstrated in several in vitro studies [12,13,14]. Positive effects of n-3 PUFA supplementation on bone mass, BMD, and bone strength have also been consistent findings in animal models [26]. Further, an indirect effect of n-3 PUFA on bone through increased calcium absorption in the intestines has been reported in experimental models [27,28]. In our previous observational study, a positive correlation was found between plasma n-3 PUFA and total calcium levels in kidney transplant recipient, which might support a similar mechanism in humans [21]. However, in our present interventional study, we were unable to demonstrate an effect of marine n-3 PUFA supplementation on biochemical measures of mineral metabolism. We also found no effect of this intervention on the TBS score, a gray-scale textural analysis of DXA images of the lumbar spine. The TBS is an index of trabecular microstructure, with the potential to deliver information on bone quality which is not readily captured by BMD [29].
The possibility of a threshold effect of n-3 PUFA on BMD has been proposed as an explanation for inconsistent results in observational studies. Two large cohorts from regions of low fish intake, the Women’s Health Initiative (WHI) [30] and the UK Framingham Osteoporosis study (FOS) [31], reported no associations between measured levels of n-3 PUFA and BMD. In contrast, positive correlations were found between n-3 PUFA levels and peak bone mass in young Swedish men [15], and total hip T-scores in Korean women [16]. Similarly, we found positive correlations between marine n-3 PUFA levels and baseline Z-scores in our study. It is possible that a significant proportion of our patients were already above a threshold of marine n-3 PUFA optimal for bone health, and that further supplementation had little potential to provide additional benefit.
Two previous studies investigated the association between n-3 PUFA and BMD after kidney transplantation. Baggio et al. reported a positive association between change in plasma n-3 PUFA level and ΔBMD over a two-year period [20], in a small study of 19 kidney transplant recipients. We could not confirm this association in our present study, though we did explore correlations between increase in plasma n-3 PUFA and change in BMD. In our previous study, we reported a positive association between plasma values of n-3 PUFA and BMD Z-scores of spine and hip in a large cohort of kidney transplant recipients at 6–8 weeks post-transplant [21]. This association was robust despite adjustment for multiple potential confounders; however, regression coefficients indicated a very modest effect-size. Our present study sample size may therefore not have been large enough, and the time frame of 44 weeks may also have been too short, to detect modest changes in BMD.
A recent meta-analysis summarized interventional trials investigating the effect of n-3, n-6 and a mixture of PUFAs on musculoskeletal health. The authors concluded that n-3 PUFA supplementation may result in a small (2.6%) increase in lumbar spine BMD, based on the combined results of five studies totaling 463 participants. No significant effect was seen on femoral neck BMD [32]. However, the overall level of evidence of the included studies in this meta-analysis was considered of low to very low quality, and the authors’ note that there was considerable heterogeneity both in the populations studied, and the doses of n-3 PUFA used. The only trial with a low risk of bias was on an Australian cohort of 202 patients with knee osteoarthritis randomized to high (4.5 g EPA + DHA) or low (0.45 g) dose n-3 PUFA supplementation. In this study, no effect of high dose n-3 PUFA was found on lumbar spine or femoral neck BMD after two years of treatment [33].
Study design and the complete follow-up of the cohort are strengths of this study. When considering BMD as an endpoint, the follow-up time was rather short, particularly as recent studies indicate highly variable changes in BMD by DXA during the first year post-transplant [34,35]. The clinical value of BMD monitoring by DXA-scans within a time-period of 1–3 years is debated [36,37], but on the other hand, recent international guidelines do suggest repeated DXA 1 year after initiation of therapy [38]. An even longer time-interval may be necessary to detect changes in the lumbar spine TBS, as the least significant change of this parameter is reported to be higher than that of BMD using the same DXA equipment [39]. Norwegians are known for a high-intake of fish, which may have diluted our intervention and potentially masked a true association. Thus, results may not be applicable to other populations with lower intakes of seafood. The supplementation protocol did, however, succeed in achieving a sizeable increase in plasma n-3 PUFA content, with a significant between-group difference after 44 weeks. Finally, the size of our cohort may not have been large enough to detect a modest effect of marine n-3 PUFA supplementation on BMD.
5. Conclusions
Our findings do not support recommending a supplement of marine n-3 PUFA to benefit bone health during the first year after kidney transplantation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13072361/s1, Figure S1: The association between baseline plasma phospholipid content of n-3 PUFA and change in lumbar spine bone mineral density (BMD) during the first year post-transplant. Table S1: Sensitivity analyses on threshold effect of polyunsaturated fatty acids (n-3 PUFA) on bone mineral density in kidney transplant recipients.
Author Contributions
Conceptualization, M.S., I.A.E. and H.S.J.; methodology, M.S., J.B., K.G., E.B.S.; software, K.G., H.S.J.; validation, E.B.S. and K.G.; formal analysis, H.S.J.; investigation, I.A.E., M.S., T.J.; resources, T.J., A.Å., J.B., A.H., E.B.S.; writing—original draft preparation, H.S.J., M.S.; writing—review and editing, H.S.J., I.A.E., T.J., A.Å., J.B., K.G., A.H., E.B.S., M.S.; supervision, M.S., I.A.E.; project administration, I.A.E., T.J., A.Å., M.S.; funding acquisition, I.A.E., M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the South-Eastern Norway Regional Health Authority; Gidske and Peter Jacob Sørensen Research Fund; the Norwegian National Association for Kidney Patients and Transplant Recipients Research Fund; the Raagholt Foundation; the Freia Corporation Medical Fund; Nathalia and Knut Juul Christensen Research Fund; Signe and Albert Bergsmarken Research Fund; Gertrude and Jack Nelsons Research Fund. Pronova Biopharma provided study drugs. Neither funding organizations nor Pronova Biopharma had any role in study design, data collection, data analysis and interpretation, manuscript preparation, or decision to submit this manuscript.
Institutional Review Board Statement
The ORENTRA trial was approved by the Regional Committees for Medical and Health Research Ethics (identifier 2012/1419) and The Norwegian Medicines Agency, and was performed in accordance with Good Clinical Practice and the Declaration of Helsinki. The clinical trial identifier was NCT01744067 (Clinical.Trials.gov) and the European Union Drug Regulating Authorities Clinical Trials Database identifier was 2012-004992-37.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy of participants.
Acknowledgments
We acknowledge the skilled assistance of May Ellen Lauritsen, Kirsten Lund, Els Breistein, and Sebastian Müller at The Renal Physiology Laboratory, Oslo University Hospital, as well as Rikke Eschen, Birthe Thomsen, and Anne-Mette Christensen at The Lipid Research Laboratory, Aalborg University Hospital. We sincerely thank all patients for their participation in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, D.; Olesen, J.B.; Gislason, G.; Abrahamsen, B.; Hommel, K. Risk of fracture in adults on renal replacement therapy: A Danish national cohort study. Nephrol. Dial. Transplant. 2016, 31, 1654–1662. [Google Scholar] [CrossRef]
- Vautour, L.M.; Melton, L.J.; Clarke, B.L.; Achenbach, S.J.; Oberg, A.L.; McCarthy, J.T. Long-term fracture risk following renal transplantation: A population-based study. Osteoporos. Int. 2004, 15, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ramsey-Goldman, R.; Dunn, J.E.; Dunlop, D.D.; Stuart, F.P.; Abecassis, M.M.; Kaufman, D.B.; Langman, C.B.; Salinger, M.H.; Sprague, S.M. Increased Risk of Fracture in Patients Receiving Solid Organ Transplants. J. Bone Miner. Res. 1999, 14, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kwan, J.T.C.; McCloskey, E.; McGee, G.; Thomas, G.; Johnson, D.; Wills, R.; Ogunremi, L.; Barron, J. Prevalence and Causes of Low Bone Density and Fractures in Kidney Transplant Patients. J. Bone Miner. Res. 2001, 16, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Nikkel, L.E.; Hollenbeak, C.S.; Fox, E.J.; Uemura, T.; Ghahramani, N. Risk of Fractures After Renal Transplantation in the United States. Transplantation 2009, 87, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Stehman-Breen, C.O.; Sherrard, D.J.; Alem, A.M.; Gillen, D.L.; Heckbert, S.R.; Wong, C.S.; Ball, A.; Weiss, N.S. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int. 2000, 58, 2200–2205. [Google Scholar] [CrossRef]
- Perrin, P.; Caillard, S.; Javier, R.M.; Braun, L.; Heibel, F.; Borni-Duval, C.; Muller, C.; Olagne, J.; Moulin, B. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am. J. Transplant. 2013, 13, 2653–2663. [Google Scholar] [CrossRef]
- Nisbeth, U.; Lindh, E.; Ljunghall, S.; Backman, U.; Fellström, B. Increased Fracture Rate in Diabetes Mellitus And Females After Renal Transplantation. Transplantation 1999, 67, 1218–1222. [Google Scholar] [CrossRef]
- Nikkel, L.E.; Mohan, S.; Zhang, A.; McMahon, D.J.; Boutroy, S.; Dube, G.; Tanriover, B.; Cohen, D.; Ratner, L.; Hollenbeak, C.S.; et al. Reduced Fracture Risk With Early Corticosteroid Withdrawal After Kidney Transplant. Am. J. Transplant. 2012, 12, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutriments 2019, 11, 2974. [Google Scholar] [CrossRef] [Green Version]
- Bao, M.; Zhang, K.; Wei, Y.; Hua, W.; Gao, Y.; Li, X.; Ye, L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2019, 53, e12735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coetzee, M.; Haag, M.; Joubert, A.; Kruger, M. Effects of arachidonic acid, docosahexaenoic acid and prostaglandin E2 on cell proliferation and morphology of MG-63 and MC3T3-E1 osteoblast-like cells. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Pierson, D.; Mehta, S.; Gonda, S.; Smith, S.M. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-kappaB activation: From cells to bed rest to astronauts. J. Bone Miner. Res. 2009, 25, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Casado-Díaz, A.; Santiago-Mora, R.; Dorado, G.; Quesada-Gómez, J.M. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: Potential implication in osteoporosis. Osteoporos. Int. 2012, 24, 1647–1661. [Google Scholar] [CrossRef]
- Hogstrom, M.; Nordström, P.; Nordström, A. n−3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 Study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.-J.; Kim, T.-H.; Byun, D.-W.; Park, Y. Positive Correlation between Erythrocyte Levels of n–3 Polyunsaturated Fatty Acids and Bone Mass in Postmenopausal Korean Women with Osteoporosis. Ann. Nutr. Metab. 2012, 60, 146–153. [Google Scholar] [CrossRef]
- Harris, T.B.; Song, X.; Reinders, I.; Lang, T.F.; Garcia, E.M.; Siggeirsdottir, K.; Sigurdsson, S.; Gudnason, V.; Eiriksdottir, G.; Sigurdsson, G.; et al. Plasma phospholipid fatty acids and fish-oil consumption in relation to osteoporotic fracture risk in older adults: The Age, Gene/Environment Susceptibility Study. Am. J. Clin. Nutr. 2015, 101, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, M.C.; Coetzer, H.; De Winter, R.; Gericke, G.; Van Papendorp, D.H. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging 1998, 10, 385–394. [Google Scholar] [CrossRef]
- Bassey, E.J.; Littlewood, J.J.; Rothwell, M.C.; Pye, D.W. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre- and postmenopausal women:two randomized controlled trials of Efacal® v. calcium alone. Br. J. Nutr. 2000, 83, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggio, B.; Budakovic, A.; Ferraro, A.; Checchetto, S.; Priante, G.; Musacchio, E.; Manzato, E.; Zaninotto, M.; Maresca, M.-C. Relationship between Plasma Phospholipid Polyunsaturated Fatty Acid Composition and Bone Disease in Renal Transplantation. Transplantation 2005, 80, 1349–1352. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Eide, I.A.; Hartmann, A.; Åsberg, A.; Christensen, J.H.; Schmidt, E.B.; Godang, K.; Bollerslev, J.; Svensson, M. Plasma n-3 Polyunsaturated Fatty Acids and Bone Mineral Density in Renal Transplant Recipients. J. Ren. Nutr. 2016, 26, 196–203. [Google Scholar] [CrossRef]
- Eide, I.A.; Reinholt, F.P.; Jenssen, T.; Hartmann, A.; Schmidt, E.B.; Åsberg, A.; Bergan, S.; Brabrand, K.; Svensson, M. Effects of marine n-3 fatty acid supplementation in renal transplantation: A randomized controlled trial. Am. J. Transplant. 2019, 19, 790–800. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wright, P.; Jones, A.E.; Wootton, S. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000, 84, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Gjesdal, C.G.; Aanderud, S.J.; Haga, H.-J.; Brun, J.G.; Tell, G.S. Femoral and whole-body bone mineral density in middle-aged and older Norwegian men and women: Suitability of the reference values. Osteoporos. Int. 2004, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.; Cohen, D.J.; Ward, W.E.; Ma, D.W. Investigating the Role of Polyunsaturated Fatty Acids in Bone Development Using Animal Models. Molecules 2013, 18, 14203–14227. [Google Scholar] [CrossRef] [Green Version]
- Haag, M.; Magada, O.N.; Claassen, N.; Böhmer, L.H.; Kruger, M.C. Omega-3 fatty acids modulate ATPases involved in duodenal Ca absorption. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 423–429. [Google Scholar] [CrossRef]
- Coetzer, H.; Claassen, N.; van Papendorp, D.; Kruger, M. Calcium transport by isolated brush border and basolateral membrane vesicles: Role of essential fatty acid supplementation. Prostaglandins Leukot. Essent. Fat. Acids 1994, 50, 257–266. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.; Leslie, W.; Hans, D.; Johansson, H.; Kanis, J.A. Adjusting Fracture Probability by Trabecular Bone Score. Calcif. Tissue Int. 2015, 96, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Ing, S.W.; Lu, B.; Belury, M.A.; Johnson, K.; Wactawski-Wende, J.; Jackson, R.D. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J. Bone Miner. Res. 2012, 28, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, E.K.; Kiel, D.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The framingham osteoporosis study. J. Bone Miner. Res. 2012, 27, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, A.; the PUFAH Group; Hooper, L.; Sivakaran, R.; Hayhoe, R.P.G.; Welch, A. The Relationship Between Omega-3, Omega-6 and Total Polyunsaturated Fat and Musculoskeletal Health and Functional Status in Adults: A Systematic Review and Meta-analysis of RCTs. Calcif. Tissue Int. 2019, 105, 353–372. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Hill, C.L.; Lester, S.; Ruediger, C.D.; Battersby, R.; Jones, G.; Cleland, L.G.; March, L.M. Supplementation with omega-3 fish oil has no effect on bone mineral density in adults with knee osteoarthritis: A 2-year randomized controlled trial. Osteoporos. Int. 2015, 27, 1897–1905. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Cavalier, E.; Kuypers, D. Natural history of mineral metabolism, bone turnover and bone mineral density in de novo renal transplant recipients treated with a steroid minimization immunosuppressive protocol. Nephrol. Dial. Transplant. 2018, 35, 697–705. [Google Scholar] [CrossRef]
- Smerud, K.T.; Dolgos, S.; Olsen, I.C.; Åsberg, A.; Sagedal, S.; Reisaeter, A.V.; Midtvedt, K.; Pfeffer, P.; Ueland, T.; Godang, K.; et al. A 1-Year Randomized, Double-Blind, Placebo-Controlled Study of Intravenous Ibandronate on Bone Loss Following Renal Transplantation. Am. J. Transplant. 2012, 12, 3316–3325. [Google Scholar] [CrossRef]
- Bell, K.J.L.; Hayen, A.; Macaskill, P.; Irwig, L.; Craig, J.; Ensrud, K.; Bauer, D.C. Value of routine monitoring of bone mineral density after starting bisphosphonate treatment: Secondary analysis of trial data. BMJ 2009, 338, b2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, N.B.; Lewiecki, E.M.; Bonnick, S.L.; Laster, A.J.; Binkley, N.; Blank, R.D.; Geusens, P.P.; Miller, P.D.; Petak, S.M.; Recker, R.R.; et al. Clinical Value of Monitoring BMD in Patients Treated with Bisphosphonates for Osteoporosis. J. Bone Miner. Res. 2009, 24, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Compston, J.; Carey, J.J.; Wu, C.-H.; Ibrahim, A.; Lewiecki, E.M. Repeating Measurement of Bone Mineral Density when Monitoring with Dual-energy X-ray Absorptiometry: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Schwartz, E.N.; Chung, Y.-S.; Lewiecki, E.M. Dual-energy X-ray Absorptiometry Monitoring with Trabecular Bone Score: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 501–505. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).