Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland
Abstract
1. Introduction
2. Materials and Methods
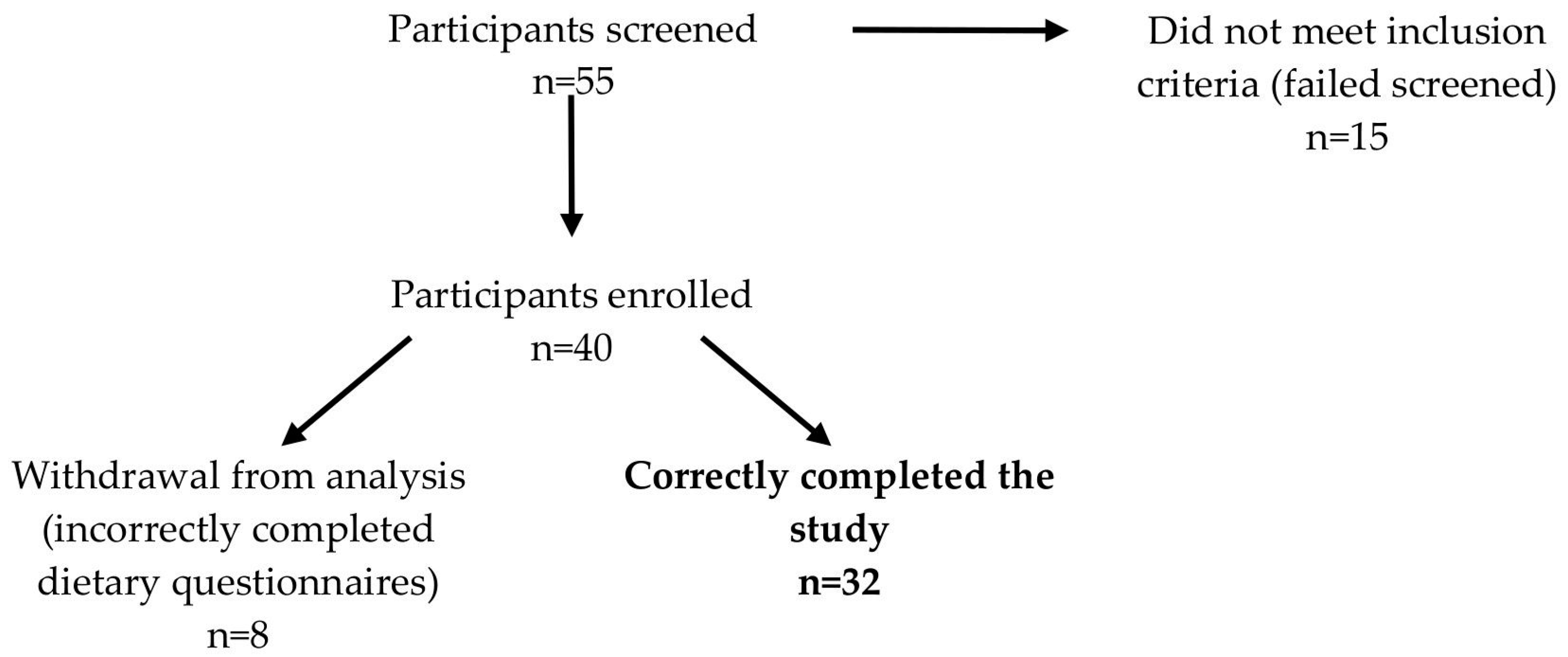
2.1. Participants and Study Design
2.2. Maternal Dietary Intake
2.3. Anthropometric Measurements and Body Composition Analysis
2.4. Milk Sample Collection and Composition Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Human Milk Composition and Estimated Nutrients Intakes
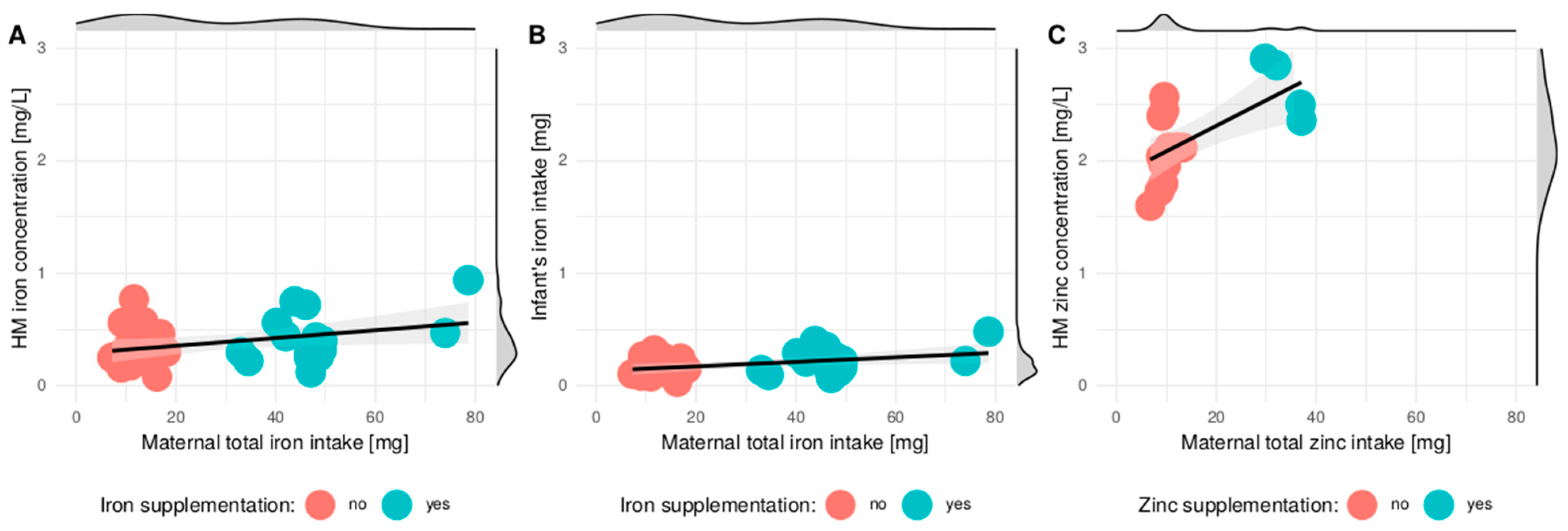
3.3. Correlation between Maternal Diet and Iron and Zinc Concentration in HM
3.4. Multivariable Regression Models Evaluating the Relationship between Maternal Factors and Mineral HM Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Swizerland, 2013. [Google Scholar]
- Picciano, M.F. Nutrient Composition of Human Milk. Pediatr. Clin. North Am. 2001, 48, 53–67. [Google Scholar] [CrossRef]
- Eussen, S.; Alles, M.; Uijterschout, L.; Brus, F.; van der Horst-Graat, J. Iron intake and status of children aged 6–36 months in Europe: A systematic review. Ann. Nutr. Metab. 2015, 66, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Hutton, E.K.; Hassan, E.S. Late vs early clamping of the umbilical cord in full-term neonates: Systematic review and meta-analysis of controlled trials. J. Am. Med. Assoc. 2007, 297, 1241–1252. [Google Scholar] [CrossRef]
- Institute of Medicine. Nutrition during Lactation; The National Academic Press: Washington, DC, USA, 1991. [Google Scholar]
- Chaparro, C.M. Setting the stage for child health and development: Prevention of iron deficiency in early infancy. J. Nutr. 2008, 138, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, S.S.; Okada, I.A.; Rondo, P.H.; Duran, M.C.; Paiva, A.A.; Neto, J.M. Concentrations of Fe, K, Na, Ca, P, Zn and Mg in maternal colostrum and mature milk. J. Trop. Pediatr. 2006, 52, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Iron and copper in human milk. Nutrition 2000, 16, 209–220. [Google Scholar] [CrossRef]
- Wasowicz, W.; Gromadzinska, J.; Szram, K.; Rydzynski, K.; Cieslak, J.; Pietrzak, Z. Selenium, zinc, and copper concentrations in the blood and milk of lactating women. Biol. Trace Elem. Res. 2001, 79, 221–233. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Dietary Reference Values for the Polish Population and their Application; National Institute of Public Health: Warsaw, Poland, 2020. [Google Scholar]
- Ackland, L.; Michalczyk, A. Zinc and infant nutrition. Arch. Biochem. Biophys. 2016, 611, 51–57. [Google Scholar] [CrossRef]
- Black, R.E.; Fischer Walker, C. Role of zinc in child health and survival. Nestle Nutr. Inst. Workshop 2012, 70, 37–42. [Google Scholar]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844–3876. [Google Scholar] [CrossRef]
- Brown, K.H.; Engle-Stone, R.; Krebs, N.F.; Peerson, J.M. Dietary intervention strategies to enhance zinc nutrition: Promotion and support of breastfeeding for infants and young children. Food Nutr. Bull. 2009, 30, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.; Pott, A.; Hiane, P.A.; Freitas, K.C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Westcott, J. Zinc and breastfed infants: If and when is there a risk of deficiency? Adv. Exp. Med. Biol. 2002, 503, 69–75. [Google Scholar] [PubMed]
- Maguire, J.L.; Salehi, L.; Birken, C.S.; Carsley, S.; Mamdani, M.; Thorpe, K.E. Association between total duration of breastfeeding and iron deficiency. Pediatrics 2013, 131, 1530–1537. [Google Scholar] [CrossRef]
- Dumrongwongsiri, O.; Suthutvoravut, U.; Chatvutinun, S.; Phoonlabdacha, P.; Sangcakul, A.; Siripinyanond, A. Maternal zinc status is associated with breast milk zinc concentration and zinc status in breastfed infants aged 4–6 months. Asia Pac. J. Clin Nutr. 2015, 24, 273–280. [Google Scholar]
- Clark, K.M.; Li, M.; Zhu, B.; Liang, F.; Shao, J.; Zhang, Y. Breastfeeding, mixed, or formula feeding at 9 months of age and the prevalence of iron deficiency and iron deficiency anemia in two cohorts of infants in China. J. Ped. 2017, 181, 56–61. [Google Scholar] [CrossRef]
- Lönnerdal, B. Regulation of mineral and trace elements in human milk: Exogenous and endogenous factors. Nutr. Rev. 2000, 58, 223–229. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.M.; Lee, J.E.; Cho, M.S.; Kang, B.S.; Choi, H.; Kim, Y. Association of maternal diet with zinc, copper, and iron concentrations in transitional human milk produced by Korean mothers. Clin. Nutr. Res. 2016, 5, 15–25. [Google Scholar] [CrossRef]
- Hannan, M.A.; Faraji, B.; Tanguma, J.; Longoria, N.; Rodriguez, R.C. Maternal milk concentration of zinc, iron, selenium, and iodine and its relationship to dietary intakes. Biol. Trace Elem. Res. 2009, 127, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.S.; Baykan, A.; Yurdakök, K.; Yalçin, S.; Gücüş, A.I. The factors that affect milk-to-serum ratio for iron during early lactation. J. Pediatr. Hematol. Oncol. 2009, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Nikniaz, L.; Gayemmagami, S.J. Association between zinc, copper, and iron concentrations in breast milk and growth of healthy infants in Tabriz, Iran. Biol. Trace Elem. Res. 2010, 135, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.D.; Lagarda, M.J.; Farre, R.; Martinez-Costa, C.; Brines, J.; Molina, A.; Clemente, G. A study of factors that may influence the determination of copper, iron, and zinc in human milk during sampling and in sample individuals. Biol. Trace Elem. Res. 2000, 76, 217–227. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Cadmium, Lead, Copper and Zinc in Breast Milk in Poland. Biol. Trace. Elem. Res. 2014, 57, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Woolridge, M.W.; Butte, N.; Dewey, K.G.; Ferris, A.M.; Garza, C.; Keller, E.P. Methods for the measurement of milk volume intake of the breastfed infant. In Human Lactation: Milk Components and Methodologies; Jensen, R.G., Neville, M.C., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 5–21. [Google Scholar]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Albums of Photographs of Food Products and Dishes; National Food and Nutrition Institute: Warsaw, Poland, 2011. [Google Scholar]
- World Health Organization (WHO). Fourth WHO Coordinated Survey of Human Milk for Persistent Organic Pollutants in Cooperation with UNEP. Guidelines for Developing a National Protocol. Available online: http://www.who.int/foodsafety/chem/POPprotocol.pdf (accessed on 20 December 2020).
- Euro WHO. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 21 December 2020).
- Heyvard, V.H.; Stolarczyk, L.M. Applied Body Composition Assessment; Human Kinetics Publisher: Champaign, IL, USA, 1996; pp. 1–215. [Google Scholar]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Olędzka, G.; Szostak-Węgierek, D.; Weker, H.; Wesołowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of Infant and Maternal Factors on Energy and Macronutrient Composition of Human Milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef]
- Domellöf, M.; Lönnerdal, B.; Dewey, K.G.; Cohen, R.J. Hernell, Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 2004, 79, 111–115. [Google Scholar] [CrossRef]
- Gibson, R.S.; Rahmannia, S.; Diana, A.; Leong, C.; Haszard, J.J.; Hampel, D.; Reid, M.; Erhardt, J.; Suryanto, A.H.; Sofiah, W.N.; et al. Association of maternal diet, micronutrient status, and milk volume with milk micronutrient concentrations in Indonesian mothers at 2 and 5 months postpartum. Am. J. Clin. Nutr. 2020, 112, 1039–1050. [Google Scholar] [CrossRef]
- Dumrongwongsiri, O.; Chongviriyaphan, N.; Chatvutinun, S.; Phoonlabdacha, P.; Sangcakul, A.; Siripinyanond, A.; Suthutvoravut, U. Dietary Intake and Milk Micronutrient Levels in Lactating Women with Full and Partial Breastfeeding. Matern. Child Health J. 2020. [Google Scholar] [CrossRef]
- Nakamori, M.; Ninh, N.X.; Isomura, H.; Yoshiike, N.; Hien, V.T.T.; Nhug, B.T.; Van Nhien, N.; Nakano, T.; Khan, N.C.; Yamamoto, S. Nutritional status of lactating mothers and their breast milk concentration of iron, zinc and copper in rural Vietnam. J. Nutr. Sci. Vitaminol. 2009, 55, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Bavrins, K.; Borisova, A. Zinc Content in Breast Milk and Its Association with Maternal Diet. Nutrients 2018, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Doneray, H.; Olcaysu, E.; Yildirim, A.; Ozden, A. The effect of the zinc concentration in breast milk on neonatal weight gain. J. Trace Elem. Med. Biol. 2017, 41, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, J.; Li, J.Z.; Suzuki, T.; Karita, K.; Abe, M.; Fujii, H.; Mishina, J.; Morita, M. Trace elements in human transitory milk. Variation caused by biological attributes of mother and infant. Biol. Trace Elem. Res. 1991, 31, 159–170. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, M.; Jacobs, M.A.; Mylet, S. Zinc in human milk: Diurnal and within-feeding patterns. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 227–229. [Google Scholar] [CrossRef]
- Celada, A.; Busset, R.; Gutierrez, J.; Herreros, V. No correlation between iron concentration in breast milk and maternal iron stores. Helv. Paediatr. Acta 1982, 37, 11–16. [Google Scholar]
- Fransson, G.B.; Agarwal, K.N.; Gebre, M.M.; Hambraeus, L. Increased breast milk iron in severe maternal anemia: Physiological “trapping” or leakage? Acta Paediatr. Scand. 1985, 74, 290–291. [Google Scholar]
- Tamura, T.; Yoshimura, Y.; Arakawa, T. Human milk folate status in lactating mothers and their infants. Am. J. Clin. Nutr. 1980, 33, 193–197. [Google Scholar] [CrossRef]
- Mello-Neto, J.; Carvalho Rondó, P.H.; Oshiiwa, M.; Morgano, M.A.; Zago Zacari, C.; Lima dos Santos, M. Iron Supplementation in Pregnancy and Breastfeeding and Iron, Copper and Zinc Status of Lactating Women from a Human Milk Bank. J. Trop. Ped. 2013, 59, 140–144. [Google Scholar] [CrossRef]
- O’Brien, K.O.; Zavaleta, N.; Caulfield, L.E.; Wen, J.; Abrams, S.A. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J. Nutr. 2000, 130, 2251–2255. [Google Scholar] [CrossRef]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Lopez-Alarcon, M.G.; Garza, C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant During the First Six Months of Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Fomon, S.J. Nutrition of Normal Infants; Mosby: St. Louis, MO, USA, 1993. [Google Scholar]
- Samuel, T.M.; Thomas, T.; Thankachan, P.; Bhat, S.; Virtanen, S.M.; Kurpad, A.V. Breast milk zinc transfer and early post-natal growth among urban South Indian term infants using measures of breast milk volume and breast milk zinc concentrations. Matern. Child Nutr. 2014, 10, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Simmer, I.; Ahmed, S.; Carlsson, L.; Thompson, R.P.H. Breast milk zinc and copper concentrations in Bangladesh. Br. J. Nutr. 1990, 63, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Karra, M.V.; Kirksey, A.; Galal, O.; Bassily, N.S.; Harrison, G.G.; Jerome, N.W. Zinc, calcium, and magnesium concentrations in milk from American and Egyptian women throughout the first 6 mo of lactation. Am. J. Clin. Nutr. 1988, 47, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Prual, A.; Preziosi, P.; Cherouvrier, F.; Favier, A.; Galan, P.; Hercberg, S. Effect of iron supplementation during pregnancy on trace element (Cu, Se, Zn) concentrations in serum and breast milk from Nigerian women. Ann. Nutr. Metab. 1993, 37, 262–271. [Google Scholar] [CrossRef]
- Krebs, N.F.; Reidinger, C.J.; Hartley, S.; Robertson, A.D.; Hambidge, K.M. Zinc supplementation during lactation: Effects on maternal status and milk zinc concentrations. Am. J. Clin Nutr. 1995, 61, 1030–1036. [Google Scholar] [CrossRef]
- Sian, L.; Krebs, N.F.; Westcott, J.E.; Fengliang, L.; Tong, L.; Miller, L.V. Zinc homeostasis during lactation with a low zinc intake. Am. J. Clin. Nutr. 2002, 75, 99–103. [Google Scholar] [CrossRef]
- Leotsinidis, M.; Alexopoulos, A.; Kostopoulou-Farri, E. Toxic and essential trace elements in human milk from Greek lactating women: Association with dietary habits and other factors. Chemosphere 2005, 61, 238–247. [Google Scholar] [CrossRef]
- Vuori, E.; Makinen, S.M.; Kara, R.; Kuitunen, P. The effects of the dietary intakes of copper, iron, manganese, and zinc on the trace element content of human milk. Am. J. Clin. Nutr. 1980, 33, 227–231. [Google Scholar] [CrossRef]
- Dempsey, C.; McCormick, N.H.; Croxford, T.P.; Seo, Y.A.; Grider, A.; Kelleher, S.L. Marginal maternal zinc deficiency in lactating mice reduces secretory capacity and alters milk composition. J. Nutr. 2012, 142, 655–660. [Google Scholar] [CrossRef]
- Petry, N.; Olofin, I.; Boy, E.; Donahue Angel, M.; Rohner, F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients 2016, 30, 773. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Black, R.E.; Robertson, A.D.; Akhtar, N.A.; Ahmed, G.; Becker, S. Clinical and field studies of human lactation: Methodological considerations. Am. J. Clin. Nutr. 1982, 35, 745–756. [Google Scholar] [CrossRef] [PubMed]
| Number | Time (Minutes) | Microwave Power (W) | Temperature (°C) |
|---|---|---|---|
| 1 | 15 | 1800 | 0–180 |
| 2 | 10 | 1800 | 180 |
| 3 | 10 | 0 | 180–0 |
| Parameter | Description |
|---|---|
| Spray Chamber | Scott, Quartz |
| Atomizer | Coaxial (Mainhardt) |
| Plasma torch | Quartz |
| Sampling cone | Nickel |
| The forming cone | Nickel |
| Frequency of the generator | 40 MHz |
| Resolution of mass signals | (0.7 ± 0.05) AMU 1 |
| Generator power | 1600 W |
| Deflector voltage | −9 V |
| Voltage on the analog detector | −1800 |
| Voltage on the pulse detector | 950 V |
| Plasma gas flow | 18.00 L/min |
| Auxiliary gas flow | 1.2 L/min |
| The flow of atomizing gas | 1.03 L/min |
| Stop time | 50 ms |
| Number of sweeps | 20 |
| Number of repetitions | 3–5 |
| Solution dispensing speed | 1 mL/min |
| Parameters | Mean ± SD | Median (Interquartile Ranges) |
|---|---|---|
| Age (years) | 33.8 ± 4.6 | 32 (30.8–36) |
| Gestational age (weeks) | 38 ± 1.2 | 39 (38–39) |
| Weight before pregnancy (kg) | 62.2 ± 11.8 | 58 (53.4–70.6) |
| Weight gain during pregnancy (kg) | 15.1 ± 4.7 | 14 (12–17) |
| Pre-pregnancy BMI (kg/m2) | 22.6 ± 3.4 | 22.1 (19.6–24.2) |
| Current weight (kg) | 65.5 ± 13.2 | 64.9 (54.5–74.5) |
| Current BMI (kg/m2) | 23.6 ± 3.7 | 23.1 (20.4–26.3) |
| Rest metabolic rate (kcal) | 1528.7 ± 72.1 | 1515 (1477.5–1578) |
| Fat free mass (%) | 70.9 ± 9.0 | 70.2 (65.6–79.1) |
| Fat mass (%) | 29.1 ± 9.0 | 29.7 (20.9–34.44) |
| Total body water (%) | 50.9 ± 5.5 | 50.0 (46.2–55.5) |
| Extracellular body water (%) | 46.4 ± 3.0 | 46.7 (45.4–48.0) |
| Intracellular body water (%) | 53.6 ± 3.0 | 53.3 (52.0–54.6) |
| Body cell mass (kg) | 24 ± 3.1 | 24.0 (22.2–26.9) |
| Proteins (kg) | 8.8 ± 1.4 | 8.9 (8.3–9.7) |
| Minerals (kg) | 3.7 ± 0.6 | 3.7 (3.4–4.0) |
| Muscles (kg) | 19.8 ± 2.0 | 19.6 (18.5–21.3) |
| Infants’ birth weight (grams) | 3548 ± 383 | 3530 (3225–3862.5) |
| Mean ± SD | Median (Interquartile Ranges) | |
|---|---|---|
| HM Composition | ||
| Energy (kcal/100 mL) | 65.67 ± 10.73 | 65.5 (62–73.08) |
| Total protein (g/100 mL) | 1.21 ± 0.23 | 1.17 (1.09–1.3) |
| True protein (g/100 mL) | 0.96 ± 0.19 | 0.93 (0.8–1.01) |
| Fat (g/100 mL) | 3.54 ± 1.04 | 3.55 (3.12–4.34) |
| Carbohydrates (g/100 mL) | 6.94 ± 0.34 | 6.98 (6.77–7.18) |
| Iron (mg/L) | 0.39 ± 0.2 | 0.33 (0.26–0.46) |
| Zinc (mg/L) | 2.21 ± 0.37 | 2.12 (1.97–2.45) |
| Daily HM volume (ml) | 492.5 ± 48 | 492.5 (466–528.5) |
| Number of daily feedings | 10.3 ± 3.3 | 10 (8–11.25) |
| Infants Intake of Energy and Nutrients 1 | ||
| Energy (kcal/d) | 324.55 ± 66.83 | 326.62 (284.78–363.35) |
| True protein (g/d) | 4.74 ± 1.24 | 4.65 (3.96–5.18) |
| Fat (g/d) | 17.54 ± 5.72 | 17.66 (15.35–21.1) |
| Carbohydrates (g/d) | 34.15 ± 3.27 | 33.87 (31.79–36.71) |
| Iron (mg/d) | 0.19 ± 0.1 | 0.16 (0.12–0.24) |
| Energy and Nutrients in Maternal Diets | Mean ± SD | Median (Interquartile Ranges) | Spearman/Pearson Correlation Coefficient r 1 | |
|---|---|---|---|---|
| HM Iron Concentration | HM Zinc Concentration | |||
| Energy (kcal) | 1831 ± 485. | 1841 (1493–2104) | −0.201 | −0.148 |
| Protein (g) | 78.6 ± 22.3 | 78.1 (61.8–92.4) | −0.011 | 0.12 |
| Protein (% kcal) | 17.5 ± 3.5 | 17.8 (14.7–19.9) | 0.14 | 0.388 |
| Fat (g) | 63.8 ± 21 | 62.7 (50.4–73.9) | −0.184 | −0.165 |
| Fat (% kcal) | 31 ± 5.9 | 30.1 (27.4–34.4) | 0.045 | −0.308 |
| PUFA (g) | 0.33 ± 0.49 | 0.08 (0.04–0.46) | −0.028 | 0.03 |
| Carbohydrates (g) | 255.1 ± 72.1 | 267.3 (190.3–296.7) | −0.183 | 0.009 |
| Carbohydrates (% kcal) | 51.5 ± 6.6 | 52.1 (48.1–55.7) | −0.149 | −0.145 |
| Sucrose (g) | 49.6 ± 32.5 | 39.5 (24.3–61.4) | 0.003 | −0.167 |
| Lactose (g) | 13.2 ± 11.6 | 11.8 (5.3–20.5) | 0.026 | −0.167 |
| Fiber (g) | 21.3 ± 5.9 | 20.8 (16.8–25.4) | −0.023 | −0.012 |
| Sodium (mg) | 2666 ± 874 | 2620 (2062–3160) | 0.241 | −0.001 |
| Potassium (mg) | 3023 ± 659 | 2924 (2516–3476) | −0.001 | 0.017 |
| Calcium (mg) | 746 ± 358 | 710 (517–914) | 0.129 | −0.113 |
| Phosphorus (mg) | 1331 ± 356 | 1288 (1042–1482) | −0.012 | −0.112 |
| Magnesium (mg) | 317 ± 83 | 306 (267–336) | −0.089 | −0.088 |
| Iron (mg) | 11.2 ± 2.8 | 10.9 (9.3–13) | −0.055 | <0.001 |
| Iron 2 (mg) | 29.2 ± 20.3 | 17.5 (11.6–46.7) | 0.258 | 0.452 |
| Zinc (mg) | 9.9 ± 1.8 | 9.6 (8.8–11) | 0.104 | 0.001 |
| Zinc 2 (mg) | 13.6 ± 9 | 9.8 (9–12.3) | 0.237 | 0.688 * |
| Iodine (µg) | 106.3 ± 34.6 | 102.2 (86.3–127.2) | 0.124 | 0.067 |
| Vitamin A (µg) | 1160.5 ± 579 | 1023.9 (825.8–1275.1) | −0.134 | −0.4 |
| Vitamin D (µg) | 3 ± 2.4 | 1.9 (1.3–3.5) | 0.081 | 0.366 |
| Vitamin E (mg) | 10.1 ± 4.2 | 9.3 (6.7–12.9) | 0.129 | −0.181 |
| Vitamin B1 (mg) | 1.2 ± 0.4 | 1.1 (1–1.3) | −0.199 | −0.188 |
| Vitamin B2 (mg) | 1.7 ± 0.6 | 1.7 (1.3–2) | 0.02 | 0.135 |
| Vitamin PP (mg) | 15.7 ± 6.1 | 14.6 (10.8–17.7) | −0.071 | 0.341 |
| Vitamin B6 (mg) | 1.8 ± 0.5 | 1.7 (1.4–2.1) | −0.074 | 0.13 |
| Folic acid (mg) | 300.3 ± 82.6 | 281.1 (239.2–371.3) | 0.04 | 0.007 |
| Vitamin B12 (µg) | 3.7 ± 1.7 | 3.4 (2.3–4.7) | 0.061 | 0.245 |
| Vitamin C (mg) | 125 ± 106.7 | 92 (63.6–130.8) | 0.154 | 0.437 |
| Food Group | Median Values (Interquartile Ranges) of the Food Consumption Frequency 1 | Spearman Correlation Coefficient r | |
|---|---|---|---|
| HM Iron Concentration | HM Zinc Concentration | ||
| Grain based products (include 6 food items) | 20 (17.5–22) | 0.159 | 0.227 |
| Milk and milk products (include 10 food items) | 28 (13.5–33) | 0.159 | 0.113 |
| Eggs (include 1 food item) | 3.5 (3–4) | 0.400 * | −0.069 |
| Meat (include 8 food items) | 28 (23.75–31.25) | −0.01 | 0.12 |
| Fish and seafood (include 3 food items) | 28 (23.75–31.25) | −0.136 | 0.025 |
| Vegetables and legumes (include 4 food items) | 16 (10.75–16.25) | 0.497 * | 0.114 |
| Fruits (include 5 food items) | 18 (16–20) | 0.012 | 0.408 |
| Nuts and seeds (include 2 food items) | 7 (5.75–8) | −0.105 | 0.23 |
| Vegetable oils (include 6 food items) | 10.5 (8–15) | 0.165 | −0.092 |
| Sweets and snacks (include 8 food items) | 22 (16.75–26) | 0.169 | 0.443 |
| Estimate | Standard Error | p-Value | |
|---|---|---|---|
| Model A (Dependent Variable: HM Iron Concentration) | |||
| Supplemented iron (mg/d) | 0.003 | 0.002 | 0.497 |
| Model B (Dependent Variable: Iron Infants’ Intake) | |||
| Total daily iron intake (mg/d) | 0.002 | 0.0008 | 0.0198 |
| Model C (Dependent Variable: HM Iron Concentration) | |||
| Meat 2 | 0.018 | 0.008 | 0.046 * |
| Fish and seafood 2 | −0.020 | 0.007 | 0.013 * |
| Vegetables and legumes 2 | 0.032 | 0.007 | <0.001 * |
| Nuts and seeds | −0.029 | 0.013 | 0.037 * |
| Total daily iron intake (mg/d) | 0.005 | 0.001 | <0.001 * |
| Model D (Dependent Variable: Iron Infants’ Intake) | |||
| Meat 2 | 0.009 | 0.003 | 0.030 |
| Fish and seafood 2 | −0.011 | 0.004 | 0.005 |
| Vegetables and legumes 2 | 0.015 | 0.003 | <0.001 * |
| Nuts and seeds 2 | −0.014 | 0.006 | 0.039 * |
| Total daily iron intake (mg/d) | 0.002 | 0.001 | <0.001 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Model E (Dependent Variable: HM Zinc Concentration) | |||
| Body fat mass (%) | 0.89 | 0.63–1.11 | 0.357 |
| Percentage of energy delivered from protein (%) | 2.00 | 1.02–7.81 | 0.141 |
| Total zinc intake (mg/d) | 1.18 | 1.04–1.52 | 0.056 |
| Model F (Dependent Variable: HM Zinc Concentration) | |||
| Meat2 | 1.382 | 0.96–2.69 | 0.161 |
| Vegetables and legumes2 | 1.279 | 0.87–2.76 | 0.340 |
| Total zinc intake (mg/d) | 1.227 | 1.06–1.65 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzikowska-Jura, A.; Sobieraj, P.; Michalska-Kacymirow, M.; Wesołowska, A. Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland. Nutrients 2021, 13, 303. https://doi.org/10.3390/nu13020303
Bzikowska-Jura A, Sobieraj P, Michalska-Kacymirow M, Wesołowska A. Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland. Nutrients. 2021; 13(2):303. https://doi.org/10.3390/nu13020303
Chicago/Turabian StyleBzikowska-Jura, Agnieszka, Piotr Sobieraj, Magdalena Michalska-Kacymirow, and Aleksandra Wesołowska. 2021. "Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland" Nutrients 13, no. 2: 303. https://doi.org/10.3390/nu13020303
APA StyleBzikowska-Jura, A., Sobieraj, P., Michalska-Kacymirow, M., & Wesołowska, A. (2021). Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland. Nutrients, 13(2), 303. https://doi.org/10.3390/nu13020303





