Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications
Abstract
1. Introduction
2. Oral Vitamin D2, Oral 1 Alpha-HydroxyvitaminD3 (1(OH)D3), Oral Calcitriol, Topical Calcitriol and Oral Vitamin D3 Safely Treat Psoriasis—1930s to 2019
2.1. Sunshine and Oral Vitamin D2 in the 1930s—Krafka
2.2. Oral 1 Alpha-HydroxyvitaminD3, Oral Calcitriol and Topical Calcitriol in the 1980s—Morimoto
2.3. Oral and Topical Calcitriol in the 1980s and 1990s—Smith, Huckins, Perez and Holick
2.4. Oral and Topical Calcitriol 12-Month Study, 1988—Smith
- (a)
- On cultures of fibroblasts and keratinocytes from patients with psoriasis
- (b)
- Orally in 14 patients with moderate to severe psoriasis
- (c)
- Topically in 3 patients with psoriasis.
2.5. Oral Calcitriol 6-Month Study in Psoriatic Arthritis, 1990—Huckins
2.6. Oral Calcitriol 3-Year Dose Titration Safety Study, 1996—Perez
2.7. Serum 25(OH)D3 Concentrations in 2 Patients with Plaque Psoriasis after 5 Months’ Oral Vitamin D3 in 2012—McCullough
2.8. Serum 25(OH)D3 Concentrations after 6 Months of Taking 35,000 IU/Day of Oral Vitamin D3 in 25 Patients with Either Plaque Psoriasis or Vitiligo, 2013—Finamor
2.9. Serum 25(OH)D2 Concentrations in a Patient with Plaque Psoriasis after 42 Months of Taking 50,000 IU/Day of Oral Vitamin D2, 2019—McCullough
- (a)
- patients receive very little sunshine in the hospital
- (b)
- there is very little vitamin D in the food they eat
- (c)
- (d)
- vitamin D, sunshine and UVB phototherapy were shown to be effective treatments for several diseases in the 1930s and 1940s, and again beginning in the 1980s as discussed earlier [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,45,46,47,48,49,50,51,52,53,54]. In addition, several patients received daily doses of vitamin D2 or vitamin D3 ranging from 20,000 to 50,000 IU/day based on specific disease concerns.
3. Changes in Serum 25(OH)D3 Concentrations in Psoriasis Patients Treated with UVB Phototherapy and Sunshine—1996, 2009, and 2010
3.1. Serum 25(OH)D3 Concentrations in Psoriasis Patients after 8 Weeks of UVB Phototherapy, 1996—Prystowsky
3.2. Serum 25(OH)D3 Concentrations in Psoriasis Patients after 1–4 Months of NB-UVB Phototherapy, 2010—Ryan
3.3. Serum 25(OH)D3 Concentrations in Psoriasis Patients after 8–12 Weeks of NB-UVB and BB-UVB Phototherapy, 2009—Osmancevic
3.4. Serum 25(OH)D3 Concentrations in Psoriasis Patients after 15 Days of Sunshine or 8–12 Weeks of NB-UVB or BB-UVB Phototherapy, 2010—Osmancevic
- (a)
- To increase the knowledge about the effects of phototherapy on vitamin D production during the treatment of psoriasis,
- (b)
- To see if there were differences between the effect of BB-UVB, NB-UVB and heliotherapy on vitamin D synthesis in psoriasis patients.
4. Summary and Discussion of Key Findings in the Reviewed Reports
- (1)
- Four different oral forms of vitamin D are safe and effective treatments for plaque psoriasis
- (2)
- Normal serum 25(OH)D concentrations (>20 ng/mL) were common pretreatment but insufficient to improve psoriatic lesions
- (3)
- High serum 25(OH)D concentrations (>100 ng/mL) were often reported with safe control of psoriasis
- (4)
- Changes in serum 25(OH)D concentrations after treatment vary significantly with the treatment used
- (5)
- A therapeutic dose response of psoriasis to vitamin D appears to be present
- (6)
- Calcitriol formation is the common endpoint after treatment with vitamin D, sunshine and UVB phototherapy
- (7)
- Psoriasis can recur with cessation of treatment with vitamin D, sunshine or UVB phototherapy
- (8)
- Psoriasis can improve again with resumption of treatment with vitamin D, sunshine or UVB phototherapy
- (9)
- Post treatment serum 25(OH)D concentrations are higher after UVB phototherapy compared to sunshine
- (10)
- A paucity of adverse reactions was observed with vitamin D supplementation in the reviewed studies
- (11)
- Clinical efficacy and safety of oral and topical vitamin D treatments are comparable to UVB phototherapy and sunshine treatments
- (12)
- All authors reviewed stated unequivocal support for the safety and efficacy of vitamin D in treating psoriasis
- (13)
- Estimates of vitamin D production in the 1970s are significantly lower than doses used clinically in treating diseases in the 1930s and 1940s— but significantly higher than the doses recommended for use today.
4.1. Four Different Oral Forms of Vitamin D Are Safe and Effective Treatments for Plaque Psoriasis
4.2. Normal Serum 25(OH)D Concentrations (>20 ng/mL) Are Often Insufficient for Disease Control in Psoriasis Patients
4.3. High Serum 25(OH)D Concentrations (>100 ng/mL) Were often Reported with Safe Control of Psoriasis
4.4. Changes in Serum 25(OH)D Concentrations after Treatment Vary Significantly with the Treatment Used
4.5. A Therapeutic Dose Response to Vitamin D Appears to Be Present
4.6. Calcitirol Formation Is the Common Endpoint after Treatment with Vitamin D, UVB Phototherapy and Sunshine
4.7. Psoriasis Can Recur with Cessation of Vitamin D or UVB Phototherapy
4.8. Psoriasis Can Improve Again with Resumption of Treatment with Vitamin D or UVB Phototherapy
4.9. Post Treatment Serum 25(OH)D Concentrations Are Higher after UVB Phototherapy Compared to Sunshine
4.10. A Paucity of Adverse Reactions Was Observed in the Reviewed Studies
4.11. Sunshine and Topical Vitamin D Appear to Work More Quickly Than UVB Phototherapy and Oral Vitamin D
4.12. Authors Reported Views Support the Safety and Efficacy of Vitamin D for the Treatment of Psoriasis
4.13. Estimates of Vitamin D Production in the 1970s Are Much Lower Than Doses Used in the 1930s and 1940s
5. Implications for the Treatment of Other Vitamin D Deficiency Related Diseases with Vitamin D or Phototherapy
5.1. Inadequacy of 4000 IU/Day of Vitamin D3 in the Treatment of Asthma and of 2000 IU/Day in the Prevention of Cancer
5.2. Case Reports and Clinical Trials of Diseases Showing Clinical Improvement with Vitamin D Intake > 4000 IU/Day
- (1)
- (2)
- Control of chronic pain in children suffering from sickle cell disease using 50,000 IU twice weekly of vitamin D3 for 8 weeks, followed by once weekly for 32 months in a 2011 case report [296], and subsequently in a weight-based dosing study using 40,000 IU to 100,000 IU/ week for 6 weeks in 20 children in a 6 month placebo controlled trial published in 2012 [297];
- (3)
- Chronic fatigue in a 2014 prospective study of 171 adult patients with low serum 25(OH)D concentrations (<30 ng/mL) using 50,000 IU of vitamin D2 three times a week for 5 weeks [303], which averages out to 21,429 IU/day;
- (4)
- Prevention of statin intolerance secondary to myalgia, myositis, myopathy or necrosis in 171 previously statin intolerant patients with low serum 25(OH)D concentrations (<32 ng/mL) using either 50,000 or 100,000 IU/week of vitamin D2 for 24 months published in 2015 [308];
- (5)
- 282 patients treated with these same doses in a prospective one-year clinical safety trial from this same group using vitamin D3 instead of vitamin D2, and again found to be safe in 2016 [309];
- (6)
- Prevention of progression of a case of advanced pancreatic cancer using 50,000 IU/day of vitamin D3 for 9 months reported in 2016 [310];
- (7)
- (8)
- (9)
- The need for ICU treatment in patients requiring hospitalization due to proven COVID-19 infections in 50 patients treated with 25(OH)D (532 µg (21,280 IU) on day 1; 266 µg (10,640 IU) on days 3 and 7, followed by 266 µg weekly until discharge) which resulted in one ICU admission (2%), versus a group of 26 untreated patients of which thirteen (50%) required admission for care in an ICU [80].
5.3. Need to Better Define the Therapeutic Index of Vitamin D
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Psoriasis Foundation. Psoriasis Statistics. Available online: https://www.psoriasis.org/content/statistics (accessed on 15 August 2019).
- Brezinski, E.A.; Dhillon, J.S.; Armstrong, A.W. The economic burden of psoriasis in the United States. J. Am. Acad. Dermatol. 2015, 151, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Psoriasis Treatments. National Psoriasis Foundation. June 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments (accessed on 15 August 2019).
- National Psoriasis Foundation. Moderate to Severe Psoriasis and Psoriatic Arthritis: Biologic Drugs. 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments/biologics (accessed on 15 August 2019).
- National Psoriasis Foundation. Moderate to Severe Psoriasis and Psoriatic Arthritis: Bio-Similar Medicines. 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments/biosimilars (accessed on 15 August 2019).
- National Psoriasis Foundation. Oral Treatments. 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments/oral-treatments (accessed on 15 August 2019).
- National Psoriasis Foundation. Traditional Systemic Medications. 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments/systemics (accessed on 15 August 2019).
- National Psoriasis Foundation. Phototherapy. June 2018. Available online: https://www.psoriasis.org/about-psoriasis/treatments/phototherapy (accessed on 15 August 2019).
- National Psoriasis Foundation. Topical Treatments. 2019. Available online: https://www.psoriasis.org/about-psoriasis/treatments/topicals (accessed on 15 August 2019).
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Codoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.R.; Gelfand, J.M.; Lichten, J.; Mehta, N.N. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Krafka, J. A Simple Treatment for Psoriasis. J. Clin. Lab. Med. 1936, 21, 1147–1148.2. [Google Scholar]
- Morimoto, S.; Kumahara, Y. A Patient with psoriasis cured by 1 alpha-hydroxyvitamin D3. Med. J. Osaka Univ. 1985, 35, 51–54. [Google Scholar]
- Morimoto, S.; Yoshikawa, K.; Kozuka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An open study of vitamin D3 treatment in psoriasis vulgaris. Br. J. Dermatol. 1986, 115, 421–429. [Google Scholar] [CrossRef]
- Takamoto, S.; Onishi, T.; Moromoto, S.; Imanaka, S.; Yukawa, S.; Kozuka, T.; Kitano, Y.; Seino, Y.; Kumahara, Y. Effect of 1 alpha-hydroxycholecalciferol on psoriasis vulgaris: A pilot study. Calcif. Tissue Int. 1986, 39, 360–364. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Kozuka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Onishi, T.; Kumahara, Y. Treatment of psoriasis vulgaris with oral 1 alpha,25-dihydroxyvitamin D3—Report of two cases. J. Dermatol. 1987, 14, 59–62. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K. Psoriasis and vitamin D3. A review of our experience. Arch. Dermatol. 1989, 125, 231–234. [Google Scholar] [CrossRef]
- Holick, M.F.; Smith, E.; Pincus, S. Skin as the Site of Vitamin D Synthesis and Target Tissue for 1,25-Dihydroxyvitamin D3 Use of Calcitriol (1,25-Dihydroxyvitamin D3) for Treatment of Psoriasis. Arch. Dermatol. 1987, 123, 1677–1683a. [Google Scholar] [CrossRef]
- Smith, E.L.; Pincus, S.H.; Donovan, L.; Holick, M.F. A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J. Am. Acad. Dermatol. 1988, 19, 516–528. [Google Scholar] [CrossRef]
- Holick, M.F. Will 1,25-Dihydroxyvitamin D3, MC 903, and Their Analogues Herald a New Pharmacologic Era for the Treatment of Psoriasis? Arch. Dermatol. 1989, 125, 1692–1697. [Google Scholar] [CrossRef]
- Huckins, D.; Felson, D.; Holick, M.F. Treatment of Psoriatic Arthritis with Oral 1,25-dihydroxyvitamin D3: A Pilot Study. Arthritis Rheum. 1990, 33, 1723–1727. [Google Scholar] [CrossRef]
- Perez, A.; Raab, R.; Chen, T.C.; Turner, A.; Holick, M.F. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br. J. Dermatol. 1996, 134, 1070–1078. [Google Scholar] [CrossRef]
- Holick, M.F. McCollum Award Lecture, 1994: Vitamin D-new horizons for the 21st century. Am. J. Clin. Nutr. 1994, 60, 619–630. [Google Scholar] [CrossRef]
- Ezquerra, G.M.; Regaña, M.S.; Millet, P.U. Combination of acetretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Derm. Venereol. 2007, 87, 449–450. [Google Scholar] [CrossRef]
- McCullough, P.J.; Arnold-Long, M. Marked improvement in psoriasis skin lesions within 5 months in two patients after treatment with oral vitamin D3 in doses ranging from 10,000 international units (IU’s) to 40,000 IUs daily. Poster Presentation. In Proceedings of the 15th Vitamin D Workshop, Houston, TX, USA, 20–22 June 2012. [Google Scholar]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Guiterrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Derm. Endocrinol. 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Kamangari, F.; Koo, J.; Heller, M.; Lee, E.; Bhutani, T. Oral vitamin D, still a viable treatment option for psoriasis. J. Dermatol. Treat. 2013, 24, 261–267. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 to 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Rappaport, B.Z.; Reed, C.I.; Hathaway, M.L.; Struck, H.C. The Treatment of Hay Fever and Asthma with Viosterol of High Potency. J. Allergy. 1935, 5, 541–553. [Google Scholar] [CrossRef]
- Dreyer, I.; Reed, C. The Treatment of Arthritis with Massive Doses of Vitamin D. Arch. Phys. Ther. 1935, 16, 537–543. [Google Scholar]
- Howard, J.E.; Meyer, R.J. Intoxication with vitamin D. J. Clin. Endocrinol. 1948, 8, 895–910. [Google Scholar] [CrossRef]
- Parks, E.A. The therapy of rickets. JAMA 1940, 115, 370–379. [Google Scholar] [CrossRef]
- Dowling, G.B.; Prosser Thomas, E.W. Lupus vulgaris treated with calciferol. Proc. R. Soc. Med. 1945, 39, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.B.; Prosser Thomas, E.W.; Wallace, H.J. Lupus Vulgaris treated with Calciferol. Proc. R. Soc. Med. 1946, 39, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.B.; Prosser, E.W. Treatment of lupus vulgaris with Calciferol. Lancet 1946, 22, 919–922. [Google Scholar] [CrossRef]
- Raab, W. Vitamin D—Its bactericidal action. Chest 1946, 12, 409–415. [Google Scholar] [CrossRef]
- Michelson, H.E.; Steves, R.J. Treatment of cutaneous tuberculosis with large doses of vitamin D. Arch. Derm. Syphilol. 1947, 56, 317–324. [Google Scholar] [CrossRef]
- Tomlinson, K.M. Calcium content of skin in lupus vulgaris treated with Calciferol. Lancet 1948, 251, 327–328. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S. Vitamin D, cod liver oil, sunshine and phototherapy: Safe, effective and forgotten tools for treating and curing tuberculosis infections—A comprehensive review. J. Steroid Biochem. Mol. Biol. 2018, 177, 21–29. [Google Scholar] [CrossRef]
- Grzybowski, A.; Pietrzak, K. From patient to discoverer—Niels Ryberg Finsen (1860–1904)—The founder of phototherapy in dermatology. Clin. Dermatol. 2012, 30, 451–455. [Google Scholar] [CrossRef]
- Honigsmann, H. History of phototherapy in dermatology. Photochem. Photobiol. Sci. 2013, 12, 16–21. [Google Scholar] [CrossRef]
- Honigsmann, H. Phototherapy. J. Invest Dermatol. 2013, 133, E18–E20. [Google Scholar] [CrossRef][Green Version]
- Grzybowski, A.; Sak, J.; Pawlikowski, J. A brief report on the history of phototherapy. Clin Dermatol. 2016, 34, 532–537. [Google Scholar] [CrossRef]
- Matos, T.R.; Sheth, V. The symbiosis of phototherapy and photoimmunology. Clin. Dermatol. 2016, 34, 538–547. [Google Scholar] [CrossRef]
- Hess, A.F.; Unger, L.J. The cure of infantile rickets by sunshine. JAMA 1921, 77, 39. [Google Scholar]
- Rajakumar, K. Vitamin D, cod-liver oil, sunlight, and rickets: A historical perspective. Pediatrics 2003, 112, e132–e135. [Google Scholar] [CrossRef]
- Williams, C.J.B. On the use and administration of cod-liver oil in pulmonary consumption. Lond. J Med. Jan. 1849, 2, 50. [Google Scholar] [CrossRef]
- Morner, K.A.H. Nobel Prize for Physiology or Medicine Award Ceremony Speech. 10 December 1903. Available online: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/press.html (accessed on 10 January 2021).
- Biography of Dr Neils Ryberg-Finsen. Available online: https://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/finsen-bio.html (accessed on 10 January 2021).
- Aitken, R. Lupus vulgaris with special reference to its treatment with the finsenhomholt lamp. Br. Med. J. 1937, 23, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Masten, A.R. Good Climate—An Asset in the Treatment of Tuberculosis. Chest 1937, 3, 20–24. [Google Scholar] [CrossRef]
- Van Der Lugt, L.; Rottier, P.B. Finsen therapy and vitamin D. Acta Derm. Venerol. 1958, 38, 264–273. [Google Scholar] [PubMed]
- Tavera-Mendoza, L.E.; White, J.H. Cell Defenses and the Sunshine Vitamin. Sci. Am. 2007, 297, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Gotzsche, P.C. Niels Finsen’s treatment for lupus vulgaris. J. R. Soc. Med. 2011, 104, 41–42. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; IOM (Institute of Medicine): Washington, DC, USA; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R.P. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Araki, T.; Holick, M.F.; Alfonso, B.D.; Charlap, E.; Romero, C.M.; Rizk, D.; Newman, L.G. Vitamin D Intoxication with Severe Hypercalcemia due to Manufacturing and Labeling Errors of Two Dietary Supplements Made in the United States. J. Clin. Endocrinol. Metab. 2011, 96, 3603–3608. [Google Scholar] [CrossRef]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB phototherapy and oral calcitriol (1,25-dihydroxyvitamin D3) on vitamin D photosynthesis in patients with psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar] [CrossRef]
- Ryan, C.; Moran, B.; McKenna, M.J.; Murray, B.F.; Brady, J.; Collins, P.; Rogers, S.; Kirby, B. The Effect of Narrowband UV-B Treatment for Psoriasis on Vitamin D Status during Wintertime in Ireland. Arch. Dermatol. 2010, 146, 836–842. [Google Scholar] [CrossRef]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larko, O.; Wennberg, A.M.; Krogstad, A.L. Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol. Photoimmunol. Photomed. 2009, 25, 112–123. [Google Scholar] [CrossRef]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larko, O.; Krogstad, A.L. Vitamin D status in psoriasis patients during different treatments with phototherapy. J. Photochem. Photobiol. B Biol. 2010, 101, 117–123. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Di Gioia, G. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021, 44, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Kurian, S.J.; Bagchi, D.; Manu, M.K.; Saravu, K.; Unnikrishnan, M.K.; Mukhopadhyay, C.; Rao, M.; Miraj, S.S. Vitamin D in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. J. Am. Coll. Nutr. 2020, 1–14. [Google Scholar] [CrossRef]
- Benskin, L.L. A Basic Review of the Preliminary Evidence that COVID-19 Risk and Severity is Increased in Vitamin D Deficiency. Front. Public Health 2020, 8, 513. [Google Scholar] [CrossRef]
- Crane-Godreau, M.A.; Clem, K.J.; Payne, P.; Fiering, S. Vitamin D Deficiency and Air Pollution Exacerbate COVID-19 Through Suppression of Antiviral Peptide LL37. Front. Public Health 2020, 8, 232. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S. Commentary. Eliminating vitamin D deficiency during the COVID-19 pandemic: A call to action. Metab. Clin. Exp. 2020, 112, 154322. [Google Scholar] [CrossRef]
- Giménez, V.M.M.; Inserra, F.; Ferder, L.; García, J.; Manucha, W. Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2. J. Hum. Hypertens. 2021, 35, 378–380. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Pirab, K.; Van Den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalized older patients with COVID-19. Postgrad. Med. J. 2020, 1–6. [Google Scholar] [CrossRef]
- Davies, G.; Garami, A.R.; Byers, J. Evidence Supports a Causal Model for Vitamin D in COVID-19 Outcomes. MedRxiv 2020, 1–40. [Google Scholar] [CrossRef]
- Slominski, R.M.; Stefan, J.; Athar, M.; Holick, M.F.; Jetten, A.M.; Raman, C.; Slominski, A.T. COVID-19 and Vitamin D: A lesson from the skin. Exp. Dermatol. 2020, 29, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Slominski, R.M.; Goepfert, P.A.; Kim, T.; Holick, M.F.; Jetten, A.M.; Raman, C. Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? Am. J. Physiol. Endocrinol. Metab. 2020, 319, E455–E457. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25- hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Hadizadeh, F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2021, 79, 200–208. [Google Scholar] [CrossRef]
- Ferder, L.; Martín Giménez, V.M.; Inserra, F.; Tajer, C.; Antonietti, L.; Mariani, J.; Manucha, W. Vitamin D Supplementation As A Rational Pharmacological Approach In The Covid-19 Pandemic. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L941–L948. [Google Scholar] [CrossRef]
- Castillo, M.E.; Costa, L.M.E.; Barrios, J.M.V.; Díaz, J.F.A.; Miranda, J.L.; Bouillon, R.; Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Morimoto, S.; Onishi, T.; Imanaka, S.; Yukawa, H.; Kozuka, T.; Kitano, Y.; Yoshikawa, K.; Kumahara, Y. Topical administration of 1,25-dihydroxyvitamin D3 for psoriasis: Report of five cases. Calcif. Tissue Int. 1986, 38, 119–122. [Google Scholar] [CrossRef]
- Lugo-Somolinos, A.; Sanchez, J.L.; Haddock, L. Efficacy of 1, alpha 25-dihidroxyvitamin D (Calcitriol) in the treatment of psoriasis vulgaris: An open study. Bol. Asoc. Med. Puerto Rico 1990, 82, 450–453. [Google Scholar]
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Investig. 1986, 78, 557–566. [Google Scholar] [CrossRef]
- Milde, P.; Hauser, U.; Simon, T.; Mall, G.; Ernst, V.; Haussler, M.R.; Frosch, P.; Rauterberg, E.W. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J. Invest. Dermatol. 1991, 97, 230–239. [Google Scholar] [CrossRef]
- Araujo, O.E.; Flowers, F.P.; Brown, K.D. Vitamin D Therapy in Psoriasis. DICP Ann. Pharmacother. 1991, 25, 835–839. [Google Scholar] [CrossRef]
- Berth-Jones, J.; Hutchinson, P.E. Vitamin D analogues and psoriasis. Br. J. Dermatol. 1992, 127, 71–78. [Google Scholar] [CrossRef]
- Boisseau-Gersaud, A.M.; Legrain, V.; Hehunstre, J.P.; Maleville, J.; Taieb, A. Treatment of psoriasis by oral calcitriol. A study of 5 cases and review of the literature. Ann. Dermatol. Venereol. 1993, 120, 669–674. [Google Scholar]
- El-Azhary, R.A.; Peters, M.S.; Pittelkow, M.R.; Kao, P.C.; Muller, S.A. Efficacy of Vitamin D3 Derivatives in the Treatment of Psoriasis Vulgaris: A Preliminary Report. Mayo Clin. Proc. 1993, 68, 835–841. [Google Scholar] [CrossRef]
- Samini, M.; Seirafi, H.; Eftekhari, H.R. Efficacy and safety of topical cholecalciferol, calcitriol, and calcipitol in the treatment of plaque psoriasis: A comparative study. Acta Med. Iran. 1998, 36, 19–27. [Google Scholar]
- Takahashi, H.; Ibe, M.; Kinouchi, M.; Ishida-Yamamoto, A.; Hashimoto, Y.; Lizuka, H. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J. Dermatol. Sci. 2003, 31, 21–28. [Google Scholar] [CrossRef]
- Lebwohl, M.; Menter, A.; Weiss, J.; Clark, S.D.; Flores, J.; Powers, J.; Balin, A.K.; Kempers, S.; Glinert, R.J.; Fleming, T.; et al. Calcitriol 3 microg/g ointment in the management of mild to moderate plaque type psoriasis: Results from 2 placebo-controlled, multicenter, randomized double-blind, clinical studies. J. Drugs Dermatol. 2007, 6, 428–435. [Google Scholar]
- Gold, L.F. Calcitriol ointment: Optimizing psoriasis therapy. J. Drugs Dermatol. 2009, 8, s23–s27. [Google Scholar]
- Abramovitz, W. Calcitriol 3 microg/g ointment: An effective and safe addition to the armamentarium in topical psoriasis therapy. J. Drugs Dermatol. 2009, 8, s17–s22. [Google Scholar]
- Kircik, L. Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis. J. Drugs Dermatol. 2009, 8, s9–s16. [Google Scholar] [PubMed]
- Lehmann, B. Role of the vitamin D3 pathway in healthy and diseased skin—Facts, contradictions and hypotheses. Exp. Dermatol. 2009, 18, 97–108. [Google Scholar] [CrossRef]
- Gaal, J.; Lakos, G.; Zodoray, P.; Kiss, J.; Horvath, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm. Venereol. 2009, 89, 140–144. [Google Scholar] [PubMed]
- O’Neill, J.L.; Feldman, S.R. Vitamin D analogue-based therapies for psoriasis. Drugs Today 2010, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Laws, P.M.; Young, H.S. Topical treatment of psoriasis. Expert Opin. Pharmacother. 2010, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Meurer, M. Vitamin D metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Oquendo, M.; Abramovits, W.; Morrell, P. Topical vitamin D analogs available to treat psoriasis. Skinmed 2012, 10, 356–360. [Google Scholar]
- Lebwohl, M.; Preston, N.; Gottschalk, R.W. Impact of Baseline Disease Severity Over 26 and 52 Weeks of Treatment with Calcitriol Ointment 3mcg/g in Patients with Mild-to-moderate Plaque Psoriasis. J. Clin. Aesthetic Dermatol. 2012, 5, 28–33. [Google Scholar]
- Sawyer, L.; Samarasekera, E.J.; Wonderling, D.; Smith, C.H. Topical therapies for the treatment of localized plaque psoriasis in primary care: A cost-effectiveness analysis. Br. J. Dermatol. 2013, 168, 1095–1105. [Google Scholar] [CrossRef]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef]
- Wadhwa, B.; Relhan, V.; Kochar, A.M.; Garg, V.K. Vitamin D and skin diseases: A review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef]
- Barrea, L.; Savanelli, M.C.; Somma, C.; Napolitano, M.; Megna, A.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Hambly, R.; Kirby, B. The relevance of serum vitamin D in psoriasis: A review. Arch. Dermatol. Res. 2017, 309, 499–517. [Google Scholar] [CrossRef]
- Paul, C.; Leonardi, C.; Menter, A. Calcipotriol Plus Betamethasone Dipropionate Aerosol Foam in Patients with Moderate-to-Severe Psoriasis: Sub-Group Analysis of the PSO-ABLE Study. Am. J. Clin. Dermatol. 2017, 18, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Stamp, T.C.B.; Haddad, J.G.; Twigg, C.A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1977, 309, 1341–1343. [Google Scholar] [CrossRef]
- Holick, M. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638S–645S. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and Skin Physiology: A D-Lightful Story. J. Bone Miner. Res. 2007, 22, V28–V33. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A D-Lightful Solution for Health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Amend, J. Results of daily oral dosing with up to 60,000 international units of vitamin D3 for 2 to 6 years in 3 adult males. J. Steroid Biochem. Mol. Biol. 2017, 173, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Hearn, R.M.R.; Kerr, A.C.; Rahim, K.F.; Ferguson, J.; Dawe, R.S. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br. J. Dermatol. 2008, 159, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Koek, M.; Buskens, E.; van Weelden, H.; Steegmans, P.H.A.; Bruijnzeel-Koomen, C.A.F.M.; Sigurdsson, V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: Pragmatic multicenter randomized controlled non-inferiority trial (PLUTO study). BMJ 2009, 338, b1542. [Google Scholar] [CrossRef]
- Koek, M.; Sigurdsson, V.; van Weelden, H.; Steegmans, P.H.A.; Bruijnzeel-Koomen, C.A.F.M.; Buskens, E. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: Economic evaluation of a randomized controlled trial (PLUTO study). BMJ 2010, 340, c1490. [Google Scholar] [CrossRef]
- Vahavihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Renunala, T. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.M.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J. Am. Acad. Dermatol. 2010, 62, 114–135. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Ishida-Yamamoto, A.; Lizuka, H. Comparison of clinical effects of psoriasis treatment regimens among calcipotriol alone, narrowband ultraviolet B phototherapy alone, combination of calcipotriol and narrowband ultraviolet B phototherapy once a week, and combination of calcipotriol and narrowband ultraviolet B phototherapy more than twice a week. J. Dermatol. 2013, 40, 424–427. [Google Scholar] [CrossRef]
- Wong, T.; Hsu, L.; Liao, W. Phototherapy in Psoriasis: A Review of Mechanisms of Action. J. Cutan. Med. Surg. 2013, 17, 6–12. [Google Scholar] [CrossRef]
- Shors, A.; Williams, L.; Fishman, P. Cost of prevalent psoriasis. J. Am. Acad. Dermatol. 2014, 70, ABBO. [Google Scholar]
- Lim, H.W.; Silpa-archa, N.; Amadi, U.; Menter, A.; Van Vorhees, A.S.; Lebwohl, M. Phototherapy in dermatology: A call for action. J. Am. Acad. Dermatol. 2015, 72, 1078–1080. [Google Scholar] [CrossRef]
- Foerster, J.; Boswell, K.; West, J.; Cameron, H.; Fleming, C.; Ibbotson, S.; Dawe, R. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE 2017, 12, e0181813. [Google Scholar] [CrossRef]
- Reichrath, J.; Saternusa, R.; Vogta, T. Challenge and perspective: The relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases. Photochem. Photobiol. Sci. 2017, 16, 433–444. [Google Scholar] [CrossRef]
- Boswell, K.; Cameron, H.; West, J.; Fleming, C.; Ibbotson, S.; Dawe, R.; Foerster, J. Narrowband ultraviolet B treatment for psoriasis is highly economical and causes significant savings in cost for topical treatments. Br. J. Dermatol. 2018, 179, 1148–1156. [Google Scholar] [CrossRef]
- Hyde, K.; Cardwell, L.A.; Stotts, R.; Feldman, S.R. Psoriasis Treatment Cost Comparison: Biologics Versus Home Phototherapy. Am. J. Pharm. Benefits 2018, 10, 18–21. [Google Scholar]
- Tanew, A.; Lim, H.W. Phototherapy for psoriasis—Outdated or underused? Br. J. Dermatol. 2018, 179, 1019–1020. [Google Scholar] [CrossRef]
- Kimball, S.M.; Lee, J.; Vieth, R. Sunbeds with UVB radiation can produce physiological levels of serum 25-Hydroxyvitamin D in healthy volunteers. Derm. Endocrinol. 2018, 9, e1375635. [Google Scholar] [CrossRef]
- Hart, P.H.; Norval, M.; Byrne, S.N.; Rhodes, L.E. Exposure to Ultraviolet Radiation in the Modulation of Human Diseases. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 55–81. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Norman, A. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Norman, A.; Bouillon, R. Vitamin D nutritional policy needs a vision for the future. Exp. Biol. Med. 2010, 235, 1034–1045. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef]
- Pike, J.W. Genome-wide principles of gene regulation by the vitamin D receptor and it’s activating ligand. Mol. Cell. Endocrinol. 2011, 347, 3–10. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha, 25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef]
- Penna, G.; Amuchastegui, S.; Giarrantana, N.; Daniel, K.C.; Vulcano, M.; Sozzani, S.; Adorini, L. 1,25-Dihydroxyvitamin D3 Selectively Modulates Tolerogenic Properties in Myeloid but Not Plasmacytoid Dendritic Cells. J. Immunol. 2007, 178, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and innate immunity. Curr. Opin. Investig. Drugs 2008, 9, 485–490. [Google Scholar] [PubMed]
- Sezeles, L.; Keresztes, G.; Torocsik, D.; Balajthy, Z.; Krenacs, L.; Poliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-Dihydroxyvitamin D3 Is an Autonomous Regulator of the Transcriptional Changes Leading to a Tolerogenic Dendritic Cell Phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Ødum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Coussens, A. Immunomodulatory Actions of Vitamin D Metabolites and their Potential Relevance to Human Lung Disease. Curr. Respir. Med. Rev. 2011, 7, 444–453. [Google Scholar] [CrossRef]
- Khoo, A.; Joosten, I.; Michels, M.; Woestenenk, R.; Preijers, F.; He, X.; Netea, M.H.; van der Ven, A.J.A.M.; Koenen, H.J.P.M. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology 2011, 134, 459–468. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and innate and adaptive immunity. Vitam. Horm. 2011, 86, 23–62. [Google Scholar] [CrossRef]
- Peelen, E.; Knippenberg, S.; Muris, A.H.; Thewissen, M.; Smolders, J.; Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun. Rev. 2011, 10, 733–743. [Google Scholar] [CrossRef]
- Hewison, M. An update of vitamin D and human immunity. Clin. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, T.; Roep, B.O. Regulatory multitasking of tolerogenic dendritic cells—lessons taken from vitamin D3-treated tolerogenic dendritic cells. Front. Immunol. 2013, 4, 113. [Google Scholar] [CrossRef]
- Brosbøl-Ravnborg, A.; Bundgaard, B.; Hoollsberg, P. Synergy between Vitamin D3 and Toll-Like Receptor Agonists Regulates Human Dendritic Cell Response during Maturation. Clin. Dev. Immunol. 2013, 2013, 807971. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function:lessons learned from genome-wide analysis. Front. Physiol. Integr. Physiol. 2014, 5, 151. [Google Scholar]
- Bscheider, M.; Butcher, E.C. Vitamin D immunoregulation through dendritic cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef]
- Vanherwegen, A.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Gorman, S.; Kuritsky, L.A.; Judge, M.A.; Dixon, K.M.; McGlade, J.P.; Mason, R.S.; Finlay, J.J.; Hart, P.H. Topically Applied 1,25-Dihydroxyvitamin D3 Enhances the Suppressive Activity of CD4 + CD25+ Cells in the Draining Lymph Nodes. J. Immunol. 2007, 179, 6273–6283. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Bach, P.; Obst, J.; Komba, M.; Fleet, J.C.; Dutz, J.P. Expansion of Antigen-Specific Regulatory T Cells with the Topical Vitamin D Analog Calcipotriol. J. Immunol. 2009, 182, 6071–6078. [Google Scholar] [CrossRef]
- Gorman, S.; Judge, M.; Hart, P. Immune-modifying properties of topical vitamin D: Focus on dendritic cells and T cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 247–249. [Google Scholar] [CrossRef]
- Bakdash, G.; Schneider, L.P.; van Capel, T.; Kapsenberg, M.L.; Teunissen, M.; de Jong, E. Intradermal application of vitamin D3 increases migration of CD14+ dermal dendritic cells and promotes the development of Foxp3+ regulatory T cells. Hum. Vaccines Immunother. 2013, 9, 250–258. [Google Scholar] [CrossRef]
- Lovatoa, P.; Norsgaarda, H.; Tokurac, Y.; Røpke, M.A. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J. Dermatol. Sci. 2016, 81, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Shimizu, T.; Tada, Y.; Kamata, M.; Takeoka, S.; Shibata, S.; Mitsut, A.; Asano, Y.; Sugaya, M.; Kadono, T.; et al. The vitamin D3 analog, maxacalcitol, reduces psoriasiform skin inflammation by inducing regulatory T cells and downregulating IL-23 and IL-17 production. J. Dermatol. Sci. 2018, 92, 117–126. [Google Scholar] [CrossRef]
- Adorini, L.; Giarratana, N.; Penna, G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin. Immunol. 2004, 16, 127–134. [Google Scholar] [CrossRef]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.; et al. Reversing the defective induction of IL-10 secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef]
- Ardalan, M.; Maljaei, H.; Shoja, M.M.; Piri, A.R.; Khosroshahi, H.T.; Noshad, H.; Argani, H. Calcitriol Started in the Donor, Expands the Population of CD4+ CD25+ T Cells in Renal Transplant Recipients. Transplant. Proc. 2007, 39, 951–953. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Gorman, S.; Judge, M.A.; Hart, P.H. Gene Regulation by 1,25-Dihydroxyvitamin D3 in CD4+ CD25+ Cells Is Enabled by IL-2. J. Investig. Dermatol. 2010, 130, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.; Judge, M.A.; Burchell, J.T.; Turner, D.J.; Hart, P.H. 1,25-dihydroxyvitamin D3 enhances the ability of transferred CD4+ CD25+ cells to modulate T helper type 2-driven asthmatic responses. Immunology 2010, 130, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Pilz, S.; Wolf, M.; Tomaschitz, A.; Obermayer-Pietsch, B.; Graninger, W.; Pieber, T.R. Vitamin D Supplementation and Regulatory T Cells in Apparently Healthy Subject: Vitamin D Treatment for Autoimmune Diseases. IMAJ 2010, 12, 136–139. [Google Scholar] [PubMed]
- Smolders, J.; Peelen, E.; Thewissen, M.; Tervaert, J.W.C.; Menheere, P.; Hupperts, R.; Dmoiseaux, J. Safety and T Cell Modulating Effects of High Dose Vitamin D3 Supplementation in Multiple Sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef]
- Zold, E.; Szodoray, P.; Kappelmayer, J.; Gaal, J.; Csathy, L.; Barath, S.; Gyimesi, E.; Hajas, A.; Zeher, M.; Szegedi, G.; et al. Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand. J. Rheumatol. 2010, 39, 490–497. [Google Scholar] [CrossRef]
- Zold, E.; Szodoray, P.; Nakken, B.; Barath, S.; Kappelmayer, J.; Csathy, L.; Hajas, A.; Sipka, S.; Gyimesi, E.; Gaal, J.; et al. Alfacalcidol treatment restores derailed immune-regulation in patients with undifferentiated connective tissue disease. Autoimmun. Rev. 2011, 10, 155–162. [Google Scholar] [CrossRef]
- Bobryshev, Y. Vitamin D3 Suppresses Immune Reactions in Atherosclerosis, Affecting Regulatory T Cells and Dendritic Cell Function. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2317–2319. [Google Scholar] [CrossRef]
- Van der Aar, D.A.M.; Sibiryak, D.S.; Bakdash, G.; van Capel, T.M.M.; van der Kleij, H.P.M.; Opstelten, J.E.; Teunissen, M.B.M.; Kapsenberg, M.L.; de Jong, E.C. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J. Allergy Clin. Immunol. 2011, 127, 1532–1540.e7. [Google Scholar] [CrossRef]
- Zold, E.; Barta, Z.; Bodolay, E. Vitamin D Deficiency and Connective Tissue Disease. Vitam. Horm. 2011, 86, 261–286. [Google Scholar] [CrossRef]
- Takeda, M.; Yamashita, T.; Sasaki, N.; Nakajima, K.; Kita, T.; Shinohara, M. Oral Administration of an Active Form of Vitamin D3 (Calcitriol) Decreases Atherosclerosis in Mice by Inducing Regulatory T Cells and Immature Dendritic Cells with Tolerogenic Functions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2495–2503. [Google Scholar] [CrossRef]
- Chambers, E.S.; Hawrylowicz, C.M. The impact of vitamin D on regulatory T cells. Curr. Allergy Asthma Rep. 2011, 11, 29–36. [Google Scholar] [CrossRef]
- Bock, G.; Prietl, B.; Mader, J.K.; Holler, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-pietsch, B.M.; Pieber, T.R. The effect of vitamin D supplementation on peripheral regulatory T cells and B cell function in healthy humans: A randomized controlled trial. Diabetes Metab. Res. Rev. 2011, 27, 942–945. [Google Scholar] [CrossRef]
- Kilick, J.; Hay, J.; Morandi, E.; Vermeren, S.; Kari, S.; Angles, T.; Williams, A.; Damoiseaux, J.; Astier, A.L. Vitamin D/CD46 Crosstalk in Human T Cells in Multiple Sclerosis. Front. Immunol. 2020. [Google Scholar] [CrossRef]
- Cantorna, M. Vitamin D, Multiple Sclerosis and Inflammatory Bowel Disease. Arch. Biochem. Biophys. 2012, 523, 103–106. [Google Scholar] [CrossRef]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef]
- Ooi, J.H.; Chen, J.; Cantorna, M.T. Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol. Asp. Med. 2012, 33, 77–82. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S. Availability of 25-hydroxyvitamin D3 to antigen presenting cells controls the balance between regulatory and inflammatory T cell responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Farias, A.S.; Spagnol, G.S.; Bordeaux-Rego, P.; Oliveira, C.O.F.; Fontana, A.G.M.; de Paula, R.F.O.; Santos, M.P.A.; Pradella, F.; Moraes, A.S.; Oliveira, E.C.; et al. Vitamin D3 Induces IDO+ Tolerogenic DCs and Enhances Treg, Reducing the Severity of EAE. CNS Neurosci. Ther. 2013, 19, 269–277. [Google Scholar] [CrossRef]
- Baráth, S.; Nagy, G.; Zöld, E.; Csípo, I.; Gyimesi, E.; Zeher, M.; Bodolay, E. Conductor of regulatory cells: Does vitamin D restore the shifted balance of the distinct regulatory cell types in undifferentiated connective tissue disease? Immunol. Lett. 2013, 153, 71–72. [Google Scholar] [CrossRef][Green Version]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin D receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Gupta, A.; Dimeloe, S.; Richards, D.F.; Chambers, E.S.; Black, C.; Urry, Z.; Ryanna, K.; Xystrakis, E.; Bush, A.; Saglani, S.; et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax 2014, 69, 508–515. [Google Scholar] [CrossRef]
- Chambers, E.S.; Suwannasaen, D.; Mann, E.H.; Urry, Z.; Richards, D.F.; Lertmemongkolchai, G.; Hawrylowicz, C.M. 1a,25-dihydroxyvitamin D3 in combination with transforming growth factor-b increases the frequency of Foxp3+ regulatory T cells through preferential expansion and usage of interleukin-2. Immunology 2014, 143, 52–60. [Google Scholar] [CrossRef]
- Cantorna, M.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Mader, J.K.; Hoeller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur. J. Nutr. 2014, 53, 751. [Google Scholar] [CrossRef] [PubMed]
- Keating, P.; Munim, A.; Hartmann, J.X. Effect of vitamin D on T-helper type 9 polarized human memory cells in chronic persistent asthma. Ann. Allergy Asthma Immunol. 2014, 112, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, T.L.; Vanherwegen, A.S.; Feyaerts, D.; De Clercq, P.; Verstuyf, A.; Korf, H.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 and Its Analog TX527 Promote a Stable Regulatory T Cell Phenotype in T Cells from Type 1 Diabetes Patients. PLoS ONE 2014, 9, e109194. [Google Scholar] [CrossRef]
- Cantorna, M.; Snyder, L.; Lin, Y.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J. Vitamin D as an Immunomodulator: Risks with Deficiencies and Benefits of Supplementation. Healthcare 2015, 3, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.E.; Hubler, S.L.; Moore, J.R. Vitamin D actions on CD4CT cells in autoimmune disease. Front. Immunol. TCell Biol. 2015, 6, 100. [Google Scholar] [CrossRef]
- Bizzaro, G.; Shoenfeld, Y. Vitamin D: A panacea for autoimmune diseases? Can. J. Physiol. Pharmacol. 2015, 93, 395–397. [Google Scholar] [CrossRef]
- Ghoryania, M.; Sahebarib, M.; Mahmoudia, M.; Abdollahib, N.; Reihania, H.; Rabe, S.Z.T.; Tabasi, N.; Rastin, M. Immunomodulatory vitamin D effects on regulatory T-cells and cytokines in an in vitro study on patients with systemic lupus erythematosus. Food Agric. Immunol. 2016, 27, 377–387. [Google Scholar] [CrossRef][Green Version]
- Konijeti, G.G.; Arora, P.; Boylan, M.R.; Song, Y.; Huang, S.; Harrell, F.; Newton-Cheh, C.; O’Neill, D.; Korzenik, J.; Wang, T.J.; et al. Vitamin D Supplementation Modulates T Cell–Mediated Immunity in Humans: Results from a Randomized Control Trial. J. Clin. Endocrinol. Metab. 2016, 101, 533–538. [Google Scholar] [CrossRef]
- Gorman, S.; Geldenhuys, S.; Judge, M.; Weeden, C.E.; Waithman, J.; Hart, P.H. Dietary Vitamin D Increases Percentages and Function of Regulatory T Cells in the Skin-Draining Lymph Nodes and Suppresses Dermal Inflammation. J. Immunol. Res. 2016, 2016, 1426503. [Google Scholar] [CrossRef]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNbeta; the SOLARIUM study. J. Neuroimmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef]
- Fawaz, L.; Mrad, M.F.; Kazan, J.M.; Sayagh, S.; Akika, R.; Khoury, S.J. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin. Immunol. 2016, 166, 59–71. [Google Scholar] [CrossRef]
- Şıklar, Z.; Karataş, D.; Doğu, F.; Hacıhamdioğlu, B.; İkincioğulları, A.; Berberoğlu, M. Regulatory T Cells and Vitamin D Status in Children with Chronic Autoimmune Thyroiditis. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 276–281. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell. Endocrinol. 2017, 453, 68–78. [Google Scholar] [CrossRef]
- Zhoua, Q.; Qinb, S.; Zhanga, J.; Zhona, L.; Pena, Z.; Xinga, T. 1,25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-gamma1/TGF-beta1/pathway. Mol. Immunol. 2017, 91, 156–164. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is vitamin D Receptor (vDR) polymorphism the Culprit? IMAJ 2017, 19, 438–443. [Google Scholar] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.; Geldenhuys, S.; Weeden, C.E.; Grimbaldeston, M.A.; Hart, P.H. Investigating the roles of regulatory T cells, mast cells and interleukin-9 in the control of skin inflammation by vitamin D. Arch. Dermatol. Res. 2018, 310, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Smolders, J. The Engagement Between Vitamin D and the Immune System: Is Consolidation by a Marriage to Be Expected? EBioMedicine 2018, 31, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.; Eelenb, G.; Ferreira, G.B.; Ghesquiere, B.; Cook, D.P.; Nikolic, T.; Roep, B.; Carmeliet, P.; Telang, S.; Mathieu, C.; et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019, 187, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Heier, I.; Soyland, E.; Krogstad, A.; Rodriguez-Gallego, C.; Nenseter, M.S.; Jahnsen, F.L. Sun exposure rapidly reduces plasmacytoid dendritic cells and inflammatory dermal dendritic cells in psoriatic skin. Br. J. Dermatol. 2011, 165, 792–801. [Google Scholar] [CrossRef]
- Soyland, E.; Heier, I.; Rodriguez-Gallego, C.; Molines, T.E.; Johansen, F.-E.; Holven, K.B.; Halvorsen, B.; Aukrust, P.; Jahnsen, F.L.; de la Rosa Carrillo, D.; et al. Sun exposure induces rapid immunological changes in skin and peripheral blood in patients with psoriasis. Br. J. Dermatol. 2011, 164, 344–355. [Google Scholar] [CrossRef]
- Khoo, A.L.; Koenen, H.J.; Chai, L.Y.; Sweep, F.C.; Netea, M.G.; van der Ven, A.J.; Joosten, I. Seasonal Variation in Vitamin D3 Levels Is Paralleled by Changes in the Peripheral Blood Human T Cell Compartment. PLoS ONE 2012, 7, e29250. [Google Scholar] [CrossRef]
- Schwarz, A.; Maeda, A.; Wild, M.K.; Kernebeck, K.; Gross, N.; Aragane, Y.; Beissert, S.; Vestweber, D.; Schwarz, T. Ultraviolet Radiation-Induced Regulatory T Cells Not Only Inhibit the Induction but Can Suppress the Effector Phase of Contact Hypersensitivity. J. Immunol. 2004, 172, 1036–1043. [Google Scholar] [CrossRef]
- Schwarz, T. Regulatory T cells induced by ultraviolet radiation. Int. Arch. Allergy Immunol. 2005, 137, 187–193. [Google Scholar] [CrossRef]
- Schwarz, T. 25 years of UV-induced Immunosuppression Mediated by T Cells—From Disregarded T Suppressor Cells to Highly Respected Regulatory T Cells. Photochem. Photobiol. 2008, 84, 10–18. [Google Scholar] [CrossRef]
- Shintani, Y.; Yasuda, Y.; Kobayashi, K.; Maeda, A.; Morita, A. Narrowband ultraviolet B radiation suppresses contact hypersensitivity. Photoderm. Photoimmunol. Photomed. 2008, 24, 32–37. [Google Scholar] [CrossRef]
- Loser, K.; Beissert, S. Regulation of cutaneous immunity by the environment: An important role for UV irradiation and vitamin D. Int. Immunopharmacol. 2009, 9, 587–589. [Google Scholar] [CrossRef]
- McGlade, J.P.; Strickland, D.H.; Lambert, M.J.; Gorman, S.; Thomas, J.A.; Judge, M.A.; Burchell, J.T.; Zosky, G.R.; Hart, P.H. UV inhibits allergic airways disease in mice by reducing effector CD4 T cells. Clin. Exp. Allergy 2010, 40, 772–785. [Google Scholar] [CrossRef]
- Gorman, S.; McGlade, J.P.; Lambert, M.J.M.; Strickland, D.H.; Thomas, J.A.; Hart, P.H. UV exposure and protection against allergic airways disease. Photochem. Photobiol. Sci. 2010, 9, 571–577. [Google Scholar] [CrossRef]
- Racz, E.; Prens, E.P.; Kurek, D.; Kant, M.; de Ridder, D.; Mourits, S.; Barveldt, E.M.; Ozgur, Z.; van IJcken, R.F.J.; Laman, J.D.; et al. Effective treatment of psoriasis with narrowband UVB phototherapy is linked to suppression of the IFN and Th17 pathways. J. Ibvest. Dermatol. 2011, 131, 1547–1558. [Google Scholar] [CrossRef]
- Milliken, S.V.I.; Wassall, H.; Lewis, B.J.; Logie, J.; Barker, R.N.; Macdonald, H.; Vickers, M.A.; Ormerod, A.D. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J. Allergy Clin. Immunol. 2012, 129, 1554–1561. [Google Scholar] [CrossRef]
- Furuhashi, T.; Saito, C.; Torii, K.; Nishida, E.; Yamazaki, S.; Morita, A. Photo(chemo)therapy Reduces Circulating TH17 Cells and Restores Circulating Regulatory T Cells in Psoriasis. PLoS ONE 2013, 8, e54895. [Google Scholar] [CrossRef]
- Yamazaki, S.; Nishioka, A.; Kasuya, S.; Ohkura, N.; Hemmi, H.; Kaisho, T.; Taguchi, O.; Sakaguchi, S.; Morita, A. Homeostasis of Thymus-Derived Foxp3+ Regulatory T Cells Is Controlled by Ultraviolet B Exposure in the Skin. J. Immunol. 2014, 193, 5488–5497. [Google Scholar] [CrossRef]
- Breuer, J.; Schwab, N.; Schneider-Hohendorf, T.; Marziniak, M.; Mohan, H.; Bhatia, U.; Gross, C.C.; Clausen, B.E.; Weishaupt, C.; Luger, T.A.; et al. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann. Neurol. 2014, 75, 739–758. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Gong, Y.; Liu, Y.; Gu, J.; Chen, W.; Shi, Y. Disruption of circulating CD4+ T-lymphocyte subpopulations in psoriasis patients is ameliorated by narrow-band UVB therapy. Cell Biochem. Biophys. 2015, 71, 499–507. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Besgen, P.; Wolf, R.; Szepietowski, J.C.; Prinz, J.C. The effect of phototherapy on systemic inflammatory process in patients with plaque psoriasis. J. Photochem. Photobiol. B Biol. 2016, 161, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bialecka, M.; Ostasz, R.; Kurzawski, M.; Klimowicz, A.; Fabianczyk, H.; Bojko, P.; Dziedziejko, V.; Safranow, K.; Machoy-Mokrzynska, A.; Drozdzik, M. IL17A and IL17F gene polymorphism association with psoriasis risk and response to treatment in a polish population. Dermatology 2016, 232, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Gober, M.; Yang, X.; Katlinski, K.V.; Marshall, C.M.; Sharma, M.; Werth, V.P.; Baker, D.P.; Rui, H.; Seykora, J.T.; et al. Therapeutic elimination of the type 1 interferon receptor for treating psoriatic skin inflammation. J. Investig. Dermatol. 2016, 136, 1990–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Y.; Chen, L.; Yang, R.; Wang, L.; Liu, W.; Zhai, Z.; Shen, Z. Ultraviolet irradiation promotes FOXP3 transcription via p53 in psoriasis. Exp. Dermatol. 2016, 25, 513–518. [Google Scholar] [CrossRef]
- Bora, S.; Cantorna, M.T. The role of UVR and vitamin D on T cells and inflammatory bowel disease. Photochem. Photobiol. Sci. 2017, 16, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Racz, E.; Prens, E.P. Phototherapy of Psoriasis, a Chronic Inflammatory Skin Disease. Adv. Exp. Med. Biol. 2017, 996, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Odanaka, M.; Nishioka, A.; Kasuya, S.; Shime, H.; Hemmi, H.; Imai, M.; Riethmacher, D.; Kaisho, T.; Ohkura, N.; et al. Ultraviolet B Induced Maturation of CD11b-Type Langerin- Dendritic Cells Controls the Expansion of Foxp3 + Regulatory T Cells in the Skin. J. Immunol. 2018, 200, 119–129. [Google Scholar] [CrossRef]
- Kotb, I.S.; Lewis, B.J.; Barker, R.N.; Ormerod, A.D. Differential effects of phototherapy, adalimumab and betamethasone–calcipotriol on effector and regulatory T cells in psoriasis. Br. J. Dermatol. 2018, 179, 127–135. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef]
- Tan, S.Y.; Buzney, E.; Mostaghimi, A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J. Am. Acad. Dermatol. 2018, 79, 672–679. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M. ACD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 16, 389. [Google Scholar]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. Interleukin 10 is a growth factor for a population of regulatory T cells. Nature 1997, 389, 737–741. [Google Scholar] [CrossRef]
- Dejaco, C.; Duftner, C.; Grubeck- Loebenstein, B.; Schirmer, M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology 2005, 117, 289–300. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Tang, Q.; Bluestone, J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef]
- Anderton, S.; Liblau, R. Regulatory T cells in the control of inflammatory demyelinating diseases of the central nervous system. Curr. Opin. Neurol. 2008, 21, 248–254. [Google Scholar] [CrossRef]
- Robinson, D.S. Regulatory T Cells and asthma. Clin. Exp. Allergy. 2009, 39, 1314–1323. [Google Scholar] [CrossRef]
- Corthay, A. How do Regulatory T Cells Work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Buckner, J.H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010, 10, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E. The Resurrection of T Cell-Mediated Suppression. J. Immunol. 2011, 186, 3805–3807. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Beissert, S. Regulatory T Cells: Banned Cells for Decades. J. Investig. Dermatol. 2012, 132, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Matozzi, C.; Salvi, M.; D’Epiro, S.; Giancristoforo, S.; Macaluso, L.; Luci, C.; Lal, K.; Calvieri, S.; Richetta, A.G. Importance of Regulatory T Cells in the Pathogenesis of Psoriasis: Review of the Literature. Dermatology 2013, 227, 134–145. [Google Scholar] [CrossRef]
- Fessler, J.; Ficjan, A.; Duftner, C.; Dejaco, C. The impact of aging on regulatoryT-cells. Front. Immunol. 2013, 4, 231. [Google Scholar] [CrossRef]
- Fessler, J.; Felber, A.; Duftner, C.; Dejaco, C. Therapeutic potential of regulatory T cells in autoimmune disorders. BioDrugs 2013, 27, 281–291. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef]
- Ohkura, N.; Kitagawa, Y.; Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 2013, 38, 414–423. [Google Scholar] [CrossRef]
- Galgani, M.; De Rosa, V.; La Cava, A.; Matarese, G. Role of Metabolism in the Immunobiology of Regulatory T Cells. J. Immunol. 2016, 197, 2567–2575. [Google Scholar] [CrossRef]
- Fessler, J.; Raicht, A.; Husic, R.; Ficjan, A.; Schwarz, C.; Duftner, C.; Schwinger, W.; Graninger, W.B.; Stradner, M.H.; Dejaco, C. Novel senescent regulatory T-cell subset with impaired suppressive Function in rheumatoid arthritis. Front. Immunol. 2017, 8, 300. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Khan, S.; Lele, S.S.; Prabhakar, B.S. Restoring Self-tolerance in Autoimmune Diseases by Enhancing Regulatory Tcells. Cell. Immunol. 2019, 339, 41–49. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Haddad, J.G.; Chyu, K.J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J. Clin. Endocrinol. 1971, 33, 992–995. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.K. Circulating Vitamin D3 and 25-hydroxyvitamin D in Humans: An Important Tool to Define Adequate Nutritional Vitamin D Status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Janneke Dijck-Brouwer, D.A.; Muskiet, F.A.J. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/L. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef]
- Holick, M. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Tsuprykov, O.; Elitok, S.; Buse, C.; Chu, C.; Krämer, B.K.; Hocher, B. Opposite correlation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D metabolites with gestational age, bone- and lipid-biomarkers in pregnant women. Sci. Rep. 2021, 11, 1923. [Google Scholar] [CrossRef]
- Shufei Zeng, S.; Chu, C.; Doebis, C.; von Baehr, V.; Hocher, B. Reference values for free 25-hydroxy-vitamin D based on established total 25-hydroxy-vitamin D reference values. J. Steroid Biochem. Mol. Biol. 2021, 210, 105877. [Google Scholar] [CrossRef]
- Garrett-Mayer, E.; Wagner, C.L.; Hollis, B.W.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation (4000 IU/d for 1 y) eliminates differences in circulating 25-hydorxyvitamin D between African American and white men. Am. J. Clin. Nutr. 2012, 96, 332–336. [Google Scholar] [CrossRef]
- Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.G.; Keane, T.E.; Hollis, B.W.; Horst, R.L.; Ambrose, L.H.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate Cancer under active surveillance. J. Clin. Endocrinol. Metab. 2012, 97, 2315–2324. [Google Scholar] [CrossRef]
- Dudenkov, D.V.; Yawn, B.P.; Oberelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing incidence of serum 25-Hydroxyvitamin d values above 50 ng/mL: A 10-Year population-based study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.M.; Mirhosseini, N.; Holick, M.F. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Derm. Endocrinol. 2017, 9, e1300213. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2016, 77, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.; Rodbro, P.; Sjö, O. “Anticonvulsant action” of vitamin D in epileptic patients. A controlled pilot study. Br. Med. J. 1974, 2, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.; Garland, F. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shabahang, M.; Buras, R.R.; Davoodi, F.; Schumaker, L.M.; Nauta, R.J.; Evans, S.R. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993, 53, 3712–3718. [Google Scholar] [PubMed]
- Shabahang, M.; Buras, R.R.; Davoodi, F.; Schumaker, L.M.; Nauta, R.J.; Uskokovic, M.R.; Brenner, R.V.; Evans, S.R. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res. 1994, 54, 4057–4064. [Google Scholar]
- Shabahang, M.; Buffan, A.E.; Nolla, J.M.; Schumaker, L.M.; Brenner, R.V.; Buras, R.R.; Nauta, R.J.; Evans, S.R. The effect of 1, 25-dihydroxyvitamin D3 on the growth of soft-tissue sarcoma cells as mediated by the vitamin D receptor. Ann. Surg. Oncol. 1996, 3, 144–149. [Google Scholar] [CrossRef]
- Evans, S.R.; Houghton, A.M.; Schumaker, L.; Brenner, R.V.; Buras, R.R.; Davoodi, F.; Nauta, R.J.; Shabahang, M. Vitamin D receptor and growth inhibition by 1,25-dihydroxyvitaminD3 in human malignant melanoma cell lines. J. Surg. Res. 1996, 61, 127–133. [Google Scholar] [CrossRef]
- Derex, L.; Trouillas, P. Reversible parkinsonism, hypophosphoremia and hypocalcemia under Vitamin D therapy. Case Rep. Mov. Disord. 1997, 12, 612–613. [Google Scholar] [CrossRef]
- Koutkia, P.; Lu, Z.; Chen, T.C.; Holick, M.F. Treatment of vitamin D deficiency due to Crohn’s disease with tanning bed ultraviolet B radiation. Gastroenterology 2001, 121, 1485–1488. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Dawson-Hughes, B.; Willett, W.; Stachelin, H.; Bazemore, M.; Zee, R.; Wong, J. Effect of vitamin D on falls. A meta-analysis. JAMA 2004, 91, 1999–2006. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a direct inducer of Antimicrobial Peptide gene expression. J. Immunol. 2004, 173, 2902–2912. [Google Scholar] [CrossRef]
- Gombert, A.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1, 25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Willett, W.; Wong, J.; Giovannucci, E.; Dietrich, T.; Dawson-Hughes, B. Fracture prevention with Vitamin D supplementation. A meta-analysis of randomized controlled trials. JAMA 2005, 293, 2257–2264. [Google Scholar] [CrossRef]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005, 16, 83–95. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Orav, E.; Dawson-Hughes, B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women. A 3-year randomized controlled trial. Arch. Intern. Med. 2006, 166, 424–430. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin d in Cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Wicherts, I.; van Schoor, N.; Boeke, A.; Visser, M.; Deeg, D.; Smit, J.; Knol, D.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.L.; Newmark, J. Vitamin d and Parkinson’s disease—A hypothesis. Mov. Disord. 2007, 22, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chen, R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch. Int. Med. 2008, 168, 771–772. [Google Scholar] [CrossRef]
- Fleet, J.C. Molecular actions of vitamin D contributing to cancer prevention. Mol. Aspects Med. 2008, 29, 388–396. [Google Scholar] [CrossRef]
- Cannell, J.J.; Zasloff, M.; Garland, C.F.; Scragg, R.; Giovannucci, E. On the epidemiology of influenza. Virol. J. 2008, 5, 29. [Google Scholar] [CrossRef]
- Gombart, A. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151. [Google Scholar] [CrossRef]
- Al-Said, Y.; Al-Rached, H.; Al-Qahtani, H.; Jan, M. Severe proximal myopathy with remarkable recovery after Vitamin D treatment. Can. J. Neurol. Sci. 2009, 36, 336–339. [Google Scholar] [CrossRef]
- Smolders, J.; Menheere, P.; Thewissen, M.; Peelen, E.; Cohen, J.W.; Tervaert, R.; Hupperts, R. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS ONE 2009, 4, e6635. [Google Scholar] [CrossRef]
- Fernandes de Abreu, D.A.; Eyles, D.; Feron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34, S265–S277. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Uhmann, A.; Niemann, H.; Lammering, B.; Henkel, C.; Heb, I.; Rosenberger, A.; Dukkin, C.; Schraepler, A.; Schulz-Schaeffer, W.; Hahn, H. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin d receptor signaling and differentiation. Mol. Cancer Ther. 2011, 10, 2179–2188. [Google Scholar] [CrossRef]
- Garland, C.F.; French, C.B.; Baggerly, L.L.; Heaney, R.P. Vitamin D supplement doses and serum 25-hydroxyvitaminD in the range associated with cancer prevention. Anticancer. Res. 2011, 31, 617–622. [Google Scholar]
- Osunkwo, I. Complete resolution of sickle cell chronic pain with high dose vitamin D therapy: A case report and review of the literature. J Pediatr. Hematol. Oncol. 2011, 33, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Osunkwo, I.; Ziegler, T.R.; Alvarez, J.; McCracken, C.; Cherry, K.; Osunkwo, C.E.; Ofori-Acquah, S.F.; Ghosh, S.; Ogunbobode, A.; Rhodes, J.; et al. High Dose Vitamin D Therapy for Chronic Pain in Children and Adolescents with Sickle Cell Disease: Results of a Randomized Double Blind Pilot Study. Br. J. Haematol. 2012, 159, 211–215. [Google Scholar] [CrossRef]
- Fleet, J.C.; Desmet, M.; Johnson, R. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–75. [Google Scholar] [CrossRef]
- Holló, A.; Clemens, Z.; Kamondi, A.; Lakatos, P.; Szűcs, A. Correction of vitamin D deficiency improves seizure control in epilepsy: A pilot study. Epilepsy Behav. 2012, 24, 131–133. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health; a global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Khare, D.; Godbole, N.M.; Pawar, S.D.; Mohan, V.; Pandey, G.; Gupta, S.; Kumar, D.; Dhole, T.N.; Godbole, M.M. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur. J. Nutr. 2013, 52, 1405–1415. [Google Scholar] [CrossRef]
- Roy, S.; Sherman, A.; Monari-Sparks, M.J.; Schweiker, O.; Hunter, K. Correction of Low Vitamin D Improves Fatigue: Effect of Correction of Low Vitamin D in Fatigue Study (EViDiF Study). N. Am. J. Med. Sci. 2014, 6, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of Vitamin D3 on Asthma Treatment Failures in Adults with Symptomatic Asthma and Lower Vitamin D Levels. The VIDA Randomized Clinical Trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef]
- Nguyen, S.; Baggerly, L.; French, C.; Heaney, R.P.; Gorham, E.D.; Garland, C.F. 25Hydroxyvitamin D in the range of 20 to 100 ng/mL and Incidence of Kidney Stones. Am. J. Public Health 2014, 104, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and Vitamin D: Necessary for Public Health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D is not as toxic as was once thought: A historical and an up-to date perspective. Mayo Clin. Proc. 2015, 90, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Khayznikov, M.; Hemachrandra, K.; Pandit, R.; Kumar, A.; Wang, P.; Glueck, C.J. Statin Intolerance Because of Myalgia, Myositis, Myopathy, or Myonecrosis Can in Most Cases be Safely Resolved by Vitamin D Supplementation. N. Am. J. Med. Sci. 2015, 7, 86–93. [Google Scholar] [PubMed]
- Jetty, V.; Glueck, C.J.; Wang, P.; Shah, P.; Prince, M.; Lee, K.; Goldenberg, M.; Kumar, A. Safety of 50,000–100,000 Units of Vitamin D3/Week in Vitamin D-Deficient, Hypercholesterolemic Patients with Reversible Statin Intolerance. N. Am. J. Med. Sci. 2016, 8, 156–162. [Google Scholar] [PubMed]
- Cannon, T.L.; Ford, J.; Hester, D.; Trump, D.L. The incidental use of high-dose vitamin D3 in pancreatic Cancer. Case Rep. Pancreat. Cancer 2016, 2, 32–35. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations ≥ 40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–790. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations>60 vs. <20ng/mL (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int. J. Mol. Sci. 2018, 19, 2245. [Google Scholar] [CrossRef] [PubMed]
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin d values and risk of all cause and cause-specific mortality: A population-based cohort study. Mayo Clin. Proc. 2018, 93, 721–730. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J.; et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children with Asthma and Low Vitamin D Levels. The VDKA Randomized Clinical Trial. JAMA 2020, 324, 752–760. [Google Scholar] [CrossRef]
- Available online: https://www.worldometers.info/coronavirus/ (accessed on 5 April 2021).
- Leake, C. Vitamin D Toxicity. Calif. West. Med. 1936, 44, 149–150. [Google Scholar]
- Bevans, M.; Taylor, H. Lesions Following the use of Ertron in Rheumatoid Arthritis. Am. J. Pathol. 1947, 23, 367–387. [Google Scholar]
- Chen, X.; Chu, C.; Doebis, C.; von Baehr, V.; Hocher, B. Sex Dependent Association of Vitamin D with Insulin Resistance. J. Clin. Endocrinol. Metab. 2021, dgab213. [Google Scholar] [CrossRef]
- Kalb, R.E.; Fiorentino, D.F.; Lebwohl, M.G.; Toole, J.; Poulin, Y.; Cohen, A.D.; Goyal, K.; Fakharzadeh, S.; Calabro, S.; Chevrier, M.; et al. Risk of Serious Infection with Biologic and Systemic Treatment of Psoriasis. Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015, 151, 961–969. [Google Scholar] [CrossRef]
- Naderi, H.R.; Sheybani, F.; Pajand, S.R. How Should We Manage Latent Tuberculosis Infection in Patients Receiving Anti-TNF-alpha Drugs: Literature Review. Iran Red Crescent Med. J. 2016, 18, e27756. [Google Scholar] [CrossRef]
- Dobry, A.S.; Quesenberry, C.P.; Ray, G.T.; Geier, J.L.; Asgari, M.M. Serious infections among a large cohort of subjects with systemically treated psoriasis. J. Am. Acad. Dermatol. 2017, 77, 838–844. [Google Scholar] [CrossRef]
- Fiorentino, D.; Ho, V.; Lebwohl, M.G.; Leite, L.; Hopkins, L.; Galindo, C.; Goyal, K.; Langhoff, W.; Fakharzadeh, S.; Srivastava, B.; et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J. Am. Acad. Dermatol. 2017, 77, 845–854. [Google Scholar] [CrossRef]
- Kirkham, B. Tumor Necrosis Factor-Alpha Inhibitors: An Overview of Adverse Effects. Up To Date. Literature Review Current through June 2019. This Topic Last Updated May 2018. Available online: https://www.uptodate.com/contents/tumor-necrosis-factor-alpha-inhibitors-an-overviewof-adverse-effects?search=tumor%20necrosis%20factor&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 10 August 2019).
- ISMP Quarter Watch. Signals for Fingolimod and Infliximab. 5 April 2012. Data from 2011 Quarter 2. Available online: https://www.ismp.org/system/files/resources/2019-02/2011Q2.pdf (accessed on 10 August 2019).
- ISMP Quarter Watch. Leading Drug Safety Issues of 2012. Three Anti-TNF Agents Dominate Manufacturer Reports of New, Serious Injuries. 17 October 2013. Data from 2012 Quarter 4 and Annual Report. Available online: https://www.ismp.org/system/files/resources/2019-02/2012Q4.pdf (accessed on 10 August 2019).
- ISMP Quarter Watch. Annual Report Issue. Two Tumor Necrosis Factor Blockers Lead Overall Report Totals in 2014. 23 September 2015. Data from 2014 Quarters 3–4. Available online: https://www.ismp.org/system/files/resources/2019-02/2014Q4.pdf (accessed on 15 August 2019).
- ISMP Quarter Watch. Cancer Risks of Biological Products for Psoriasis. 6 April 2016. Data from 2015 Quarter 3. Available online: https://pdfs.semanticscholar.org/d656/5182acc5e3eec881e32127291647783c4d6f.pdf (accessed on 15 August 2019).
- ISMP Quarter Watch. Annual Report Issue. Direct Reports to the FDA: Adalimumab (HUMIRA), A Biological Product That Suppresses Tumor Necrosis Factor (TNF), Was the Leading Suspect Drug in Reports Submitted Directly to the FDA, rather than through Drug Manufacturers. 29 June 2016. Data from 2015 Q4. Available online: https://www.ismp.org/system/files/resources/2019-02/2015Q4.pdf (accessed on 15 August 2019).
- Walker-Journey, J. Humira Generates Most Adverse Event Reports in FDA Database. Available online: http://www.rightinginjustice.com/news/2017/04/04/humira-generates-most-adverse-event-reports-in-fda-database/ (accessed on 20 August 2019).
- ISMP Quarter Watch. Complaints That a New Psoriasis Treatment Could Aggravate the Condition. 12 December 2018. Includes New Data from 2018 Q1–Q2. Available online: https://www.ismp.org/system/files/resources/2019-02/201812_0.pdf (accessed on 20 August 2019).
- Fauber, J.; Crowe, K. Biologic Medications for Arthritis and Psoriasis Have Flooded the Market and Been Linked to 34,000 Deaths. Milwaukee J. Sentinel 2019. Available online: https://www.jsonline.com/story/news/investigations/2019/05/30/arthritis-psoriasis-drugs-darker-aspect-34-000-reports-deaths/1206103001/ (accessed on 20 August 2019).
- U.S. Food and Drug Administration. FDA Drug Safety Communication: Prescription Acetaminophen Products to Be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit (accessed on 7 August 2019).
- U.S. Food and Drug Administration. Fda News Release. For Immediate Release: 13 January 2011. FDA Limits Acetaminophen in Prescription Combination Products; Requires Liver Toxicity Warnings. Available online: https://wayback.archive-it.org/7993/20170111224116/http:/www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm239894.htm (accessed on 10 August 2019).
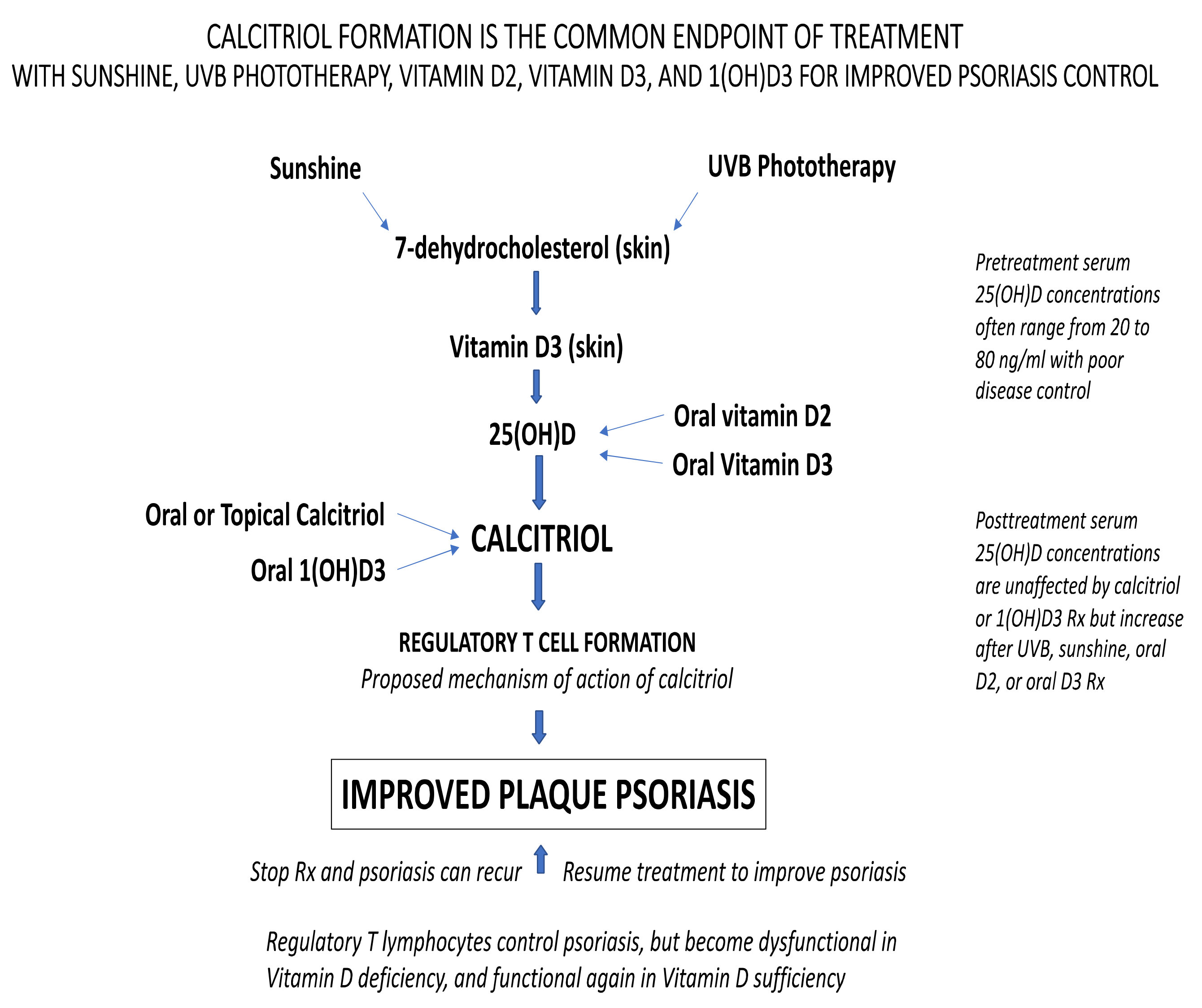
| Study | Type of | 100% | Signif | Study | |||
|---|---|---|---|---|---|---|---|
| Year | Vit D | Route | Dose/Day | N | Improv | Improv | Duration |
| 1936 | D2 | oral | NA | 3 | 2 | 3 | 6 mos |
| 1986 | 1(OH)D3 | oral | 1 µg | 17 | 5 | 13 | 6 mos |
| calcitriol | oral | 0.5 µg | 4 | 0 | 1 | 6 mos | |
| calcitriol | topical | 0.5 µg/gm | 19 | 3 | 16 | 6 mos | |
| 1988 | calcitriol | oral | 0.5 to 2 µg | 14 | 3 | 10 | 12 mos |
| calcitriol | topical | 3 µg/gm | 3 | 3 | 3 | 6 weeks | |
| 1990 | calcitriol | oral | 0.5 to 2 µg | 9 | 0 | 7 | 6 mos+ |
| 1996 | calcitriol | oral | 0.5 to 4 µg | 85 | 20 | 47 | 36 mos |
| 2012 | D3 | oral | 40,000 IU | 2 | 1 | 2 | 5 mos |
| 2013 | D3 | oral | 35,000 IU | 9 | 1 | 9 | 6 mos |
| 2019 | D2 | oral | 50,000 IU | 1 | 1 | 1 | 2+ years |
| Total | 166 | 39 | 112 |
| Baseline | Baseline | Baseline | Baseline | Post-Tx | |||
|---|---|---|---|---|---|---|---|
| Study | Type of | 25(OH)D | 25(OH)D | 25(OH)D | 25(OH)D | 25(OH)D | |
| Year | Vit D | N | Mean | Range | N > 20 | N > 50 | Peak ng/mL |
| 1936 | D2 | 3 | NA | NA | NA | NA | NA |
| 1986 | 1(OH)D3 | 17 | 23 ± 12 | NR | sig # | NR | NR |
| calcitriol | 4 | 17 ± 5 | NR | sig # | NR | NR | |
| calcitriol | 19 | 20 ± 10 | NR | sig # | NR | NR | |
| 1988 | calcitriol | 14 | 32.8 | 8 to 67 | 9 | 3 | NR |
| calcitriol | 3 | 48.5 | 30 to 67 | 2 | 1 | NR | |
| 1990 | calcitriol | 9 | NR | NR | NR | NR | NR |
| 1996 | calcitriol | 85 | 32 ± 18 | NR | sig # | sig # | NR |
| 2012 | D3 | 2 | 26 | 23 to 29 | 2 | 0 | |
| 2013 | D3 | 9 | 15 ± 7 | NR | NR | NR | 202 |
| 2019 | D2 | 1 | 70 | NA | 1 | 1 | 308 |
| 166 |
| Study | 100% | Signif | Study | ||
|---|---|---|---|---|---|
| Year | Group | N | Improv | Improv | Duration |
| 1996 | UVB + Placebo | 7 | NR | 7 | 8 weeks |
| UVB + Calcitirol | 6 | NR | 6 | 8 weeks | |
| 2010 | UVB | 29 | 29 | 29 | 4 mos |
| No UVB | 29 | 0 | 0 | 4 mos | |
| 2009 | BB-UVB | 26 | NR | 26 | 8 to 12 weeks |
| NB-UVB | 42 | NR | 42 | 8 to 12 weeks | |
| 2010 | BB-UVB | 24 | NR | 24 | 8 to 12 weeks |
| Sunshine | 20 | NR | 20 | 2 weeks | |
| Total | 183 | 29 | 154 |
| Baseline | Post-Tx | Baseline | Post-Tx | |||
|---|---|---|---|---|---|---|
| Study | 25(OH)D | 25(OH)D | 25(OH)D | 25(OH)D | ||
| Year | Group | N | Mean | Mean | Range ** | Range ** |
| 1996 | UVB + Placebo | 7 | 37.9 | 96.1 | 20 to 80 | 45 to 159 |
| UVB + Calcitirol | 6 | 27.3 | 67.1 | 15 to 40 | 45 to 123 | |
| 2010 | UVB | 29 | 23 * | 51 * | 9 to 46 | 32 to 112 |
| No UVB | 29 | 12 * | 13 * | 7 to 42 | 7 to 33 | |
| 2009 | BB-UVB | 26 | 38 ± 17 | 69 ± 20 | 17 to 82 | 45 to 118 |
| NB-UVB | 42 | 35 ± 12 | 55 ± 18 | 15 to 73 | 28 to 98 | |
| 2010 | BB-UVB | 24 | 37 ± 17 | 60 ± 19 | 18 to 88 | 25 to 90 |
| Sunshine | 20 | 23 ± 6 | 42 ± 6 | 18 to 42 | 30 to 60 | |
| Total | 183 |
| Baseline | Baseline | Post-Tx | Post-Tx | Post-Tx | |||
|---|---|---|---|---|---|---|---|
| Study | 25(OH)D | 25(OH)D | 25(OH)D | 25(OH)D | 25(OH)D | ||
| Year | Group | N | N > 20 ng/mL | N > 50 ng/mL | N > 20 ng/mL | N > 50 ng/mL | N > 100 ng/mL |
| 1996 | UVB + Placebo | 7 | 7 | 2 | 7 | 6 | 2 |
| UVB + Calcitirol | 6 | 4 | 0 | 6 | 4 | 1 | |
| 2010 | UVB | 29 | 19 | 0 | 29 | 15 | 1+ |
| No UVB | 29 | NR | 0 | 7 | 0 | 0 | |
| 2009 | BB-UVB | 26 | 65 | 5 | 68 | 48 | 3 |
| NB-UVB | 42 | ||||||
| 2010 | BB-UVB | 24 | 22 | 3 | 24 | 17 | 0 |
| Sunshine | 20 | 17 | 0 | 20 | 4 | 0 | |
| Total | 183 | 134 | 10 | 161 | 94 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCullough, P.J.; McCullough, W.P.; Lehrer, D.; Travers, J.B.; Repas, S.J. Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients 2021, 13, 1511. https://doi.org/10.3390/nu13051511
McCullough PJ, McCullough WP, Lehrer D, Travers JB, Repas SJ. Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients. 2021; 13(5):1511. https://doi.org/10.3390/nu13051511
Chicago/Turabian StyleMcCullough, Patrick J., William P. McCullough, Douglas Lehrer, Jeffrey B. Travers, and Steven J. Repas. 2021. "Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications" Nutrients 13, no. 5: 1511. https://doi.org/10.3390/nu13051511
APA StyleMcCullough, P. J., McCullough, W. P., Lehrer, D., Travers, J. B., & Repas, S. J. (2021). Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients, 13(5), 1511. https://doi.org/10.3390/nu13051511







