Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland—A New Insight into Stroke Pathophysiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection and Analysis
2.2. Statistical Analysis
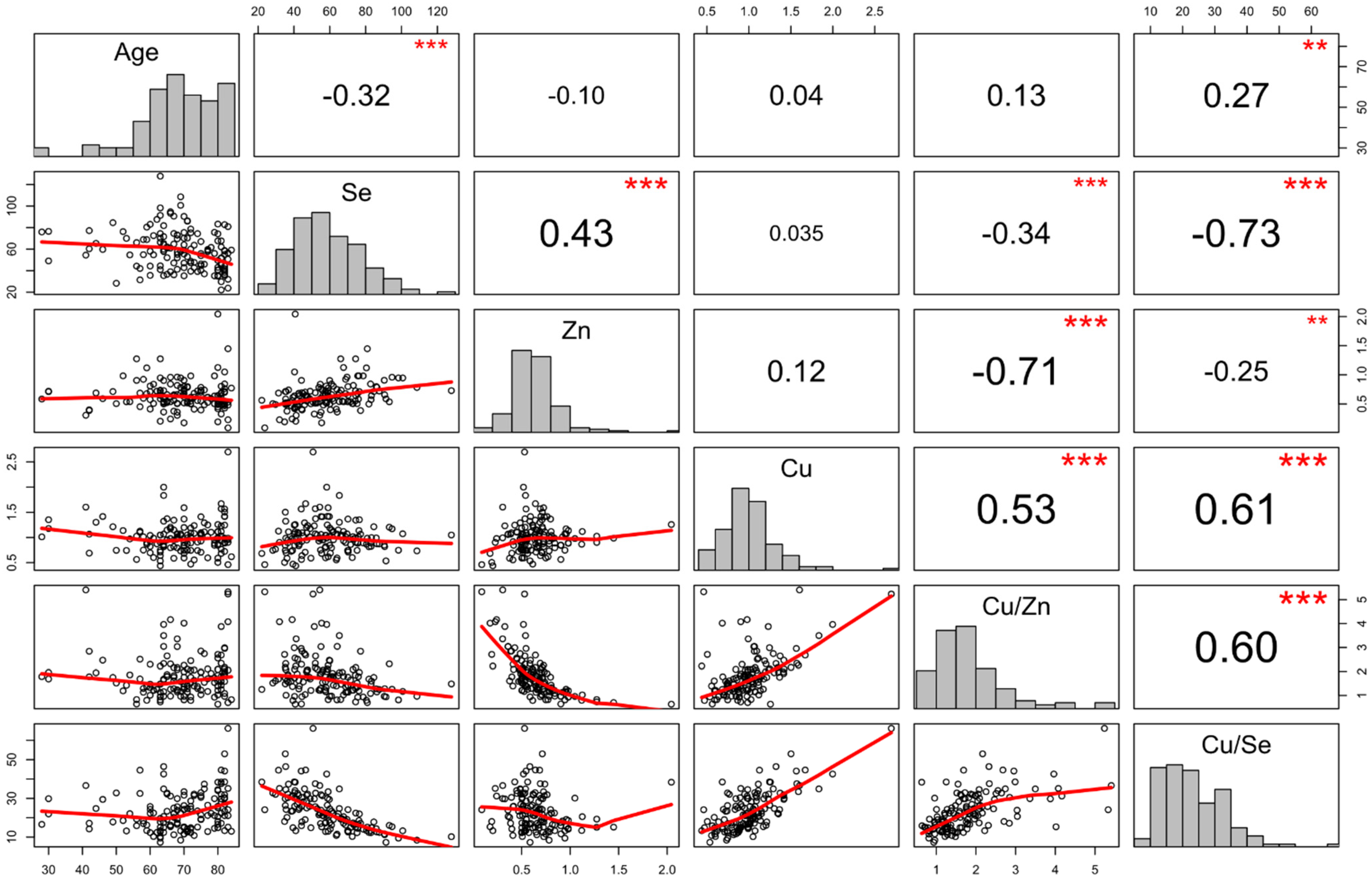
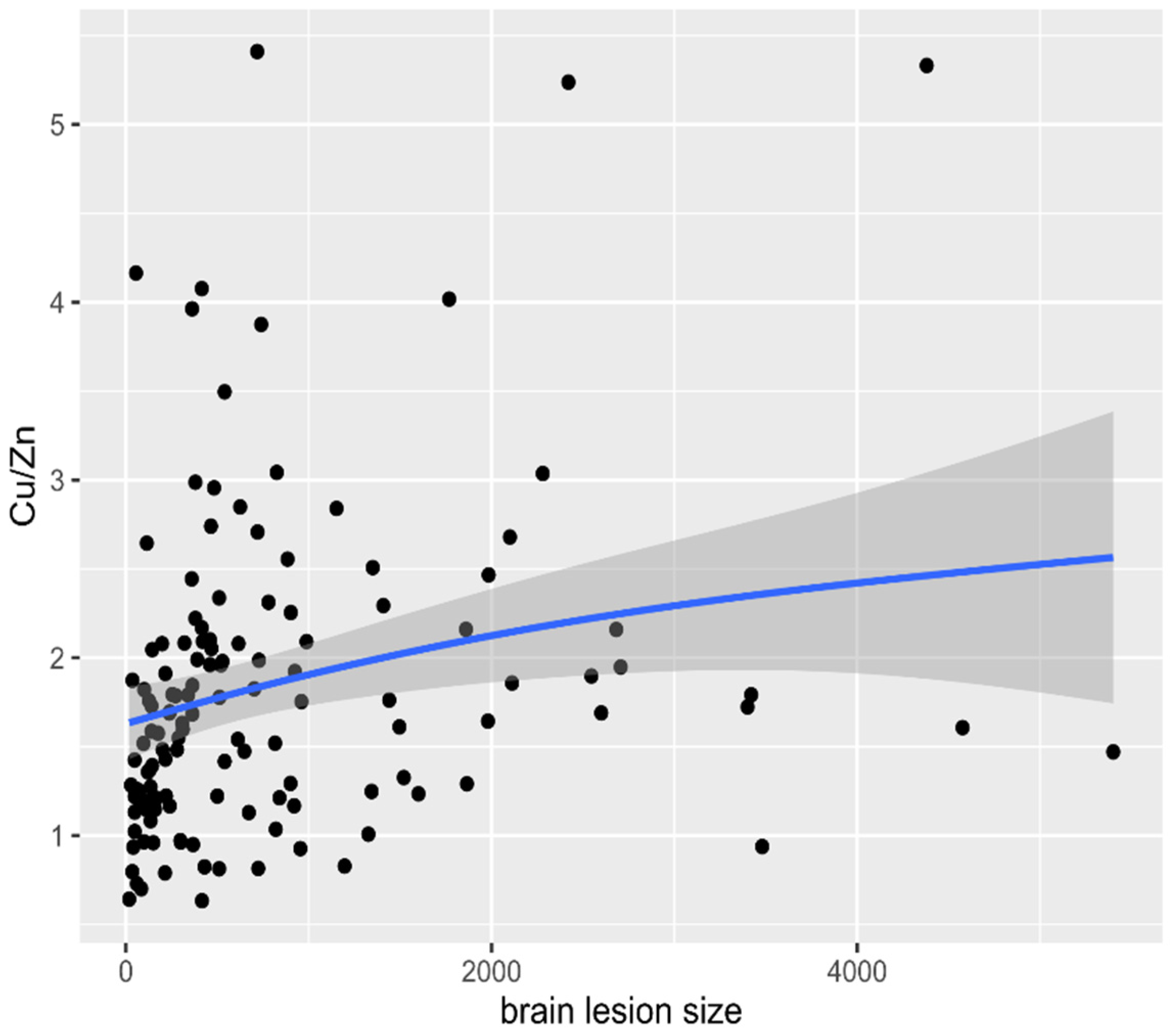
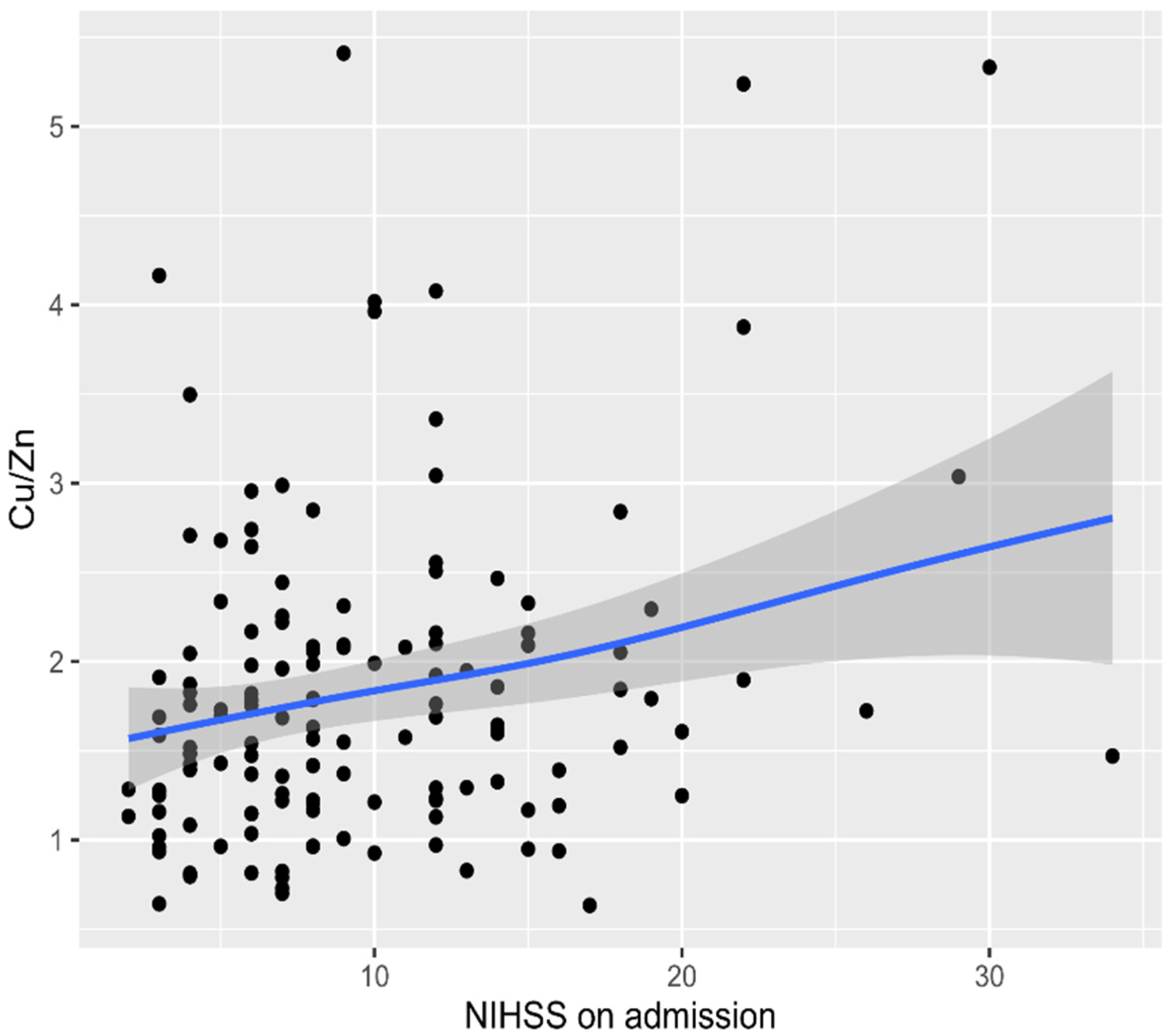
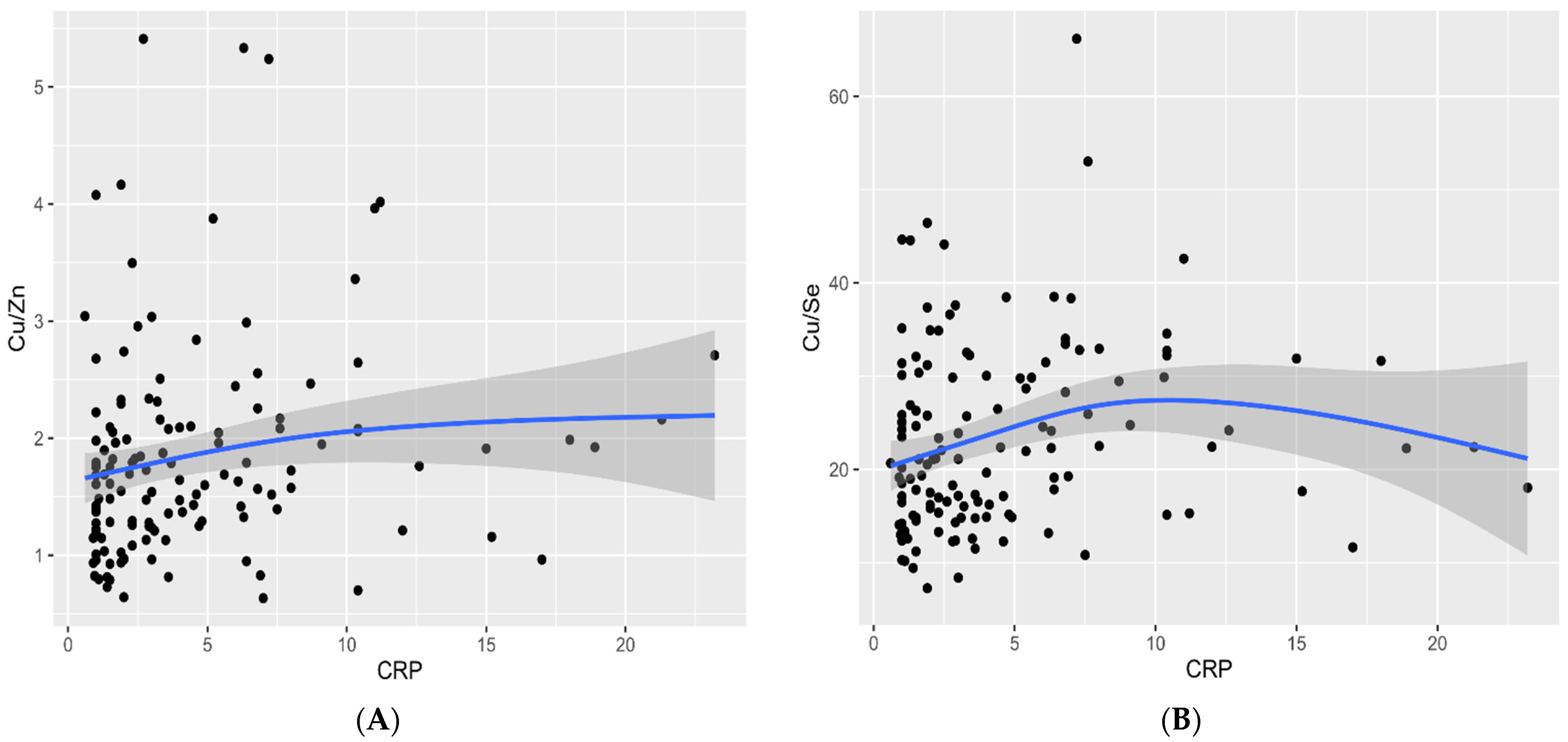
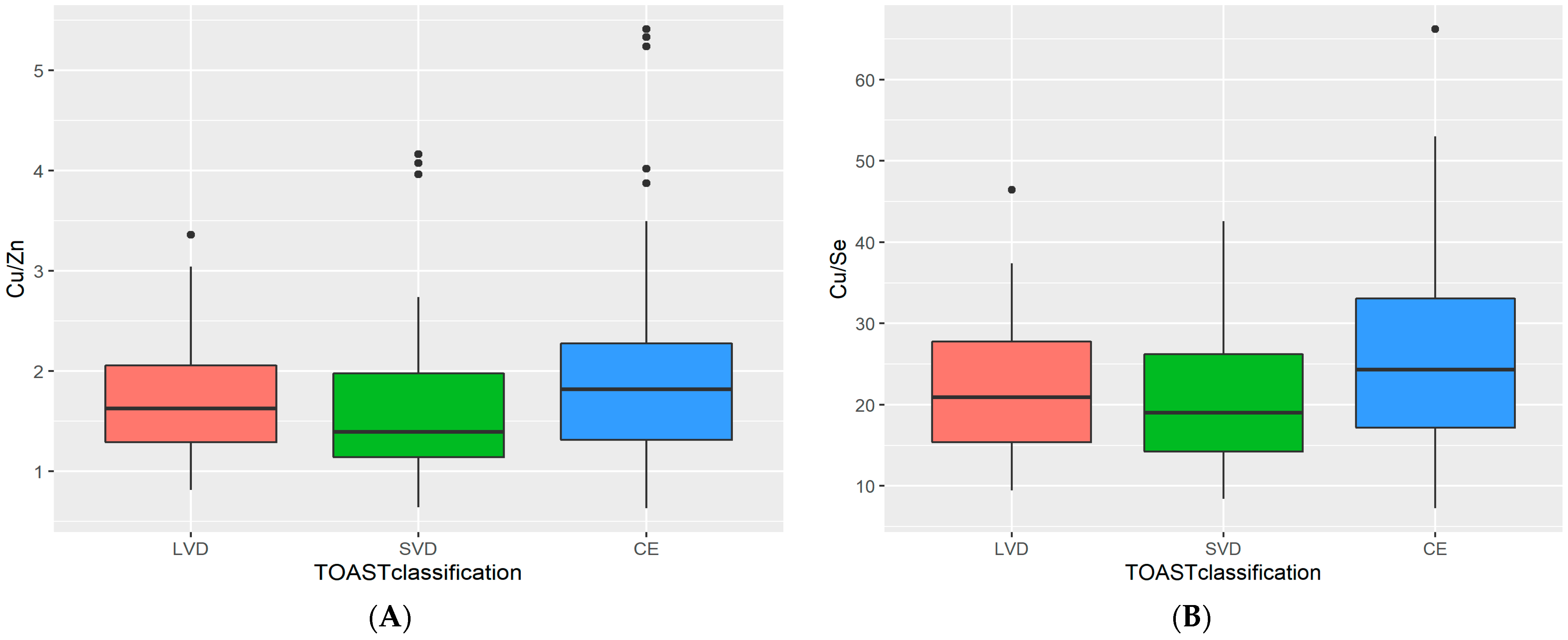
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global stroke statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef]
- Brainin, M.; Feigin, V.L.; Norrving, B.; Martins, S.C.O.; Hankey, G.J.; Hachinski, V.; World Stroke Organization Board of Directors. Global prevention of stroke and dementia: The WSO Declaration. Lancet Neurol. 2020, 19, 487–488. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e66. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.; Emmett, E.; Wang, Y.; McKevitt, W.C.; Wolfe, C. The Burden of Stroke in Europe. The Challenge for Policy Makers—King’s College London for the Stroke Alliance for Europe (SAFE). 2017. Available online: https://www.stroke.org.uk/sites/default/files/the_burden_of_stroke_in_europe_-_challenges_for_policy_makers.pdf (accessed on 29 April 2020).
- Baudry, J.; Kopp, J.F.; Boeing, H.; Kipp, A.P.; Schwerdtle, T.; Schulze, M.B. Changes of trace element status during aging: Results of the EPIC-Potsdam cohort study. Eur. J. Nutr. 2020, 59, 3045–3058. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, Y.; Liu, Y.; Yu, Y.; Jia, N.; Zhou, L.; Wang, H.; Huang, S.; Zhang, Y.; Yang, H.; et al. Circulating multiple metals and incident stroke in Chinese adults. Stroke 2019, 50, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhou, L.; Li, D.; Cheng, J. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ. Int. 2019, 125, 125–134. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.; Wang, Y.; Wang, T.; Ma, M.; Tian, C. Association between the change of serum copper and ischemic stroke: A systematic review and meta-analysis. J. Mol. Neurosci. 2020, 70, 475–480. [Google Scholar] [CrossRef]
- Munshi, A.; Babu, S.; Kaul, S.; Shafi, G.; Rajeshwar, K.; Alladi, S.; Jyothy, A. Depletion of serum zinc in ischemic stroke patients. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 433–436. [Google Scholar] [CrossRef]
- Ahmadi Ahangar, A.; Saadat, P.; Niroomand, S.; Alijanpour, S.; Sohrabnezhad, R.; Firozejahi, A.; Biani, M.A.; Arab, F.; Hosseinzadeh, H.; Faraji, S.; et al. Increased zinc serum level: New clues in babol stroke patients, northern Iran. Stroke Res. Treat. 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Angelova, E.A.; Atanassova, P.A.; Chalakova, N.T.; Dimitrov, B.D. Associations between serum selenium and total plasma homocysteine during the acute phase of ischaemic stroke. Eur. Neurol. 2008, 60, 298–303. [Google Scholar] [CrossRef]
- Mousavi-Mirzaei, S.M.; Khorasani, E.Y.; Amirabadizadeh, A.; Nakhaee, S.; Baharshahi, A.; Rajabpour-Sanati, A.; Mehrpour, O.; Talebi, A.; Lamarine, R.J.; Mehrpour, M. Comparison of blood lead concentrations in patients with acute ischemic stroke and healthy subjects. J. Trace Elem. Med. Biol. 2020, 61, 126532. [Google Scholar] [CrossRef]
- Skalny, A.V.; Klimenko, L.L.; Turna, A.A.; Budanova, M.N.; Baskakov, I.S.; Savostina, M.S.; Mazilina, A.N.; Deyev, A.I.; Skalnaya, M.G.; Tinkov, A.A. Serum trace elements are associated with hemostasis, lipid spectrum and inflammatory markers in men suffering from acute ischemic stroke. Metab. Brain Dis. 2017, 32, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Skalnaya, M.G.; Klimenko, L.L.; Mazilina, A.N.; Tinkov, A.A. Selenium in ischemic stroke. In Selenium. Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer: Cham, Switzerland, 2018; pp. 211–230. [Google Scholar] [CrossRef]
- Merrill, P.D.; Ampah, S.B.; He, K.; Rembert, N.J.; Brockman, J.; Kleindorfer, D.; McClure, L.A. Association between trace elements in the environment and stroke risk: The reasons for geographic and racial differences in stroke (REGARDS) study. J. Trace Elem. Med. Biol. 2017, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zangieva, Z.K.; Torshin, I.; Gromova, O.A.; Nikonov, A.A. Trace elements in the nervous tissue and ischemic stroke. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova. 2013, 113, 30–36. [Google Scholar]
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zincdependent NF-κB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Witt, B.; Schaumlöffel, D.; Schwerdtle, T. Subcellular localization of copper—Cellular bioimaging with focus on neurological disorders. Int. J. Mol. Sci. 2020, 21, 2341. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Prescha, A.; Płaczkowska, S.; Porębska, I.; Kosacka, M.; Pawełczyk, K. Serum and whole blood Cu and Zn status in predicting mortality in lung cancer patients. Nutrients 2020, 27, 60. [Google Scholar] [CrossRef]
- Laine, J.T.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Serum copper-to-zinc-ratio and risk of incident infection in men: The kuopio ischaemic heart disease risk factor study. Eur. J. Epidemiol. 2020, 35, 1149–1156. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An overview of the main genetic, epigenetic and environmental factors involved in autism spectrum disorder focusing on synaptic activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Li, S.O.; Wang, J.L.; Bjørklund, G.; Zhao, W.N.; Yin, C.H. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 2014, 25, 1216–1220. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M. Role of zinc and selenium in oxidative stress and immunosenescence: Implications for healthy aging and longevity. In Handbook of Immunosenescence; Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G., Eds.; Springer Nature: London, UK, 2019; pp. 2539–2573. [Google Scholar] [CrossRef]
- Malavolta, M.; Costarelli, L.; Giacconi, R.; Basso, A.; Piacenza, F.; Pierpaoli, E.; Provinciali, M.; Ogo, O.A.; Ford, D. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp. Gerontol. 2017, 99, 35–45. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Hore, N.; Eyupoglu, I.Y. Selenium and selenoproteins in neuroprotection and neuronal cell death. In Metal Ion in Stroke; Li, Y.V., Zhang, J.H., Eds.; Springer: New York, NY, USA, 2012; pp. 525–536. [Google Scholar] [CrossRef]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Hughes, D.J. The role of selenium in health and disease: Emerging and recurring trends. Nutrients 2020, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Fedor, M.; Socha, K.; Urban, B.; Soroczyńska, J.; Matyskiela, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Serum concentration of zinc, copper, selenium, manganese, and Cu/Zn ratio in children and adolescents with myopia. Biol. Trace Elem. Res. 2017, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc status and autoimmunity: A systematic review and meta-analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Valera, P.; Zavattari, P.; Sanna, A.; Pretti, S.; Marcello, A.; Mannu, C.; Targhetta, C.; Bruno, G.; Songini, M. Zinc and other metals deficiencies and risk of type 1 diabetes: An ecological study in the high risk Sardinia Island. PLoS ONE 2015, 10, e0141262. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Samsa, G.P. Reliability of the national institutes of health stroke scale. Extension to non-neurologists in the context of a clinical trial. Stroke 1997, 28, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Park, S.H.; Kim, N.; Kim, W.J.; Park, J.H.; Ko, Y.; Bae, H.J.; Yang, M.H.; Jang, M.S.; Han, M.K.; et al. Trial of ORG 10172 in acute stroke treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J. Am. Heart Assoc. 2014, 3, e001119. [Google Scholar] [CrossRef]
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, version 27.0; IBM Corp: Armonk, NY, USA.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https:https://www.R-project.org (accessed on 4 July 2020).
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2013; pp. 256–264. [Google Scholar]
- Dembińska-Kieć, A.; Solnica, B.; Naskalski, J. Diagnostyka Laboratoryjna Z Elementami Biochemii Klinicznej, 4th ed.; Edra Urban & Partner: Wroclaw, Poland, 2017; p. 23. (In Polish) [Google Scholar]
- Bhatt, A.; Farooq, M.U.; Enduri, S.; Pillainayagam, C.; Naravetla, B.; Razak, A.; Safdar, A.; Hussain, S.; Kassab, M.; Majid, A. Clinical significance of serum zinc levels in cerebral ischemia. Stroke Res. Treat. 2010, 245715. [Google Scholar] [CrossRef]
- Zimmermann, C.; Winnefeld, K.; Streck, S.; Roskos, M.; Haberl, R.L. Antioxidant status in acute stroke patients and patients at stroke risk. Eur. Neurol. 2004, 51, 157–161. [Google Scholar] [CrossRef]
- Hu, X.F.; Sharin, T.; Chan, H.M. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. (GMS) 2017, 44, 322–330. [Google Scholar] [CrossRef]
- Hu, X.F.; Stranges, S.; Chan, L.H.M. Circulating selenium concentration is inversely associated with the prevalence of stroke: Results from the Canadian health measures survey and the National Health and nutrition examination survey. J. Am. Heart Assoc. 2019, 8, e012290. [Google Scholar] [CrossRef]
- Liu, L.; Lin, G.; Wang, H.; Zhang, B.; Du, S. Selenium exposure and incident hypertension among Chinese adults (P24-020-19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1). [Google Scholar] [CrossRef]
- Bastola, M.M.; Locatis, C.; Maisiak, R.; Fontelo, P. Selenium, copper, zinc and hypertension: An analysis of the National Health and Nutrition Examination Survey (2011–2016). BMC Cardiovasc. Disord. 2020, 20, 45. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.; Unverzagt, F.W.; Cheng, Y.; Hake, A.M.; Liang, C.; Gao, S.; Ma, F.; Su, L.; Liu, J.; et al. The association between selenium and lipid levels: A longitudinal study in rural elderly Chinese. Arch. Gerontol. Geriatr. 2015, 60, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Kuo, C.S.; Lee, L.T.; Huang, K.C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res. Care 2016, 4, e000253. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Stranges, S.; Navas-Acien, A.; Ordovas, J.M.; Guallar, E. Serum selenium and plasma lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010, 210, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Chiang, C.H.; Yao, C.A.; Huang, K.C. Gender differences with dose—Response relationship between serum selenium levels and metabolic syndrome-a case-control study. Nutrients 2019, 11, 477. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, X.; Ye, H.; Jin, L.; Zhang, X.; Zhu, Y. High levels of plasma selenium are associated with metabolic syndrome and elevated fasting plasma glucose in a Chinese population: A case-control study. J. Trace. Elem. Med. Biol. 2015, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Ji, M.; Li, X.; Li, Z.; Wu, G.; Fu, X.; Yang, X.; Gao, X. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018, 37, 1512–1517. [Google Scholar] [CrossRef]
- Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 2014, 13, 62. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Borawska, M.H.; Socha, K.; Charkiewicz, A.E.; Laudański, T.; Kulikowski, M.; Kobylec, E. Antioxidant status in women who had a miscarriage. Adv. Med. Sci. 2015, 60, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum concentrations of selenium and copper in patients diagnosed with pancreatic cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xiao, Y.; Feng, W.; Liu, Y.; Yu, Y.; Zhou, L.; Qiu, G.; Wang, H.; Liu, B.; Liu, K.; et al. Plasma metal concentrations and incident coronary heart disease in Chinese adults: The Dongfeng-Tongji cohort. Environ. Health Perspect. 2017, 125, 107007. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Laclaustra, M.; Ji, C.; Cappuccio, F.P.; Navas-Acien, A.; Ordovas, J.M.; Rayman, M.; Guallar, E. Higher selenium status is associated with adverse blood lipid profile in British adults. J. Nutr. 2010, 140, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Nahan, K.S.; Walsh, K.B.; Adeoye, O.; Landero-Figueroa, J.A. The metal and metalloprotein profile of human plasma as biomarkers for stroke diagnosis. J. Trace Elem. Med. Biol. 2017, 42, 81–91. [Google Scholar] [CrossRef]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Selenium in human health and disease: An overview. In Selenium. Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar] [CrossRef]
- Altamura, C.; Squitti, R.; Pasqualetti, P.; Gaudino, C.; Palazzo, P.; Tibuzzi, F.; Lupoi, D.; Cortesi, M.; Rossini, P.M.; Vernieri, F. Ceruloplasmin/transferrin system is related to clinical status in acute stroke. Stroke 2009, 40, 1282–1288. [Google Scholar] [CrossRef]
- Lai, M.; Wang, D.; Lin, Z.; Zhang, Y. Small molecule copper and its relative metabolites in serum of cerebral ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 214–219. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef]
- Squitti, R.; Siotto, M.; Assenza, G.; Giannantoni, N.M.; Rongioletti, M.; Zappasodi, F.; Tecchio, F. Prognostic value of serum copper for post-stroke clinical recovery: A pilot study. Front. Neurol. 2018, 9, 333. [Google Scholar] [CrossRef]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, K.J. Pathophysiological role of zinc in ischemic brain injury. Oncotarget 2017, 8, 5670–5671. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Sanchez, C.; Blanco-Alvarez, V.M.; Gonzalez-Barrios, J.A.; Martinez-Fong, D.; Garcia-Robles, G.; Soto-Rodriguez, G.; Brambila, E.; Torres-Soto, M.; Gonzalez-Vazquez, A.; Aguilar-Peralta, A.K.; et al. Prophylactic chronic zinc administration increases neuroinflammation in a hypoxia-ischemia model. J. Immunol. Res. 2016, 2016, 4039837. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Kuxhaus, O.; Eichelmann, F.; Kopp, J.F.; Alker, W.; Hackler, J.; Kipp, A.P.; Schwerdtle, T.; Haase, H.; Schomburg, L.; et al. Trace element profile and incidence of type 2 diabetes, cardiovascular disease and colorectal cancer: Results from the EPIC-Potsdam cohort study. Eur. J. Nutr. 2021, 15. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Beserra, J.B.; Soares de Oiveira, A.R.; Climaco Cruz, K.J.; de Sousa Melo, S.R.; Ribeiro do Nascimento, G.V.; Soares de Macedo, G.F.; do Nascimento Marreiro, D. Association between cortisol, insulin resistance and zinc in obesity: A mini-review. Biol. Trace Elem. Res. 2019, 191, 323–330. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc deficiency—An Independent risk factor in the pathogenesis of haemorrhagic stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Shah, M.H. Multivariate statistical evaluation of trace metal levels in the blood of atherosclerosis patients in comparison with healthy subjects. Heliyon 2016, 2, e00054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and cardiovascular disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Noshin, T.F.; Ali, M.R.; Banik, S. Increased oxidative stress and altered serum macro-minerals and trace elements levels are associated with coronary artery disease. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. (GMS) 2021, 64, 126707. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.; Krempien, S.; Schwab, S.; Donneberg, S.; Hacke, W. Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury: Correlation with clinical course and infarct size. Stroke 1997, 28, 2425–2428. [Google Scholar] [CrossRef]
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of essential trace elements in nutrition and drinking water for human health and autoimmune disease risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Wandt, V.K.; Winkelbeiner, N.; Bornhorst, J.; Witt, B.; Raschke, S.; Simon, L.; Schwerdtle, T.; Ebert, F.; Kipp, A.P. A matter of concern—Trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021, 41, 101877. [Google Scholar] [CrossRef]
- Almeida, A.; Gajewska, K.; Duro, M.; Costa, F.; Pinto, E. Trace element imbalances in patients undergoing chronic hemodialysis therapy—Report of an observational study in a cohort of Portuguese patients. J. Trace Elem. Med. Biol. 2020, 62, 126580. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Wathurapatha, W.S.; Ishara, M.H.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Effects of Zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. 2015, 12, 26. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Shen, G.; Lo, K.; Huang, J.Y.; Liu, L.; Chen, C.L.; Yu, Y.L.; Sun, S.; Zhang, B.; Feng, Y.Q. Association of circulating selenium concentration with dyslipidemia: Results from the NHANES. J. Trace Elem. Med. Biol. 2020, 58, 126438. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Estecha, M.; Palazon-Bru, I.; Bodas-Pinedo, A.; Trasobares, E.; Palazon- Bru, A.; Fuentes, M.; Cuadrado-Cenzual, M.A.; Calvo-Manuel, E. Relationship between serum selenium, sociodemographic variables, other trace elements and lipid profile in an adult Spanish population. J. Trace Elem. Med. Biol. 2017, 43, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Monti, D.; Ostan, R.; Cevenini, E.; Gonos, E.S. Micronutrient-gene interactions related to infammatory/immune response antioxidant activity in ageing and infammation. A systematic review. Mech. Ageing Dev. 2014, 136–137, 29–49. [Google Scholar] [CrossRef] [PubMed]
| Clinical Parameters | AIS (n = 141) | Controls (n = 69) | p * |
|---|---|---|---|
| Gender [M n(%) / F n(%)] | 67 (47.5%) / 74 (52.5%) | 27 (39.1%) / 42 (60.9%) | 0.251 |
| Age (years) median (Q1-Q3) | 70 (63–79.5) | 55 (38.5–65.5) | <0.05 |
| BMI (Kg/M2) median (Q1-Q3) | 26.83 (24.23–30.08) | 27.19 (23.26–29.10) | 0.519 |
| Total cholesterol (TC) (mg/dL) median (Q1-Q3) | 179 (143–212) | ||
| Triglyceride (TG) (mg/dL) median (Q1-Q3) | 104 (79.75–135.25) | ||
| Low-density lipoprotein cholesterol (LDL-C) (mg/dL) median (Q1-Q3) | 119 (87.25–154.5) | ||
| High-density lipoprotein cholesterol (HDL-C) (mg/dL) median (Q1-Q3) | 45 (38–54.75) | ||
| Hypertensives n (%) | 127 (90.1%) | ||
| Diabetic subjects n (%) | 45 (31.9%) | ||
| Smokers n (%) | 62 (44%) | ||
| Obese n (%) BMI >= 25 | 97 (69%) | 32 (46%) | |
| CRP (mg/L) median (Q1-Q3) | 2.9 (1.5–6.3) | ||
| Brain lesion size (mm2) median (Q1-Q3) | 445 (170–923) | ||
| Lesion location (R n(%) /L n(%) hemisphere) | 65 (46.1%)/ 76 (53.9%) | ||
| NIHSS on admission median (Q1-Q3) | 8 (6–12) | ||
| NIHSS at discharge median (Q1-Q3) | 2 (1–5) | ||
| MRS scale median (Q1-Q3) | 2 (1–3) | ||
| HbA1c (%) median (Q1-Q3) | 5.9 (5.6–6.5) | ||
| Creatinine (mg/dL) median (Q1-Q3) | 0.86 (0.73–1.03) | ||
| Highly sensitive troponin (ng/l) median (Q1-Q3) | 5 (5–13.25) | ||
| Fibrinogen (mg/dL) median (Q1-Q3) | 375 (325.5–438) | ||
| D-dimer (µg/mL) median (Q1-Q3) | 0.86 (0.42–1.425) | ||
| Ejection fraction (EF %) median (Q1-Q3) | 56 (52–58) | ||
| Intervention treatment (T ± MT) n (%) | 60 (42%) | ||
| Trombolysis (T) n(%) | 48 (34%) | ||
| Mechanical thrombectomy (MT) n(%) | 24 (17%) | ||
| Hyperlipidemia n(%) | 102 (72.9%) | ||
| Atrial fibrillation n(%) | 45 (31.9%) | ||
| Carotid atherosclerosis | 115 (81.6%) | ||
| >30% stenosis n (%) | 30 (21.3%) | ||
| TOAST classification | 141 (100%) | ||
| LVD n(%) | 42 (29.8%) | ||
| SVD n(%) | 47 (33.3%) | ||
| CE n(%) | 52 (36.9%) |
| AIS | Controls | p * | ||
|---|---|---|---|---|
| Se [μg/L] | Total | 57.69 (44.13–70.95) | 75.48 (66.33–92.67) | <0.001 |
| Males | 59.71 (42.04–73.14) | 75.48 (61.23–99.3) | ||
| Females | 55.65 (44.39–67.98) | 75.15 (69.66–87.02) | ||
| p ** | 0.730 | 0.931 | ||
| Zn [mg/L] | Total | 0.62 (0.51–0.73) | 0.79 (0.71–0.89) | <0.001 |
| Males | 0.65 (0.51–0.73) | 0.76 (0.69–0.88) | ||
| Females | 0.59 (0.51–0.73) | 0.79 (0.72–0.90) | ||
| p ** | 0.573 | 0.658 | ||
| Cu [mg/L] | Total | 0.99 (0.82–1.12) | 0.97 (0.86–1.24) | 0.283 |
| Males | 0.97 (0.8–1.11) | 0.86 (0.78–1.11) | ||
| Females | 1.01 (0.84–1.14) | 1.06 (0.91–1.29) | ||
| p ** | 0.245 | 0.008 | ||
| Cu/Zn molar ratio *** | Total | 1.68 (1.22–2.09) | 1.34 (1.08–1.66) | <0.001 |
| Males | 1.61 (1.22–2.06) | 1.19 (1.01–1.41) | ||
| Females | 1.74 (1.20–2.20) | 1.46 (1.14–1.76) | ||
| p ** | 0.485 | 0.022 | ||
| Cu/Se molar ratio *** | Total | 21.97 (15.24–29.97) | 16.40 (13.73–20.95) | <0.001 |
| Males | 21.45 (15.17–28.28) | 13.88 (12.07–20.76) | ||
| Females | 22.33 (15.19–31.25) | 17.12 (14.94–21.29) | ||
| p ** | 0.395 | 0.037 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirończuk, A.; Kapica-Topczewska, K.; Socha, K.; Soroczyńska, J.; Jamiołkowski, J.; Kułakowska, A.; Kochanowicz, J. Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland—A New Insight into Stroke Pathophysiology. Nutrients 2021, 13, 2139. https://doi.org/10.3390/nu13072139
Mirończuk A, Kapica-Topczewska K, Socha K, Soroczyńska J, Jamiołkowski J, Kułakowska A, Kochanowicz J. Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland—A New Insight into Stroke Pathophysiology. Nutrients. 2021; 13(7):2139. https://doi.org/10.3390/nu13072139
Chicago/Turabian StyleMirończuk, Anna, Katarzyna Kapica-Topczewska, Katarzyna Socha, Jolanta Soroczyńska, Jacek Jamiołkowski, Alina Kułakowska, and Jan Kochanowicz. 2021. "Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland—A New Insight into Stroke Pathophysiology" Nutrients 13, no. 7: 2139. https://doi.org/10.3390/nu13072139
APA StyleMirończuk, A., Kapica-Topczewska, K., Socha, K., Soroczyńska, J., Jamiołkowski, J., Kułakowska, A., & Kochanowicz, J. (2021). Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland—A New Insight into Stroke Pathophysiology. Nutrients, 13(7), 2139. https://doi.org/10.3390/nu13072139







