The Chemical Composition and Health-Promoting Effects of the Grewia Species—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
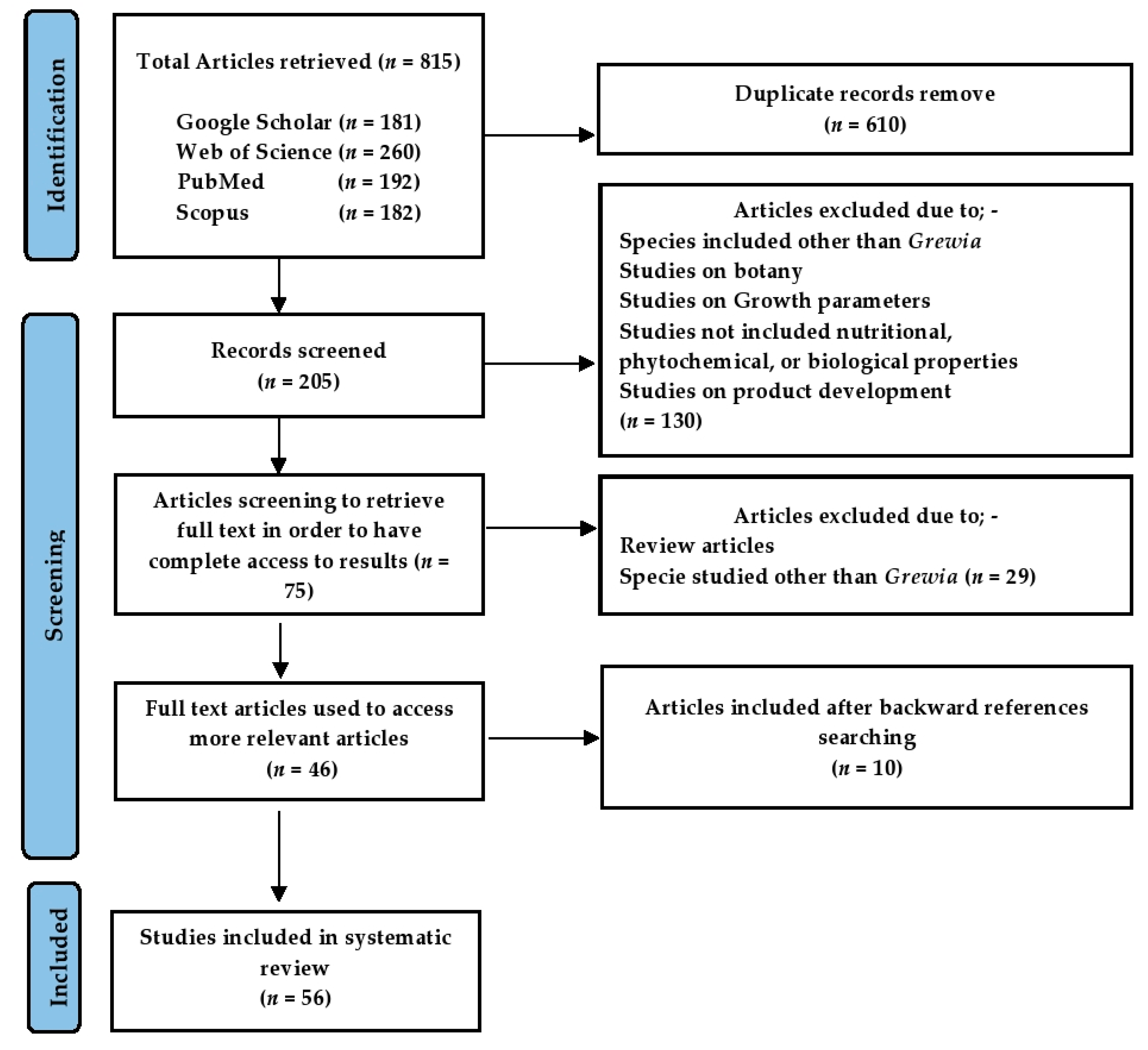
2. Materials and Methods
2.1. Literature Search and Methodology
2.2. Study Selection Criteria
- i.
- Any parts of Grewia species, such as the pulp, skin, seeds, roots, bark or leaves were described;
- ii.
- Evaluation of nutritional profiling, phytochemical composition/characterization, and pharmacological activities were provided.
2.3. Data Extraction
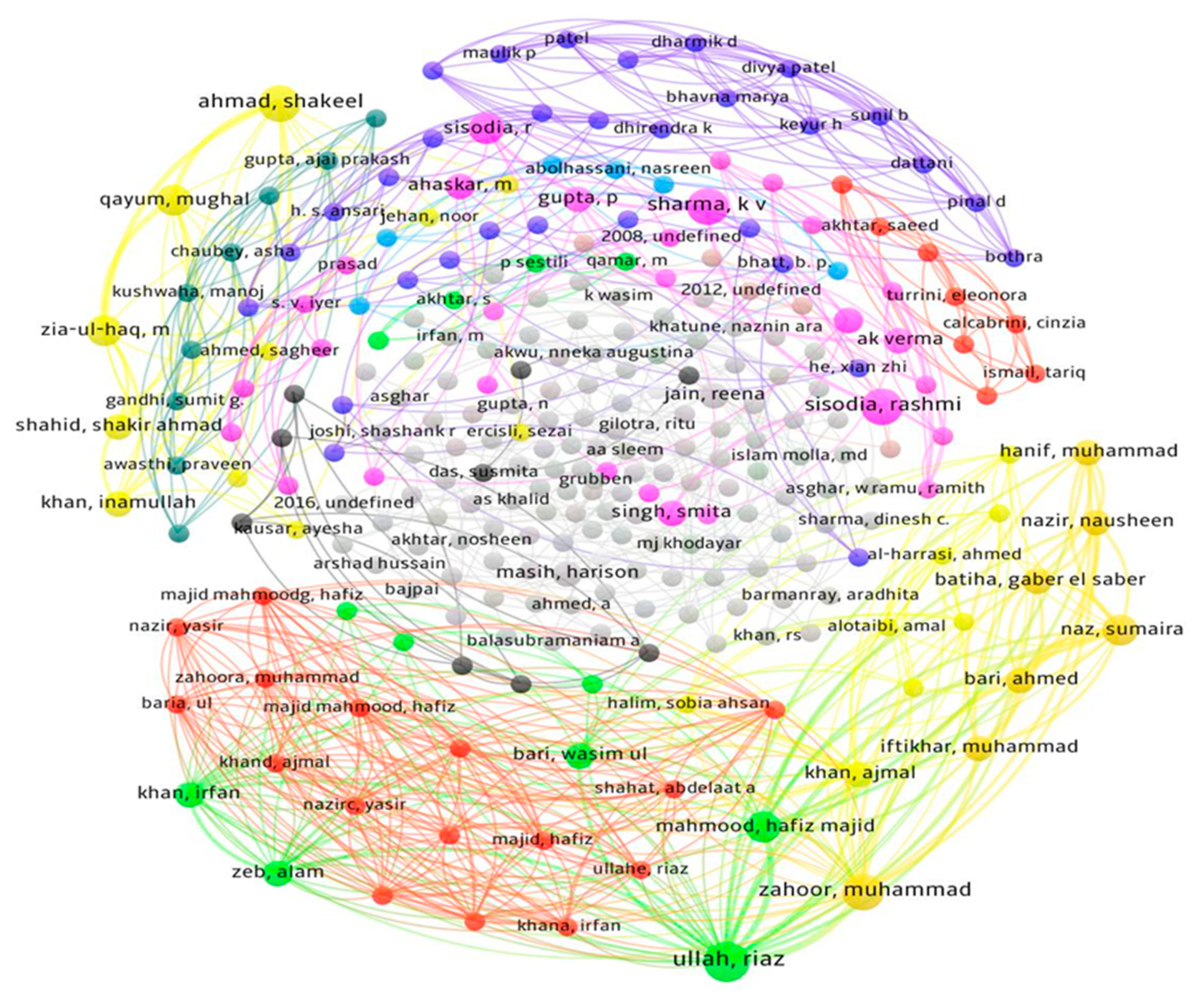
2.4. Bibliometric Analysis
3. Results
| Serial Number | Primary Metabolites | Species | Plant Part | Concentration (Dry Weight) | References | |||
|---|---|---|---|---|---|---|---|---|
| Carbohydrates | ||||||||
| 1 | Carbohydrates | G. asiatica | Fruits | 21.1% | [31] | |||
| 1 | Carbohydrates | G. asiatica | Leaves | 29.0% | [32] | |||
| 1 | Carbohydrates | G. asiatica | Seeds | 39.7% | [33] | |||
| 1 | Carbohydrates | G. tenax | Fruits | 66.0% | [34] | |||
| 1 | Carbohydrates | G. tenax | Leaves | 28.6% | [32] | |||
| 1 | Carbohydrates | G. tenax | Seeds | 66.5% | [35] | |||
| 1 | Carbohydrates | G. flavescence | Fruits | 75.0% | [34] | |||
| 1 | Carbohydrates | G. villosa | Fruits | 84.0% | [34] | |||
| 1 | Carbohydrates | G. villosa | Leaves | 33.8% | [32] | |||
| 1 | Carbohydrates | G. tilifolia | Leaves | 40.1% | [32] | |||
| 1 | Carbohydrates | G. nervosa | Leaves | 38.6% | [32] | |||
| Fat and fatty acids | ||||||||
| 2 | Fat | G. asiatica | Fruits | <0.1% (fresh weight; FW) | [31] | |||
| 2 | Fat | G. asiatica | Leaves | 2.60% | [32] | |||
| 2 | Fat | G. asiatica | Seeds | 11.1% | [33] | |||
| 2 | Fat | G. tenax | Fruits | 1.70% | [34] | |||
| 2 | Fat | G. tenax | Leaves | 3.64% | [32] | |||
| 2 | Fat | G. tenax | Seeds | 0.81% | [35] | |||
| 2 | Fat | G. flavescence | Fruits | 1.30% | [34] | |||
| 2 | Fat | G. villosa | Fruits | 1.50% | [34] | |||
| 2 | Fat | G. villosa | Leaves | 3.38% | [32] | |||
| 2 | Fat | G. tilifolia | Leaves | 3.32% | [32] | |||
| 2 | Fat | G. nervosa | Leaves | 3.86% | [32] | |||
| 3 | Oleic acid | G. asiatica | Seeds | 16.3% | [33] | |||
| 3 | Oleic acid | G. bicolor | Seeds | 19.3% | [36] | |||
| 4 | Linoleic acid | G. asiatica | Seeds | 60.1% | [33] | |||
| 4 | Linoleic acid | G. bicolor | Seeds | 53.2% | [36] | |||
| 5 | Elaidic acid | G. bicolor | Seeds | 5.70% | [36] | |||
| 6 | Palmitic acid | G. asiatica | Seeds | 12.1% | [33] | |||
| 6 | Palmitic acid | G. bicolor | Seeds | 11.4% | [36] | |||
| 7 | Stearic acid | G. asiatica | Seeds | 5.01% | [33] | |||
| 7 | Stearic acid | G. bicolor | Seeds | 5.77% | [36] | |||
| 8 | Margaric acid | G. asiatica | Seeds | 0.14% | [33] | |||
| 9 | Myristic acid | G. asiatica | Seeds | 0.41% | [33] | |||
| 10 | Behenic acid | G. asiatica | Seeds | 0.22% | [33] | |||
| 11 | Linolenic acid | G. asiatica | Seeds | 2.55% | [33] | |||
| 12 | Dihydro malvalic acid | G. asiatica | Seeds | 0.54% | [33] | |||
| 13 | Dihydro sterculic acid | G. asiatica | Seeds | 0.65% | [33] | |||
| 14 | Malvalic acid | G. asiatica | Seeds | 1.03% | [33] | |||
| 15 | Sterculic acid | G. asiatica | Seeds | 0.89% | [33] | |||
| 16 | Docosanoic acid | G. optiva | Not evaluated | [25] | ||||
| 17 | Octadecadienoic acid | G. microcos | Not evaluated | [37] | ||||
| Protein and amino acids | ||||||||
| 18 | Protein | G. asiatica | Fruits | 1.57% FW | [31] | |||
| 18 | Protein | G. asiatica | Leaves | 17.5% | [32] | |||
| 18 | Protein | G. asiatica | Seeds | 17.4% | [33] | |||
| 18 | Protein | G. tenax | Fruits | 7.70% | [34] | |||
| 18 | Protein | G. tenax | Leaves | 18.9% | [32] | |||
| 18 | Protein | G. tenax | Seeds | 7.50% | [35] | |||
| 18 | Protein | G. flavescence | Fruits | 8.70% | [34] | |||
| 18 | Protein | G. villosa | Fruits | 6.70% | [34] | |||
| 18 | Protein | G. villosa | Leaves | 18.8% | [32] | |||
| 18 | Protein | G. tilifolia | Leaves | 13.7% | [32] | |||
| 18 | Protein | G. nervosa | Leaves | 12.9% | [32] | |||
| 19 | Aspartic acid | G. asiatica | Seeds | 19.1% | [33] | |||
| 20 | Valine | G. asiatica | Seeds | 13.0% | [33] | |||
| 21 | Leucine | G. asiatica | Seeds | 11.0% | [33] | |||
| 22 | Glutamic acid | G. asiatica | Seeds | 11.0% | [33] | |||
| 23 | Isoleucine | G. asiatica | Seeds | 8.01% | [33] | |||
| 24 | Phenylalanine | G. asiatica | Seeds | 7.00% | [33] | |||
| 25 | Threonine | G. asiatica | Seeds | 4.06% | [33] | |||
| 26 | Proline | G. asiatica | Seeds | 3.01% | [33] | |||
| 27 | Tyrosine | G. asiatica | Seeds | 3.00% | [33] | |||
| 28 | Cystine | G. asiatica | Seeds | 1.08% | [33] | |||
| 29 | Alanine | G. asiatica | Seeds | 1.03% | [33] | |||
| 30 | Arginine | G. asiatica | Seeds | 2.07% | [33] | |||
| 31 | Tryptophan | G. asiatica | Seeds | 1.00% | [33] | |||
| 32 | Lysine | G. asiatica | Seeds | 2.00% | [33] | |||
| 33 | Histidine | G. asiatica | Seeds | 2.02% | [33] | |||
| 34 | Glycine | G. asiatica | Seeds | 1.02% | [33] | |||
| 35 | Serine | G. asiatica | Seeds | 4.02% | [33] | |||
| Fiber | ||||||||
| 36 | Fiber | G. asiatica | Fruits | 5.53% FW | [31] | |||
| 36 | Fiber | G. asiatica | Leaves | 38.3% | [32] | |||
| 36 | Fiber | G. asiatica | Seeds | 26.1% | [33] | |||
| 36 | Fiber | G. tenax | Fruits | 20.5% | [34] | |||
| 36 | Fiber | G. tenax | Leaves | 31.4% | [32] | |||
| 36 | Fiber | G. tenax | Seeds | 14.8% | [35] | |||
| 36 | Fiber | G. flavescence | Fruits | 42.8% | [34] | |||
| 36 | Fiber | G. villosa | Fruits | 25.5% | [34] | |||
| 36 | Fiber | G. villosa | Leaves | 28.3% | [32] | |||
| 36 | Fiber | G. tilifolia | Leaves | 29.1% | [32] | |||
| 36 | Fiber | G. nervosa | Leaves | 29.1% | [32] | |||
| Ash and minerals | ||||||||
| 37 | Ash | G. asiatica | Fruits | 1.10% FW | [31] | |||
| 37 | Ash | G. asiatica | Leaves | 6.30% | [32] | |||
| 37 | Ash | G. asiatica | Seeds | 5.08% | [33] | |||
| 37 | Ash | G. tenax | Fruits | 5.20% | [34] | |||
| 37 | Ash | G. tenax | Leaves | 11.4% | [32] | |||
| 37 | Ash | G. tenax | Seeds | 3.00% | [35] | |||
| 37 | Ash | G. flavescence | Fruits | 3.40% | [34] | |||
| 37 | Ash | G. villosa | Fruits | 4.00% | [34] | |||
| 37 | Ash | G. villosa | Leaves | 8.71% | [32] | |||
| 37 | Ash | G. tilifolia | Leaves | 7.96% | [32] | |||
| 37 | Ash | G. nervosa | Leaves | 8.00% | [32] | |||
| 38 | Sodium | G. asiatica | Fruits | 17.3 mg/100 g FW | [31] | |||
| 38 | Sodium | G. asiatica | Fruits | 0.41 mg/100 g | [38] | |||
| 38 | Sodium | G. asiatica | Seeds | 264 mg/100 g | [33] | |||
| 39 | Potassium | G. asiatica | Fruits | 372 mg/100 g FW | [31] | |||
| 39 | Potassium | G. asiatica | Fruits | 0.39 mg/100 g | [38] | |||
| 39 | Potassium | G. tenax | Fruits | 817 mg/100 g | [34] | |||
| 39 | Potassium | G. flavescence | Fruits | 877 mg/100 g | [34] | |||
| 39 | Potassium | G. villosa | Fruits | 966 mg/100 g | [34] | |||
| 40 | Calcium | G. asiatica | Fruits | 136 mg 100 g FW | [31] | |||
| 40 | Calcium | G. tenax | Fruits | 790 mg/100 g | [34] | |||
| 40 | Calcium | G. flavescence | Fruits | 269 mg/100 g | [34] | |||
| 40 | Calcium | G. villosa | Fruits | 536 mg/100 g | [34] | |||
| 40 | Calcium | G. asiatica | Seeds | 820 mg/100 g | [33] | |||
| 41 | Phosphorus | G. asiatica | Fruits | 24.2 mg/100 g FW | [31] | |||
| 41 | Phosphorus | G. asiatica | Seeds | 294 mg/100 g | [33] | |||
| 42 | Manganese | G. asiatica | Fruits | 1.08 mg/100 g | [38] | |||
| 42 | Manganese | G. tenax | Fruits | 5.10 mg/100 g | [34] | |||
| 42 | Manganese | G. flavescence | Fruits | 0.1 mg/100 g | [34] | |||
| 42 | Manganese | G. villosa | Fruits | 0.1 mg/100 g | [34] | |||
| 42 | Manganese | G. asiatica | Seeds | 1.03 mg/100 g | [33] | |||
| 43 | Copper | G. asiatica | Fruits | 16 µg/100 g | [39] | |||
| 43 | Copper | G. tenax | Fruits | 1.5 mg/100 g | [34] | |||
| 43 | Copper | G. flavescence | Fruits | 1.1 mg/100 g | [34] | |||
| 43 | Copper | G. villosa | Fruits | 1.2 mg/100 g | [34] | |||
| 43 | Copper | G. asiatica | Seeds | 1.09 mg/100 g | [33] | |||
| 44 | Iron | G. asiatica | Fruits | 1695 µg/100 g | [39] | |||
| 44 | Iron | G. tenax | Fruits | 20.8 mg/100 g | [34] | |||
| 44 | Iron | G. flavescence | Fruits | 26.9 mg/100 g | [34] | |||
| 44 | Iron | G. villosa | Fruits | 29.6 mg/100 g | [34] | |||
| 44 | Iron | G. asiatica | Seeds | 27.10 mg/100 g | [33] | |||
| 45 | Zinc | G. asiatica | Fruits | 58 µg/100 g | [39] | |||
| 45 | Zinc | G. tenax | Fruits | 1.9 mg/100 g | [34] | |||
| 45 | Zinc | G. flavescence | Fruits | 1.1 mg/100 g | [34] | |||
| 45 | Zinc | G. villosa | Fruits | 1.5 mg/100 g | [34] | |||
| 45 | Zinc | G. asiatica | Seeds | 2.04 mg/100 g | [33] | |||
| 46 | Cobalt | G. asiatica | Fruits | 33.0 µg/100 g | [39] | |||
| 46 | Cobalt | G. asiatica | Fruits | 0.46 mg/100 g | [38] | |||
| 47 | Nickel | G. asiatica | Fruits | 87.00 µg/100 g | [39] | |||
| 48 | Chromium | G. asiatica | Fruits | 36.00 µg/100 g | [39] | |||
| Vitamins | ||||||||
| 49 | Vitamin B1 | G. asiatica | Fruits | 0.02 mg/100 g FW | [31] | |||
| 50 | Vitamin B2 | G. asiatica | Fruits | 0.26 mg/100 g FW | [31] | |||
| 51 | Vitamin B3 | G. asiatica | Fruits | 0.825 mg/100 g FW | [31] | |||
| 52 | Vitamin A | G. asiatica | Fruits | 16.1 µg/100 g FW | [31] | |||
| 52 | Vitamin A | G. asiatica | Fruits | 0.89 I.U | [38] | |||
| 53 | Vitamin C | G. asiatica | Fruits | 4.38 mg/100 g | [31] | |||
| 53 | Vitamin C | G. asiatica | Fruits | 5.21 mg/100 g | [38] | |||
| Secondary metabolites | ||||||||
| Serial number | Secondary Metabolites | Category | Species | Plant Part | Type of Extract | Quantity (µg/g) | Detection Methods | References |
| Flavonoids | ||||||||
| 54 | Pelargonidin 3,5-diglucoside | Anthocyanin | G. asiatica | Fruits | Methanol | Not evaluated | Not available | [21] |
| 55 | Naringenin-7-O-β-D-glucoside | Flavanone | G. asiatica | Fruits | Methanol | Not evaluated | Not available | [21] |
| 56 | Cyanidin-3-O-arabinoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 2.29 | LC-QTOF-MS/MS | [13] |
| 57 | Cyanidin-3-O-sambubioside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 27.6 | LC-QTOF-MS/MS | [13] |
| 58 | Cyanidin-3-O-(6″-malonyl-3″-glucosylglucoside) | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 1.01 | LC-QTOF-MS/MS | [13] |
| 59 | Delphinidin-3-O-arabinoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 6.51 | LC-QTOF-MS/MS | [13] |
| 60 | Delphinidin-3-O-sambubioside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 0.80 | LC-QTOF-MS/MS | [13] |
| 61 | Petunidin | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 0.40 | LC-QTOF-MS/MS | [13] |
| 62 | Cyanidin-3-O-6″-acetylglucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 695 | HPLC (diode array detector) | [26] |
| 63 | Peonidin-3-O-6″ acetylglucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 163.6 | HPLC (diode array detector) | [26] |
| 64 | Pelargonidin-3-O-6″-acetylglucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | 140.4 | HPLC (diode array detector) | [26] |
| 65 | Malvidin-3-O-glucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | Traces | HPLC (diode array detector) | [26] |
| 66 | Delphinidin-3-O-glucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | Traces | HPLC (diode array detector) | [26] |
| 67 | Peonidin-3-O-glucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | Traces | HPLC (diode array detector) | [26] |
| 68 | Pelargonidin-3-O-malonyl glucoside | Anthocyanin | G. asiatica | Fruit | Acidified methanol | Traces | HPLC (diode array detector) | [26] |
| 69 | Calycosin | Isoflavonoid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 70 | Dihydrodaidzein-7-O-glucuronide | Isoflavonoid | G. asiatica | Fruit | Acidified methanol | 0.17 | LC-QTOF-MS/MS | [13] |
| 71 | 6,7,3′,4′- Tetrahydroxyisoflavone | Isoflavonoid | G. asiatica | Fruit | Acidified methanol | 0.12 | LC-QTOF-MS/MS | [13] |
| 72 | 5,7,8,3′,4′- Pentahydroxyisoflavone | Isoflavonoid | G. asiatica | Fruit | Acidified methanol | 0.51 | LC-QTOF-MS/MS | [13] |
| 73 | Apigenin-6-C-galactoside-8-C-arabinoside | Flavone | G. asiatica | Fruit | Acidified methanol | 0.71 | LC-QTOF-MS/MS | [13] |
| 74 | Apigenin-7-O-apiosylglucoside | Flavone | G. asiatica | Fruit | Acidified methanol | 0.33 | LC-QTOF-MS/MS | [13] |
| 75 | Luteolin-4′-glucoside | Flavone | G. asiatica | Fruit | Acidified methanol | 0.41 | LC-QTOF-MS/MS | [13] |
| 76 | Luteolin-7-O-(2-apiosyl-6- malonyl)-glucoside | Flavone | G. asiatica | Fruit | Acidified methanol | 20.09 | LC-QTOF-MS/MS | [13] |
| 77 | Hydroxyluteolin | Flavone | G. asiatica | Fruit | Acidified methanol | 0.23 | LC-QTOF-MS/MS | [13] |
| 78 | 6-Methoxyluteolin/Nepetin | Flavone | G. asiatica | Fruit | Acidified methanol | 0.60 | LC-QTOF-MS/MS | [13] |
| 79 | Genistein | Flavone | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 80 | Vitexin | Flavone | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 80 | Vitexin | Flavone | G. tiliaefolia | Bark | Methanol | Not evaluated | NMR | [22] |
| 81 | Isovitexin | Flavone | G. asiatica | Fruit | Acidified methanol | 0.33 | LC-QTOF-MS/MS | [13] |
| 82 | Narirutin | Flavanone | G. asiatica | Fruit | Acidified methanol | 0.10 | LC-QTOF-MS/MS | [13] |
| 83 | Hesperetin-3′-O-glucuronide | Flavanone | G. asiatica | Fruit | Acidified methanol | 0.64 | LC-QTOF-MS/MS | [13] |
| 84 | Naringenin | Flavanone | G. asiatica | Flowers | Chloroform | Not evaluated | Not available | [29] |
| 85 | Liquiritigenin | Flavanone | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 86 | Catechin | Flavanol | G. asiatica | Fruit | Acidified methanol | 0.14 | LC-QTOF-MS/MS | [13] |
| 86 | Catechin | Flavanol | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 87 | Epigallocatechin | Flavanol | G. asiatica | Fruit | Acidified methanol | 0.23 | LC-QTOF-MS/MS | [13] |
| 88 | Epigallocatechin-7-O-glucuronide | Flavanol | G. asiatica | Fruit | Acidified methanol | 0.16 | LC-QTOF-MS/MS | [13] |
| 89 | Epicatechin | Flavanol | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 90 | Kaempferol | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.87 | LC-QTOF-MS/MS | [13] |
| 90 | Kaempferol | Flavonol | G. asiatica | Leaves | Not mentioned | Not evaluated | Not available | [41] |
| 91 | Kaempferol-3-O-glucoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.14 | LC-QTOF-MS/MS | [13] |
| 92 | Kaempferol-3-O-xylosylglucoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.10 | LC-QTOF-MS/MS | [13] |
| 93 | Kaempferol-3-O-galactoside-7-O-rhamnoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 2.19 | LC-QTOF-MS/MS | [13] |
| 94 | Kaempferol-3-O-ß-D-glucorhamnoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.08 | LC-QTOF-MS/MS | [13] |
| 95 | Methylgalangin | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.14 | LC-QTOF-MS/MS | [13] |
| 96 | Methylgalangin | Flavonol | G. asiatica | Fruit | Acidified methanol | LC-QTOF-MS/MS | [13] | |
| 97 | Myricetin | Flavonol | G. asiatica | Fruit | Acidified methanol | 4.87 | LC-QTOF-MS/MS | [13] |
| 97 | Myricetin | Flavonol | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 98 | Myricetin-3-O-arabinoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.11 | LC-QTOF-MS/MS | [13] |
| 99 | Myricetin-3-O-rhamnoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.75 | LC-QTOF-MS/MS | [13] |
| 100 | Myricetin-3-O-galactoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.73 | LC-QTOF-MS/MS | [13] |
| 101 | Morin | Flavonol | G. asiatica | Fruit | Acidified methanol | 4.25 | LC-QTOF-MS/MS | [13] |
| 101 | Morin | Flavonol | G. optiva | Leaves | Water | Not evaluated | HPLC (diode array detector) | [27] |
| 102 | Quercetin | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.44 | LC-QTOF-MS/MS | [13] |
| 102 | Quercetin | Flavonol | G. asiatica | Fruits | Methanol | Not evaluated | Not available | [21] |
| 102 | Quercetin | Flavonol | G. asiatica | Callus | 80% Methanol | 2.42 ng/µL | TLC | [8] |
| 102 | Quercetin | Flavonol | G. asiatica | Leaves | 80% Methanol | 4.28 ng/µL | TLC | [8] |
| 102 | Quercetin | Flavonol | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 103 | Quercetin-3-O-xyloside | Flavonol | G. asiatica | Fruit | Acidified methanol | 5.07 | LC-QTOF-MS/MS | [13] |
| 104 | Quercetin-7-O-glucoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.10 | LC-QTOF-MS/MS | [13] |
| 105 | Quercetin-4′-O-glucoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.12 | LC-QTOF-MS/MS | [13] |
| 106 | Quercetin-3-O-(6”-malonyl-glucoside) | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.31 | LC-QTOF-MS/MS | [13] |
| 107 | Quercetin-3-O-glucosylxylosid | Flavonol | G. asiatica | Fruit | Acidified methanol | 34.43 | LC-QTOF-MS/MS | [13] |
| 108 | Quercetin-3-O-galactoside7-O-rhamnoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.14 | LC-QTOF-MS/MS | [13] |
| 109 | Rhamnetin | Flavonol | G. asiatica | Fruit | Acidified methanol | 2.91 | LC-QTOF-MS/MS | [13] |
| 110 | Isorhamnetin-3-O-pentaside-7-O-glucoside | Flavonol | G. asiatica | Fruit | Acidified methanol | 1.23 | LC-QTOF-MS/MS | [13] |
| 111 | Quercetin 3-O-β-D-glucoside | Flavonol | G. asiatica | Fruits | Methanol | Not evaluated | Not available | [21] |
| 112 | Isorhamnetol 5-O- [6 “ (3-hydroxy–3-methylglutarate)]-β D-glucoside | Flavonol | G. asiatica | Fruits | Ethyl acetate | Not evaluated | NMR | [2] |
| 113 | Kaempferol 3-O-β-D-glucopyranoside | Flavonol | G. asiatica | Fruits | Ethyl acetate | Not evaluated | NMR | [2] |
| 114 | Kaempferol 3-O-β-rhamnpyrnoside | Flavonol | G. asiatica | Fruits | Ethyl acetate | Not evaluated | NMR | [2] |
| 115 | Quercetin 3-O-glucoside | Flavonol | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 116 | Quercetin 3-O-rhamnoside | Flavonol | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 117 | Quercetin 3-O–β-D-2–p-coumaroylglucoside | Flavonol | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 118 | Myricetin 3-O-β-D–xyloside | Flavonol | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 119 | Salvianolic acid D | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.40 | LC-QTOF-MS/MS | [13] |
| 120 | 7-Hydroxyflavan | Flavonol | G. asiatica | Fruit | Acidified methanol | 0.10 | LC-QTOF-MS/MS | [13] |
| 121 | 7-O-Methyl cathechin | Flavanol | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 122 | Dihydroquercetin | Dihydroflavonol | G. asiatica | Fruit | Acidified methanol | 1.03 | LC-QTOF-MS/MS | [13] |
| 123 | Dihydroquercetin-3-O-hexoside | Dihydroflavonol | G. asiatica | Fruit | Acidified methanol | 0.14 | LC-QTOF-MS/MS | [13] |
| Phenolic acids | ||||||||
| 124 | Gallic acid | Phenolic acid | G. asiatica | Fruit | 50% Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 124 | Gallic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 124 | Gallic acid | Phenolic acid | G. optiva | Leaves | Water | Not evaluated | HPLC (diode array detector) | [27] |
| 125 | Caffeic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 125 | Caffeic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 126 | Quinic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 127 | Ellagic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 127 | Ellagic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 127 | Ellagic acid | Phenolic acid | G. optiva | Leaves | Water | Not evaluated | HPLC | [27] |
| 128 | Chlorogenic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 128 | Chlorogenic acid | Phenolic acid | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| 128 | Chlorogenic acid | Phenolic acid | G. optiva | Leaves | Water | Not evaluated | HPLC (diode array detector) | [27] |
| 129 | Malic acid | Phenolic acid | G. optiva | Leaves | Water | Not evaluated | HPLC (diode array detector) | [27] |
| 130 | Ascorbic acid | Phenolic acid | G. optiva | Leaves | Water | Not evaluated | HPLC (diode array detector) | [27] |
| 131 | 3, 4-Dihydroxybenzoic acid | Phenolic acid | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| Phytosterols | ||||||||
| 132 | β-Sitosterol | Phytosterol | G. asiatica | Flowers | Chloroform | Not evaluated | Not available | [29] |
| 132 | β-Sitosterol | Phytosterol | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 132 | β-Sitosterol | Phytosterol | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 133 | Stigmasterol | Phytosterol | G. asiatica | Pomace | Aqueous acetone | Not evaluated | GC/MS | [30] |
| 133 | Stigmasterol | Phytosterol | G. microcos | Roots | Ethanol | Not evaluated | NMR | [37] |
| 134 | Campesterol | Phytosterol | G. asiatica | Pomace | Aqueous acetone | Not evaluated | GC/MS | [30] |
| Triterpenes | ||||||||
| 135 | Betulin | Triterpene | G. asiatica | Stem bark | Petroleum ether | Not evaluated | GC/MS | [28] |
| 136 | Lupeol | Triterpene | G. asiatica | Stem bark | Petroleum ether | Not evaluated | GC/MS | [28] |
| 136 | Lupeol | Triterpene | G. lasiocarpa | Stem bark | Chloroform | Not evaluated | GC/MS | [15] |
| 137 | Lupenone | Triterpene | G. asiatica | Stem bark | Petroleum ether | Not evaluated | GC/MS | [28] |
| 138 | Friedelin | Triterpene | G. asiatica | Stem bark | Petroleum ether | Not evaluated | GC/MS | [28] |
| 138 | Friedelin | Triterpene | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 139 | Epi-friedelan-3-ol | Triterpene | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 140 | β-Amyrin | Triterpene | G. asiatica | Stem bark | Petroleum ether | Not evaluated | GC/MS | [28] |
| 141 | Betulinic acid | Triterpene | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 142 | Ursolic acid | Triterpene | G. microcos | Root | Ethanol | Not evaluated | NMR | [37] |
| Hydroxycinnamic acids | ||||||||
| 143 | p-Coumaroyl glycolic acid | Hydroxycinnamic acid | G. asiatica | Fruit | Acidified methanol | 0.58 | LC-QTOF-MS/MS | [24] |
| 144 | 5-Caffeoylquinic acid | Hydroxycinnamic acid | G. asiatica | Fruit | Acidified methanol | 0.25 | LC-QTOF-MS/MS | [24] |
| Carboxylic acids | ||||||||
| 145 | 1,5-Dimethyl citrate | Carboxylic acid | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 146 | Trimethyl citrate | Carboxylic acid | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 147 | Heneicosanoic acid | Carboxylic acid | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 148 | Glutaric acid | Carboxylic acid | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 149 | Hexanedioic acid | Carboxylic acid | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| Sesquiterpenoid | ||||||||
| 150 | D-Erythro-2-hexenoic acid γ-lactone | Sesquiterpenoid | G. tiliaefolia | Bark | Methanol | Not evaluated | NMR | [22] |
| 151 | Gulonic acid γ-lactone | Sesquiterpenoid | G. tiliaefolia | Bark | Methanol | Not evaluated | NMR | [22] |
| 7-Hydroxycoumarin | ||||||||
| 152 | Umbelliferone | 7-Hydroxycoumarins | G. asiatica | Fruit | Acidified methanol | 0.10 | LC-QTOF-MS/MS | [13] |
| Fatty alcohol | ||||||||
| 153 | Grewinol | Fatty alcohol | G. asiatica | Flowers | Chloroform | Not evaluated | Not available | [42] |
| Phenol | ||||||||
| 154 | Vidalenolone | Phenol | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| Xanthone | ||||||||
| 155 | Mangiferin | Xanthone | G. asiatica | Fruit | Methanol | Not evaluated | LC-ESI/MS/MS | [24] |
| Hydroxyquinol | ||||||||
| 156 | 1, 2, 3-Benzene triol | Hydroxyquinols | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| Carotenoid | ||||||||
| 157 | β-carotene | Carotenoids | G. asiatica | Fruits | Not mentioned | 0.54 µg/100 g | Not available | [38] |
| Other compounds | ||||||||
| 158 | 5,5,7,7,11,13-Hexamethyl-2-(5-methylhexyl)icosahydro-1H-cyclopenta[a]chrysen-9-ol | Other | G. optiva | Stem | Methanol | Not evaluated | GC/MS | [25] |
| 159 | 5-Hydroxymethylfurfural | Other | G. asiatica | Fruits | Water | Not evaluated | NMR | [2] |
| 160 | 3,5-Dihydroxy phenyl acrylic acid | Other | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 161 | (2,5 Dihydroxy phenyl) 3’,6’,8’-trihydroxyl-4H chromen-4’-one | Other | G. optiva | Root | Methanol | Not evaluated | NMR | [14] |
| 162 | 2,2′-(1,4-phenylene)bis(3-methylbutanoic acid | Other | G. optiva | Stem | Methanol | Not evaluated | NMR | [25] |
| 163 | N-methyl-6-β-(1′,3′,5′-trienyl)-3-β-methoxyl-3-β-methylpiperidine | Other | G. microcos | Roots | Ethanol | Not evaluated | NMR | [37] |
| 164 | Methanetriol mano formate | Other | G. optiva | Stem | Methanol | Not evaluated | GC/MS | [25] |
| 165 | Dibutyl phthalate | Other | G. microcos | Roots | Ethanol | Not evaluated | NMR | [37] |
| 166 | Propyl palmitate | Other | G. biloba | Not mentioned | Not mentioned | Not evaluated | NMR | [40] |
| 167 | (4Z, 12Z)-Cyclopentadeca-4,12-dienone | Other | G. hirsuta | Leaves | Methanol | Not evaluated | NMR | [23] |
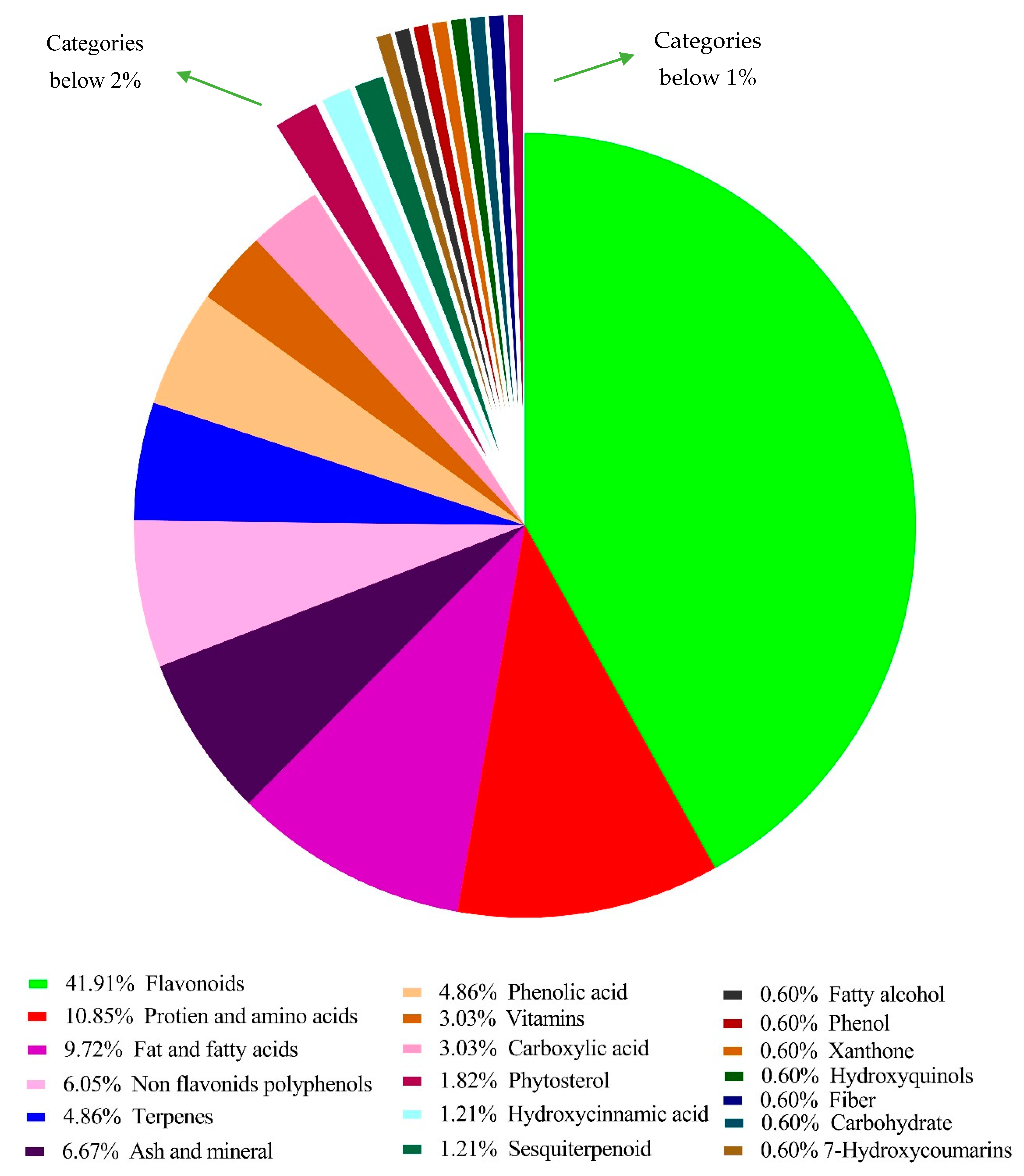
3.1. Chemical Composition
3.2. Biological Activity
3.2.1. Antioxidant Activity
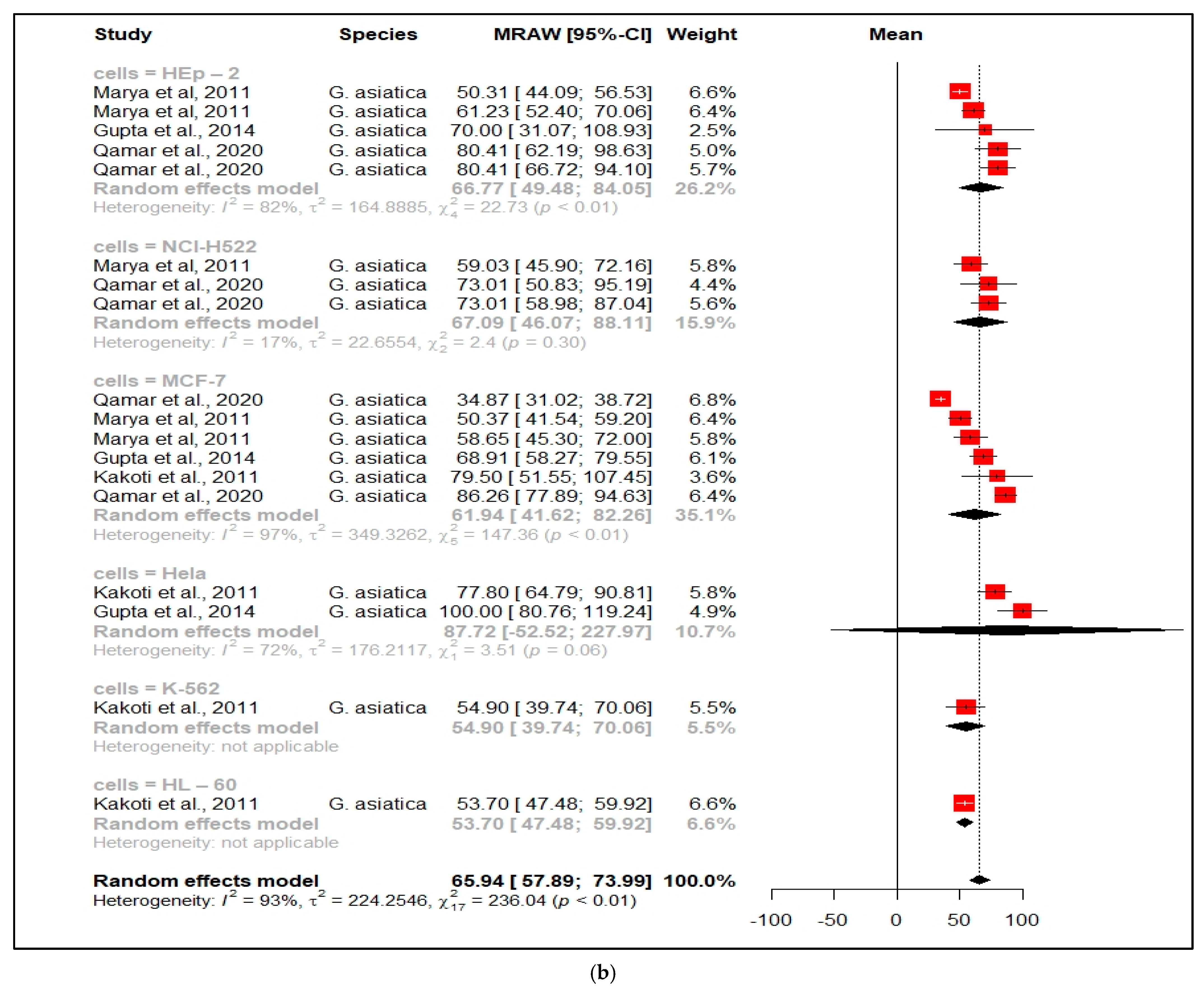
3.2.2. Anticancer Properties
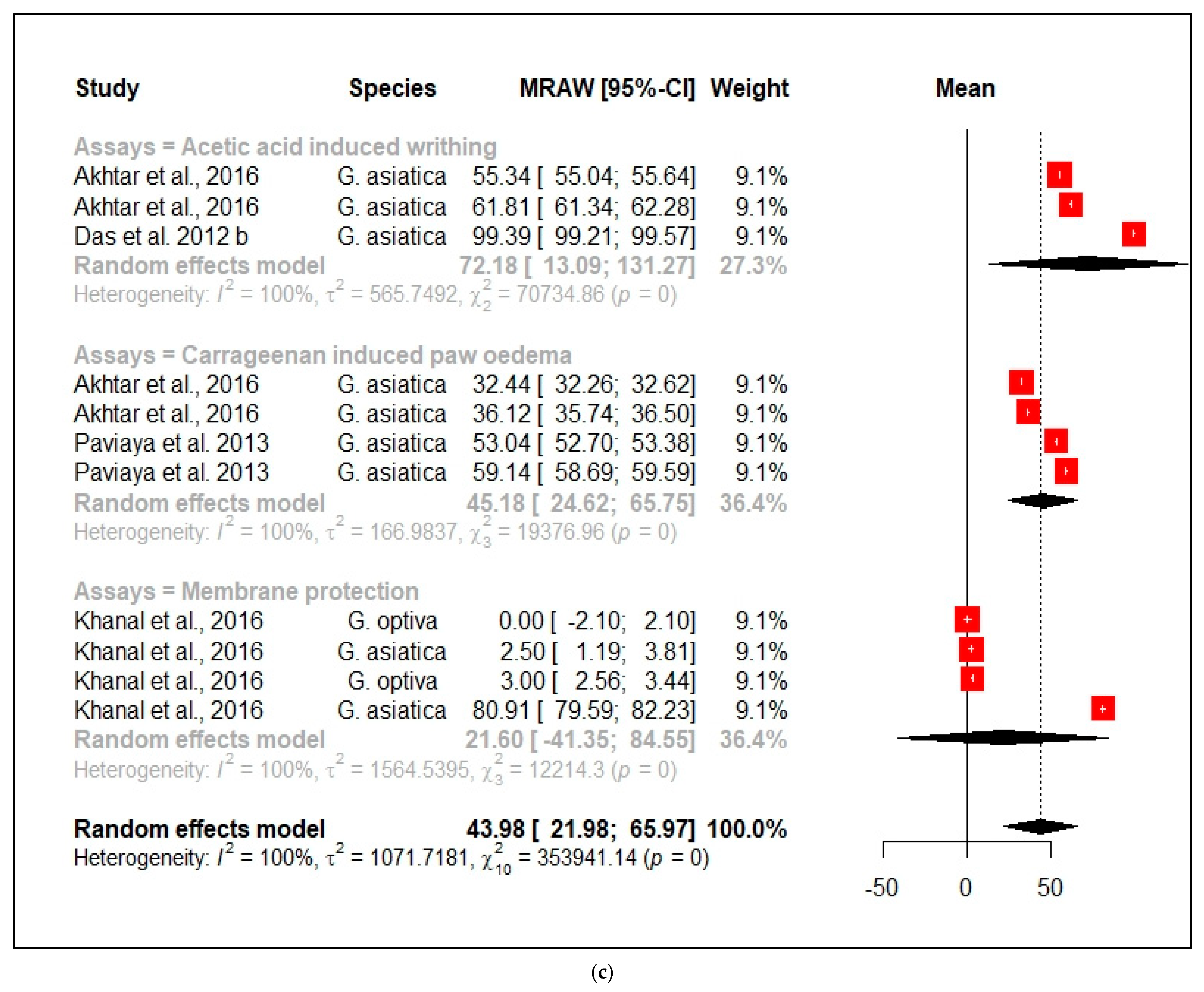
3.2.3. Anti-Inflammatory Activity
3.2.4. Antidiabetic Activity
3.2.5. Radioprotective and Hepatoprotective Potential
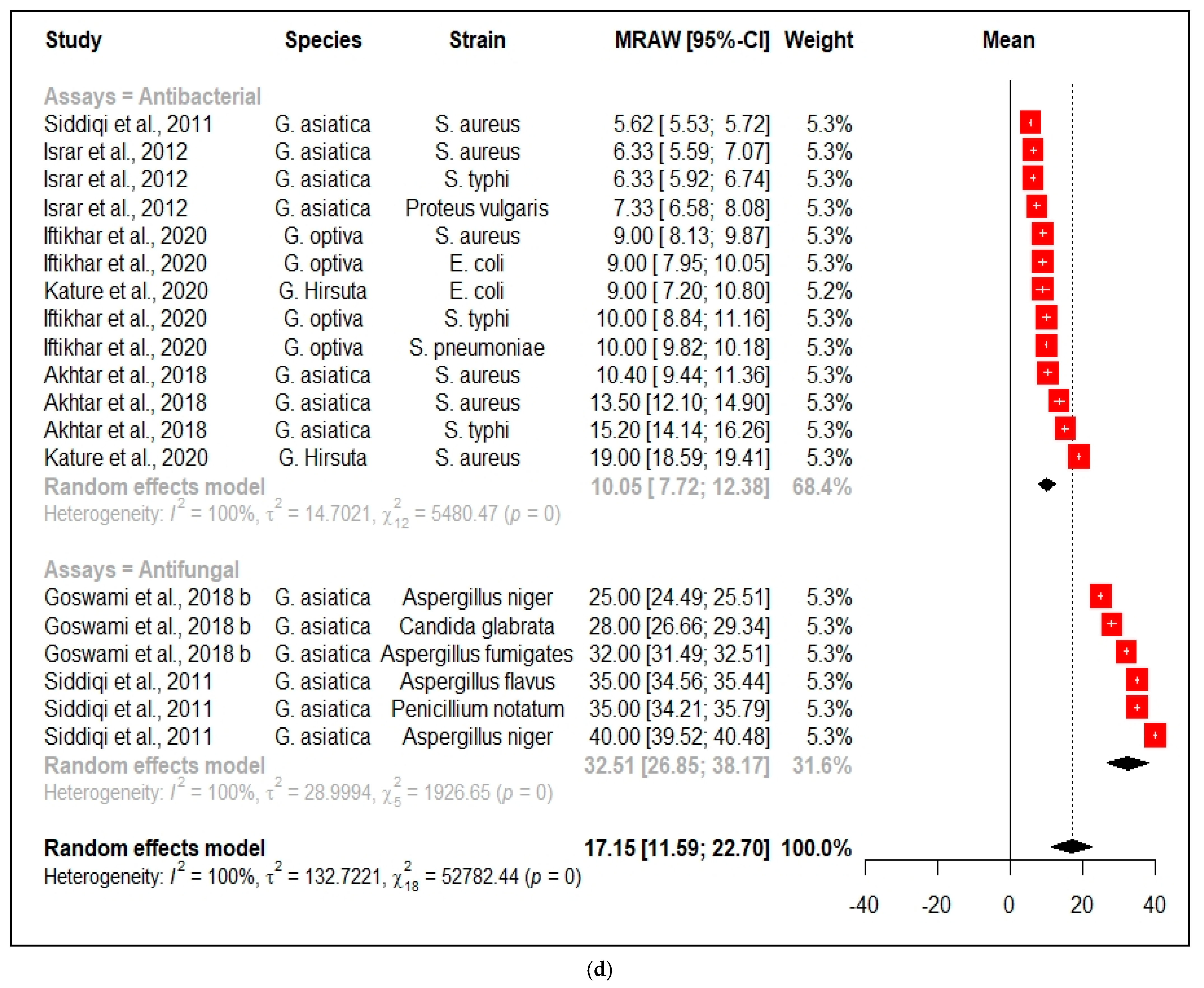
3.2.6. Antimicrobial Properties
3.2.7. Antiemetic and Antimalarial Activities
3.2.8. Other Activities: Immunomodulatory, Anti-Depressant, Anti-Neurodegenerative, Drug Delivery Polymers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, S.; Jain, R.; Masih, H. Antifungal, antioxidant and DNA protection potential of Grewia asiatica L. leaves acetone extract. J. Pharmacogn. Phytochem. 2018, 7, 212–217. [Google Scholar]
- Choudhary, I.M.; Siddiqui, J.; Abbaskhan, A.; Naheed, S.; Adhikari, A.; Awalia, J.J.A. Bio-Active Antioxidants from Plant Foods for Neutraceutical Product Development. U.S. Patent 13/759, 26 June 2018. [Google Scholar]
- Halpin, H.A.; Morales-Suárez-Varela, M.M.; Martin-Moreno, J.M. Chronic disease prevention and the new public health. Public Health Rev. 2010, 32, 120–154. [Google Scholar] [CrossRef]
- Ullah, W.; Uddin, G.; Siddiqui, B.S. Ethnic uses, pharmacological and phytochemical profile of genus Grewia. J. Asian Nat. Prod. Res. 2012, 14, 186–195. [Google Scholar] [CrossRef]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Periodical Expert’s Book Agency: New Delhi, India, 1991; 1028p. [Google Scholar]
- Louppe, A.A.; Oteng-Amoako, A.A.; Brink, M. Plant Resources of Tropical Africa 7(1); PROTA Foundation: Wageningen, The Netherlands, 2008. [Google Scholar]
- Rajan, M.D.; Sarumathy, K.; Palani, S.; Sakthivel, K.; Vijay, T. Phytochemical studies by GC-MS and cardioprotective effect of Grewia hirsute (gh) on doxorubicin induced cardiotoxicity in albino rats. Int. J. Univers. Pharm. Bio Sci. 2011, 1, 1–18. [Google Scholar]
- Sharma, N.; Patni, V. Comparative analysis of total flavonoids, quercetin content and antioxidant activity of in vivo and in vitro plant parts of Grewia asiatica Mast. Int. J. Pharm. Pharm. Res. 2013, 5, 464–469. [Google Scholar]
- Shetty, B.V.; Vijendra, S. Flora of Rajasthan; Botanical Survey of India: Calcutta, India, 1987; Volume 1. [Google Scholar]
- Singh, N.; Irchhaiya, R.; Dudhe, R.; Kumar, S. Phytochemical screening and immunomodulator activity of Grewia asiatica Linn. leaves. J. Adv. Sci. Res. 2019, 10, 166–171. [Google Scholar]
- Paul, S.; Sharma, S.; Paliwal, S.K.; Kasture, S.B. Protective action of Grewia asiatica (phalsa) berries against scopolamine-induced deficit in learning and memory using behavior paradigms in rats. Adv. Tradit. Med. 2019, 20, 243–253. [Google Scholar] [CrossRef]
- Joy, M.K.I.; Akhter, N.; Reza, R.; Antara, M.S.R.; Islam, M.S.; de la Cruz, C.V.; Ismal, M.R. Ex-vivo acetylcholinesterase and butyrylcholinesterase inhibitory activities assay of G. asiatica and G. tiliaefolia (Tiliaceae) Leaves. Annu. Res. Rev. Biol. 2019, 34, 1–10. [Google Scholar]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.; Bhatt, B.P.; Banerjee, K. Profiling of polyphenols in phalsa (Grewia asiatica L.) fruits based on liquid chromatography high resolution mass spectrometry. J. Food Sci Technol. 2020, 57, 606–616. [Google Scholar] [CrossRef]
- Bari, W.U.; Zahoor, M.; Zeb, A.; Sahibzada, M.U.K.; Ullah, R.; Shahat, A.A.; Hafiz, M.M.; Irfan, K. Isolation, pharmacological evaluation and molecular docking studies of bioactive compounds from Grewia optiva. Drug Des. Dev. Ther. 2019, 13, 3029–3036. [Google Scholar] [CrossRef]
- Akwu, N.; Naidoo, Y.; Singh, M.; Thimmegowda, S.C.; Nundkumar, N.; Lin, J. Isolation of lupeol from Grewia lasiocarpa stem bark: Antibacterial, antioxidant, and cytotoxicity activities. Biodivers. J. Biol. Divers. 2020, 21, 5684–5690. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2019, 35, 49–60. [Google Scholar] [CrossRef]
- Acedo, F.J.; Barroso, C.; Casanueva, C.; Galan, J.L. Co-authorship in management and organizational studies: An empirical and network analysis. J. Manag. Stud. 2006, 43, 957–983. [Google Scholar] [CrossRef]
- Cisneros, L.; Ibanescu, M.; Keen, C.; Lobato-Calleros, O.; Niebla-Zatarain, J. Bibliometric study of family business succession between 1939 and 2017: Mapping and analyzing authors’ networks. Scientometrics 2018, 117, 919–951. [Google Scholar] [CrossRef]
- Jan van Eck, N.; Waltman, L. VOS Viewer Manual. 2019. Available online: https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.10.pdf (accessed on 12 December 2021).
- Newman, N.M.J. Coauthorship networks and patterns of scientific collaboration. Proc. Natl. Acad. Sci. USA 2004, 101, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Pakrashi, S.C. Studies on Indian medicinal-plants. 34. Triterpenes from Grewia asiatica. J. Indian Chem. Soc. 1975, 52, 553. [Google Scholar]
- Ahamed, M.B.K.; Krishna, V.; Dandin, C.J. In vitro antioxidant and in vivo prophylactic effects of two γ-lactones isolated from Grewia tiliaefolia against hepatotoxicity in carbon tetrachloride intoxicated rats. Eur. J. Pharmacol. 2010, 631, 42–52. [Google Scholar] [CrossRef]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular docking studies of (4 Z, 12 Z)-cyclopentadeca-4, 12-dienone from Grewia hirsuta with some targets related to type 2 diabetes. BMC Complement Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef]
- Qamar, M.; Akhtar, S.; Ismail, T.; Sestili, P.; Tawab, A.; Ahmed, N. Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (Phalsa). J. Berry Res. 2020, 10, 115–131. [Google Scholar] [CrossRef]
- Ul Bari, W.; Rehman, N.U.; Khan, A.; Ahsan Halim, S.; Yuan, Y.; Blaskovich, M.A.; Ziora, M.Z. Bio-potency and molecular docking studies of isolated compounds from Grewia optiva JR Drumm. ex Burret. Molecules 2021, 26, 2019. [Google Scholar] [CrossRef]
- Talpur, M.K.; Talpur, F.N.; Balouch, A. Analysis and characterization of anthocyanin from phalsa (grewia asiatica). MOJ Food Process. Technol. 2017, 5, 299–305. [Google Scholar] [CrossRef]
- Iftikhar, M.; Zahoor, M.; Naz, S.; Nazir, N.; Batiha, G.E.S.; Ullah, R.; Bari, A.; Hanif, M.; Mahmood, H.M. Green synthesis of silver nanoparticles using Grewia optiva leaf aqueous extract and isolated compounds as reducing agent and their biological activities. J. Nanomater. 2020, 2020, 8949674. [Google Scholar] [CrossRef]
- Abou Zeid, A.H.S.; Sleem, A.A. Anti-hyperlipidemic effect and lipoidal constituents of Grewia asiatica L. leaves. Bull. Natl. Res. Cent. 2005, 30, 557–573. [Google Scholar]
- Lakshmi, V.; Chauhan, J.S. Grewinol a keto-alcohol from the flowers of Grewia asiatica [Drug plant]. Lloydia 1976, 39, 372–374. [Google Scholar]
- Gupta, P.; Bhatnagar, I.; Kim, S.K.; Verma, A.K.; Sharma, A. In-vitro cancer cell cytotoxicity and alpha amylase inhibition effect of seven tropical fruit residues. Asian Pac. J. Trop. Biomed. 2014, 4, 665–671. [Google Scholar] [CrossRef]
- Yadav, A.K. Phalsa: A Potential New Small Fruit for Georgia. In Perspectives on New Crops and New Uses; ASHS Press: Alexandria, VA, Canada, 1999; pp. 348–352. [Google Scholar]
- Pundlik, S.P.P. The study on proximate composition of different species of Genus Grewia from western Maharashtra. Eur. J. Mol. Clin. Med. 2021, 7, 3919–3924. [Google Scholar]
- Zia-Ul-Haq, M.; Ahmad, S.; Imran, I.; Ercisli, S.; Moga, M. Compositional study and antioxidant capacity of Grewia asiatica L. seeds grown in Pakistan. Comptes Rendus Acad. Bulg. Sci. 2015, 68, 191–200. [Google Scholar]
- Elhassan, G.M.; Yagi, S.M. Nutritional composition of Grewia species (Grewia tenax (Forsk.) Fiori, G. flavescens Juss and G. villosa Willd) fruits. Adv. J. Food Sci Technol. 2010, 2, 159–162. [Google Scholar]
- Abdualrahman, M.A.Y.; Ali, A.O.; Suliman, A. Nutritional evaluation of Guddaim fruits (Grewia tenax) and its utilization in ice cream production. Sudan J. Sci. Technol. 2011, 12, 38–43. [Google Scholar]
- Nyakudya, T.T.; Nosenga, N.; Chivandi, E.; Erlwanger, K.H.; Gundidza, M.; Gundidza, E.; Muredzi, P. Grewia bicolor seed oil: Putative pharmaceutical, cosmetic and industrial uses. South Afr. J. Bot. 2015, 97, 154–158. [Google Scholar] [CrossRef]
- Joshi, A.; Bhobe, M.; Sattarkar, A. Phytochemical investigation of the roots of Grewia microcos Linn. J. Chem. Pharm. 2013, 5, 80–87. [Google Scholar]
- Bala, K.; Barmanray, A. Bioactive compounds, vitamins and minerals composition of freeze-dried Grewia asiatica L. (phalsa) pulp and seed powder. Asian J. Dairy Food Res. 2019, 38, 237–241. [Google Scholar]
- Khan, A.S.; Hussain, A.; Khan, F. Nutritional importance of micronutrients in some edible wild and unconventional fruits. J. Chem. Soc. Pak. 2006, 28, 576–582. [Google Scholar]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Yan, G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. Food Chem. 2007, 105, 548–554. [Google Scholar] [CrossRef]
- Ali, S.I.; Khan, N.A.; Husain, I. Flavonoid constituents of Grewia asiatica. J. Sci. Res. 1982, 4, 55–56. [Google Scholar]
- Lakshmi, V.; Agarwal, S.K.; Chauhan, J.S. A new δ-lactone from the flowers of Grewia asiatica. Phytochemistry 1976, 15, 1397–1399. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Khan, R.S.; Asghar, W.; Khalid, N.; Nazir, W.; Farooq, M.; Ahmed, I.; Syed, Q.A. Phalsa (Grewia asiatica L.) fruit berry a promising functional food ingredient: A comprehensive review. J. Berry Res. 2019, 9, 179–193. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D. Antioxidant and free radical scavenging activities of edible weeds. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1174–1187. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lagarkha, R.; Sharma, D.K.; Sharma, P.K.; Singh, R.; Ansari, H.S. Antioxidant activity of the successive extracts of Grewia asiatica leaves. Chem. Asian J. 2007, 19, 3417. [Google Scholar]
- Asghar, M.N.; Khan, I.U.; Sherin, L.; Ashfaq, M. Evaulation of antioxidant activity of Grewia asiatica berry using 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) and N, N-dimethyl-p-phenylenediamine radical cations decolourization assays. Chem. Asian J. 2008, 20, 5123. [Google Scholar]
- Siddiqi, R.; Naz, S.; Sayeed, S.A.; Ishteyaque, S.; Haider, M.S.; Tarar, O.M.; Jamil, K. Antioxidant potential of the polyphenolics in Grewia asiatica, Eugenia jambolana and Carissa carandas. J. Agric. Sci. 2013, 5, 217–223. [Google Scholar] [CrossRef]
- Srivastava, J.; Kumar, S.; Vankar, P.S. Correlation of antioxidant activity and phytochemical profile in native plants. Nutr. Food Sci. 2012, 42, 71–79. [Google Scholar] [CrossRef]
- Imran, I.; Javaid, S.; Waheed, A.; Rasool, M.F.; Majeed, A.; Samad, N.; Saeed, H.; Alqahtani, F.; Ahmed, M.M.; Alaqil, F.A. Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 2020, 7, 587367. [Google Scholar] [CrossRef]
- Goswami, S.; Jain, R.; Masih, H. Comparative evaluation of In vitro antioxidant analysis of various leaves extracts for selected medicinal plants. J. Pharmacogn. Phytochem. 2018, 7, 1477–1481. [Google Scholar]
- Masisi, K.; Masamba, R.; Lashani, K.; Li, C.; Kwape, T.E.; Gaobotse, G. Antioxidant, cytotoxicity and cytoprotective potential of extracts of Grewia flava and Grewia bicolor Berries. J. Pharmacopunct. 2021, 24, 24–31. [Google Scholar] [CrossRef]
- Ngonda, F. In-vitro anti-oxidant activity and free radical scavenging potential of roots of Malawian Trichodesma zeylanicumm (burm. F.). Asian J. Biomed. Pharm. Sci. 2013, 3, 21–25. [Google Scholar]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Marya, B.; Dattani, K.H.; Patel, D.D.; Patel, P.D.; Patel, D.; Suthar, M.P.; Patel, V.P.; Bothara, S.B. In vitro cytotoxicity evaluation of aqueous fruit and leaf extracts of Grewia asiatica using MTT assay. Pharma Chem. 2011, 3, 282–287. [Google Scholar]
- Dattani, K.H.; Patel, D.D.; Marya, B.; Patel, P.D.; Patel, D.; Suthar, M.P.; Patel, V.P.; Bothara, S.B. In-vitro cytotoxicity evaluation of methanolic fruit extract of Grewia asiatica using MTT assay. Inventi Impact Ethnopharmacol. 2011, 2011, 282–287. [Google Scholar]
- Kakoti, B.B.; Selvan, V.T.; Manikandan, L.; Gupta, M.; Mazumder, U.K.; Das, B. Antitumor and in-vitro activity of Grewia asiatica Linn.against Ehlrich’s ascites carcinoma cell lines. Pharmacol. Online 2011, 3, 956–960. [Google Scholar]
- Das, S.; Das, S.; De, B. In vitro inhibition of key enzymes related to diabetes by the aqueous extracts of some fruits of West Bengal, India. Curr. Nutr. Food Sci. 2012, 8, 19–24. [Google Scholar]
- Akhtar, B.; Ashraf, M.; Javeed, A.; Sharif, A.; Akhtar, M.F.; Saleem, A.; Murtaza, G. Analgesic, antipyretic and antiinflammatory activities of Grewia asiatica fruit extracts in albino mice. Acta Pol. Pharm.—Drug Res. 2016, 73, 983–989. [Google Scholar]
- Paviaya, U.S.; Kumar, P.; Wanjari, M.M.; Thenmozhi, S.; Balakrishnan, B.R. Analgesic and anti-inflammatory activity of root bark of Grewia asiatica Linn. in rodents. Anc. Sci. Life. 2013, 32, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Hussain, T.; Pathak, R.; Hussain, A. Evaluation of anti-inflammatory activity of Grewia asiatica methanolic fruit extract in Wistar rats. Asian Pac. J. Trop. Biomed. 2012, 10, 1–4. [Google Scholar]
- Khanal, D.P.; Raut, B.; Kafle, M. A comparative study on phytochemical and biological activities of two Grewia species. J. Manmohan Meml. Inst. Health Sci. 2016, 2, 53–60. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, M.; Mehjabeen, J.N.; Ahmad, S.; Qayum, M.; Marwat, I.K. Antimicrobial screening of selected flora of Pakistan. Arch. Biol. Sci. 2011, 63, 691–695. [Google Scholar] [CrossRef]
- Siddiqi, R.; Naz, S.; Ahmad, S.; Sayeed, S.A. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int. J. Food Sci. Technol. 2011, 46, 250–256. [Google Scholar] [CrossRef]
- Israr, F.; Hassan, F.; Naqvi, B.S.; Azhar, I.; Jabeen, S.; Hasan, S.M.F. Studies on antibacterial activity of some traditional medicinal plants used in folk medicine. Pak. J. Pharm. Sci. 2012, 25, 669–674. [Google Scholar]
- Akhtar, N.; Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Kature, D.; Gupta, G.; Gilotra, R. Phytochemical Investigation & Antibacterial Activity of Hydroethanolic Leaf Extract of Grewia Hirsuta Collected from Forest. SGVU J. Pharm. Res. Educ. 2020, 5, 423–431. [Google Scholar]
- Dawar, S.H.A.H.N.A.Z.; Hanif, A.S.M.A.; Siddique, R. Management of root rot fungi by Grewia asiatica L. leaves and on the growth of crop plants. Pak. J. Bot. 2020, 52, 469–476. [Google Scholar] [CrossRef]
- Khattab, H.A.; El-Shitany, N.A.; Abdallah, I.Z.; Yousef, F.M.; Alkreathy, H.M. Antihyperglycemic potential of Grewia asiatica fruit extract against streptozotocin-induced hyperglycemia in rats: Anti-inflammatory and antioxidant mechanisms. Oxid. Med. Cell. Longev. 2015, 2015, 549743. [Google Scholar] [CrossRef]
- Patil, P.; Patel, M.M.; Bhavsar, C.J. Preliminary phytochemical and hypoglycemic activity of leaves of Grewia asiatica L. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 516–520. [Google Scholar]
- Zia-Ul-Haq, M.; Shahid, S.A.; Muhammed, S.; Qayum, M.; Khan, I.; Ahmad, S. Antimalarial, antiemetic and antidiabetic potential of Grewia asiatica L. leaves. J. Med. Plant. Res. 2012, 6, 3087–3092. [Google Scholar]
- Das, D.; Mitra, A.; Datta, D.; Saha, A.; Hazra, J. Evaluation of antipyretic and analgesic activity of parusaka (Grewia asiatica Linn.): An indigenous Indian plant. Int. J. Res. Ayurveda Pharm. 2012, 3, 519–523. [Google Scholar]
- Mesaik, M.A.; Ahmed, A.; Khalid, A.S.; Jan, S.; Siddiqui, A.A.; Perveen, S.; Azim, M.K. Effect of Grewia asiatica fruit on Glycemic index and phagocytosis tested in healthy human subjects. Pak. J. Pharm. Sci. 2013, 26, 85–89. [Google Scholar] [PubMed]
- Sisodia, R.; Singh, S.; Sharma, K.V.; Ahaskar, M. Post treatment effect of Grewia asiatica against radiation-induced biochemical alterations in Swiss albino mice. J. Environ. Pathol. Toxicol. 2008, 27, 113–121. [Google Scholar] [CrossRef]
- Sharma, K.V.; Sisodia, R. Hepatoprotective efficacy of Grewia asiatica fruit against oxidative stress in swiss albino mice. Int. J. Radiat. Res. 2010, 8, 175. [Google Scholar]
- Al-Dhabi, N.A.; Ghilan, A.K.M.; Esmail, G.A.; Arasu, M.V.; Duraipandiyan, V.; Ponmurugan, K. Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health 2019, 12, 549–556. [Google Scholar] [CrossRef]
- Dixon, R.A.; Howles, P.A.; Lamb, C.; He, X.Z.; Reddy, J.T. Prospects for the metabolic engineering of bioactive flavonoids and related phenylpropanoid compounds. Adv. Exp. Med. Biol. 1998, 439, 55–66. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2018, 72, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Calcabrini, C.; Turrini, E.; Sestili, P.; Fimognari, C. Nrf2: A potential therapeutic target for naturally occurring anticancer drugs? Expert Opin. Ther. Targets 2017, 21, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Calcabrini, C.; Diaz, A.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Irfan, M.; Jilani, M.S.; Wasim, K.; Kiran, M.; Muhammad, F. Lack of brine shrimp lethality and hemagglutination activity in Grewia asiatica Linn. Pharm. Negat. Results 2013, 4, 1–4. [Google Scholar] [CrossRef]
- Krebs, E.E.; Kroenke, K.; Wu, J.; Bair, M.J.; Kozak, M.A.; Yu, Z. Opioid use as a predictor of health care use and pain outcomes: Analysis of clinical trial data. Pain Med. 2016, 17, 1261–1268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zarei, M.; Mohammadi, S.; Abolhassani, N.; Asgari, N.M. The antinociceptive effects of hydroalcoholic extract of Bryonia dioica in male rats. Avicenna J. Neuro Psycho Physiol. 2015, 2, e25761. [Google Scholar] [CrossRef]
- Rodriguez, V.L.; Davoudian, T. Clinical measurement of pain, opioid addiction, and functional status. In Treating Comorbid Opioid Use Disorder in Chronic Pain; Springer International Publishing: Cham, Switzerland, 2016; pp. 47–56. [Google Scholar]
- Kazemi, S.; Shirzad, H.; Rafieian-Kopaei, M. Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr. Pharm. Des. 2018, 24, 1551–1562. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 diabetes: Demystifying the global epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef]
- Herman, W.H. The global burden of diabetes: An overview. In Diabetes Mellitus in Developing Countries and Underserved Communities; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–5. [Google Scholar]
- Khatune, N.A.; Rahman, B.M.; Barman, R.K.; Wahed, M.I.I. Antidiabetic, antihyperlipidemic and antioxidant properties of ethanol extract of Grewia asiatica Linn. bark in alloxan-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 295. [Google Scholar] [CrossRef]
- Latif, K.A.A.; Prasad, A.K.; Kumar, S.; Iyer, S.V.; Patel, H.A.; Patel, J.A. Comparative antidiabetic studies of leaves of Ipomoea carnea and Grewia asiatica on streptozotocin induced diabetic rats. Int. J. Pharm. Biol. Arch. 2012, 3, 853–857. [Google Scholar]
- Ahaskar, M.; Sisodia, R. Modulation of radiation induced biochemical changes in brain of swiss albino mice by Grewia asiatica. Asian J. Exp. Biol. Sci. 2006, 20, 399–404. [Google Scholar]
- Ahaskar, M.; Sharma, K.V.; Singh, S.; Sisodia, R. Radioprotective effect of fruit extract of Grewia asiatica in Swiss albino mice against lethal dose of γ-irradiation. Asian J. Exp. Sci. 2007, 21, 295–308. [Google Scholar]
- Sisodia, R.; Singh, S. Biochemical, behavioural and quantitative alterations in cerebellum of Swiss albino mice following irradiation and its modulation by Grewia asiatica. Int. J. Radiat. Biol. 2009, 85, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, K.V.; Sisodia, R. Radioprotective role of Grewia asiatica in mice blood. Pharmacol. Online 2007, 2, 32–43. [Google Scholar]
- Sisodia, R.; Ahaskar, M.; Sharma, K.V.; Singh, S. Modulation of radiation-induced biochemical changes in cerebrum of Swiss albino mice by Grewia asiatica. Acta Neurobiol. Exp. 2008, 68, 32. [Google Scholar]
- Sharma, K.V.; Sisodia, R. Evaluation of the free radical scavenging activity and radioprotective efficacy of Grewia asiatica fruit. J. Radiol. Prot. 2009, 29, 429. [Google Scholar] [CrossRef]
- Arasu, M.V.; Arokiyaraj, S.; Viayaraghavan, P.; Kumar, T.S.J.; Duraipandiyan, V.; Al-Dhabi, N.A.; Kavaiyarasu, K. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. 2019, 190, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.K.M.; Kaviyarasu, K.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 2019, 191, 65–74. [Google Scholar] [CrossRef]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sangita, K.; Avijit, M.; Shilpa, P.; Shivkanya, J. Studies of the antifungal and antiviral activity of methanolic extract of leaves of Grewia asiatica. Pharmacogn. J. 2009, 1, 221–223. [Google Scholar]
- Yaqueen, Z.; Sohail, T.; Rahman, A.; Salim, M.; Rahman, Z. Evaluation of antiemetic activities of alcoholic extract of Grewia asiatica in experimental model dog. Pak. J. Sci. Ind. Res. 2008, 51, 212–215. [Google Scholar]
- Zia-Ul-Haq, M.; Shahid, S.A.; Ahmed, S.; Ahmad, S.; Qayum, M.; Khan, I. Anti-platelet activity of methanolic extract of Grewia asiatica L. leaves and Terminalla chebula Retz. fruits. J. Med. Plant. Res. 2012, 6, 2029–2032. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Sim, M.O.; Lee, H.I.; Ham, J.R.; Seo, K.I.; Kim, M.J.; Lee, M.K. Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr. Res. Pract. 2015, 9, 364–369. [Google Scholar] [CrossRef]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, A.; Wang, C.; He, M.; Xu, J.; Fu, H.; Zhang, X.; Lv, W.; Guo, Z. Amelioration effects of Kaempferol on immune response following chronic intermittent cold-stress. Res. Vet. Sci. 2019, 125, 390–396. [Google Scholar] [CrossRef]
- Jebin, R.; Molla, M.I.; Chowdhury, S.M.; Rafe, M.R. Antidepressant and sedative-hypnotic activities of methanolic extract of Grewia asiatica Linn. leaves in mice. Bangladesh Pharm. J. 2019, 22, 185–191. [Google Scholar] [CrossRef]
- Gupta, N.; Kulkarni, G.T.; Kumar, P.; Awasthi, R. Grewia asiatica Mucilage: A smart gelling polymeric material for pharmaceutical applications in vitro studies. Curr. Mater. Sci. Former. Recent Pat. Mater. Sci. 2019, 12, 117–126. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- The US Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine Washington (DC); National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Deruelle, F.; Baron, B. Vitamin C: Is supplementation necessary for optimal health? J. Altern Complement. Med. 2008, 14, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Potassium. In Mineral Tolerance of Animals, 2nd ed.; National Academies Press: Washington, DC, USA, 2005; pp. 306–320. [Google Scholar]
- Preuss, H.G. Electrolytes: Sodium, chloride, and potassium. In Present Knowledge in Nutrition, 9th ed.; Bowman, B.A., Russell, R.M., Eds.; LSI Press: Washington, DC, USA, 2006; Volume I, pp. 409–421.ss. [Google Scholar]
- Kang, M.J.; Shin, M.S.; Park, J.N.; Lee, S.S. The effects of polyunsaturated: Saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005, 94, 526–532. [Google Scholar] [CrossRef]
- Salter, A.M. Dietary fatty acids and cardiovascular disease. Animal 2013, 7, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Harbingers of coronary heart disease: Dietary saturated fatty acids and cholesterol. Is chocolate benign because of its stearic acid content? Am. J. Clin. Nutr. 1999, 70, 951–952. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, N.; Lowe, J.E.; Hernandez, A.R.; Chambers, J.A.; Fucassi, F.; Cragg, P.J. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2003, 530, 27–33. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin™) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zambon, A.; Sartore, G.; Passera, D.; Francini-Pesenti, F.; Bassi, A.; Basso, C.; Crepaldi, G. Effects of hypocaloric dietary treatment enriched in oleic acid on LDL and HDL subclass distribution in mildly obese women. J. Intern. Med. 1999, 246, 191–201. [Google Scholar] [CrossRef][Green Version]
- Le Son, H.; Anh, N.P. Phytochemical composition, in vitro antioxidant and anticancer activities of quercetin from methanol extract of Asparagus cochinchinensis (Lour.) Merr. tuber. J. Med. Plant. Res. 2013, 7, 3360–3366. [Google Scholar]
- Jung, H.A.; Park, J.C.; Chung, H.Y.; Kim, J.; Choi, J.S. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch. Pharm. Res. 1999, 22, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Tiku, A.K.; Singh, G.; Koul, A.; Gupta, S.; Rana, S. In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. J. Plant Biochem. Biotechnol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Zhang, J.; Melton, L.D.; Adaim, A.; Skinner, M.A. Cytoprotective effects of polyphenolics on H 2 O 2-induced cell death in SH-SY5Y cells in relation to their antioxidant activities. Eur. Food Res. Technol. 2008, 228, 123–131. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; De la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Yonathan, M.; Asres, K.; Assefa, A.; Bucar, F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J. Ethnopharmacol. 2006, 108, 462–470. [Google Scholar] [CrossRef]
- Nardi, G.M.; Felippi, R.; DalBó, S.; Siqueira-Junior, J.M.; Arruda, D.C.; Delle Monache, F. Anti-inflammatory and antioxidant effects of Croton celtidifolius bark. Phytomedicine 2003, 10, 176–184. [Google Scholar] [CrossRef]
- Zhong, Z.; Dong, Z.; Yang, L.; Chen, X.; Gong, Z. Inhibition of proliferation of human lung cancer cells by green tea catechins is mediated by upregulation of let-7. Exp. Ther. Med. 2012, 4, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.S.; Al-Harrasi, A. Isolation and bioactivities of the flavonoids morin and morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A wild Arabian medicinal plant. Molecules 2014, 19, 17763–17772. [Google Scholar] [CrossRef]
- Yao, D.; Cui, H.; Zhou, S.; Guo, L. Morin inhibited lung cancer cells viability, growth, and migration by suppressing miR-135b and inducing its target CCNG2. Tumor Biol. 2017, 39, 1010428317712443. [Google Scholar] [CrossRef]
- Demir, S.; Turan, İ.; Demir, F.; Ayazoglu Demir, E.; Aliyazicioglu, Y. Cytotoxic effect of Laurocerasus officinalis extract on human cancer cell lines. Marmara Pharm. J. 2017, 21, 121–126. [Google Scholar] [CrossRef]
- Wang, L.; Du, H.; Chen, P. Chlorogenic acid inhibits the proliferation of human lung cancer A549 cell lines by targeting annexin A2 in vitro and in vivo. Biomed. Pharmacother. 2020, 131, 110673. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules 2018, 23, 1395. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.D.; Ahumada, M.C.; Saenz, M.T. Cytostatic activity of some phenolic acids of Scrophularia frutescens L. var. frutescens. Z. Nat. C 1998, 53, 1093–1095. [Google Scholar] [CrossRef]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytother Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef]
- Rajendran, P.; Maheshwari, U.; Muthukrishnan, A.; Muthuswamy, R.; Anand, K.; Ravindran, B.; Dhanaraj, P.; Balamuralikrishnan, B.; Chang, S.W.; Chung, W.J. Myricetin: Versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS–reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol. Cell. Biochem. 2020, 476, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 13–14. [Google Scholar] [CrossRef]
- Park, M.Y.; Kang, D.H. Antibacterial activity of caffeic acid combined with UV-A light against Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e0063121. [Google Scholar] [CrossRef]
- Kopacz, M.; Woznicka, E.; Gruszecka, J. Antibacterial activity of morin and its complexes with La (III), Gd (III) and Lu (III) ions. Acta Pol. Pharm. Drug Res. 2005, 62, 65–67. [Google Scholar]
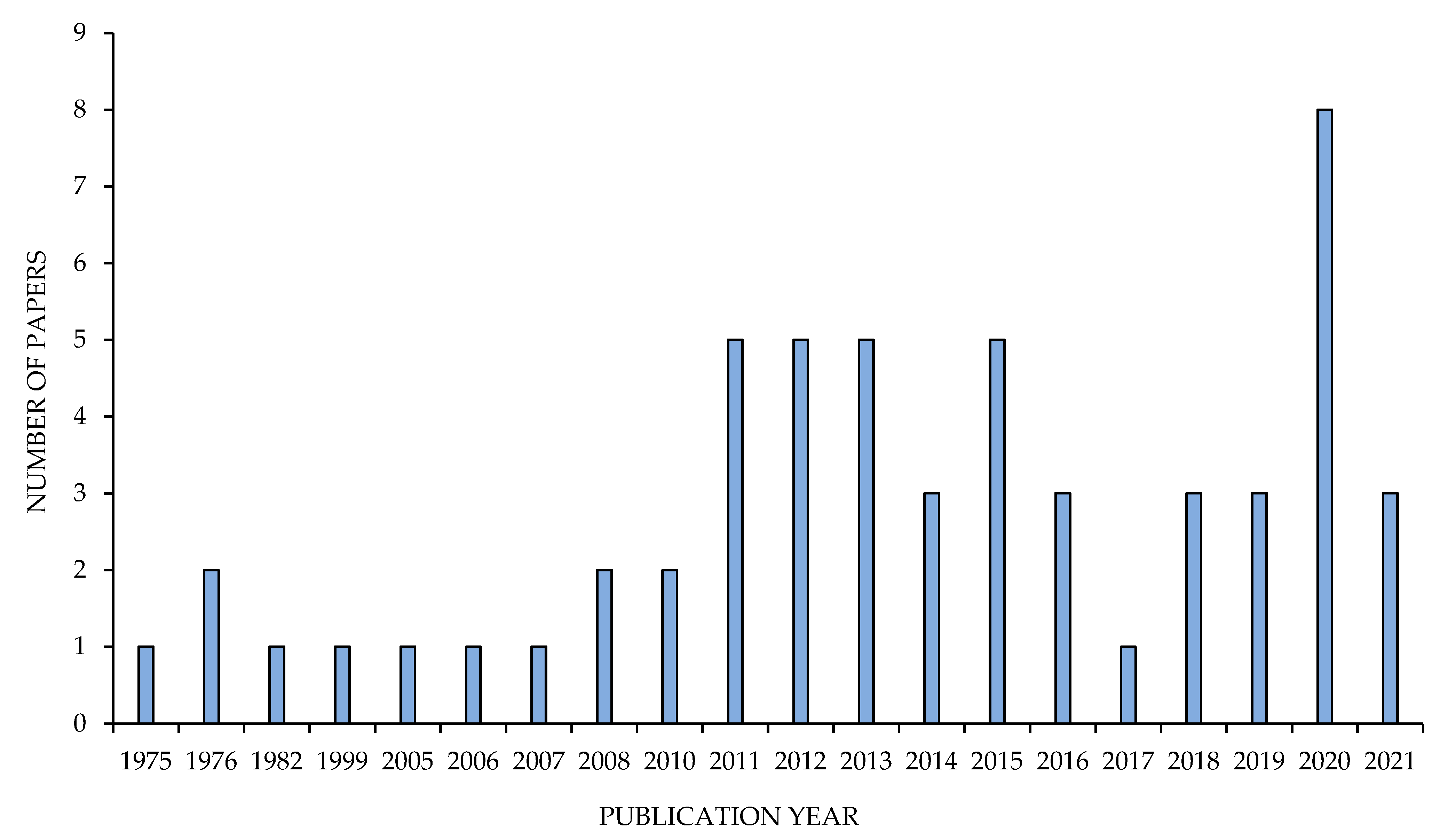
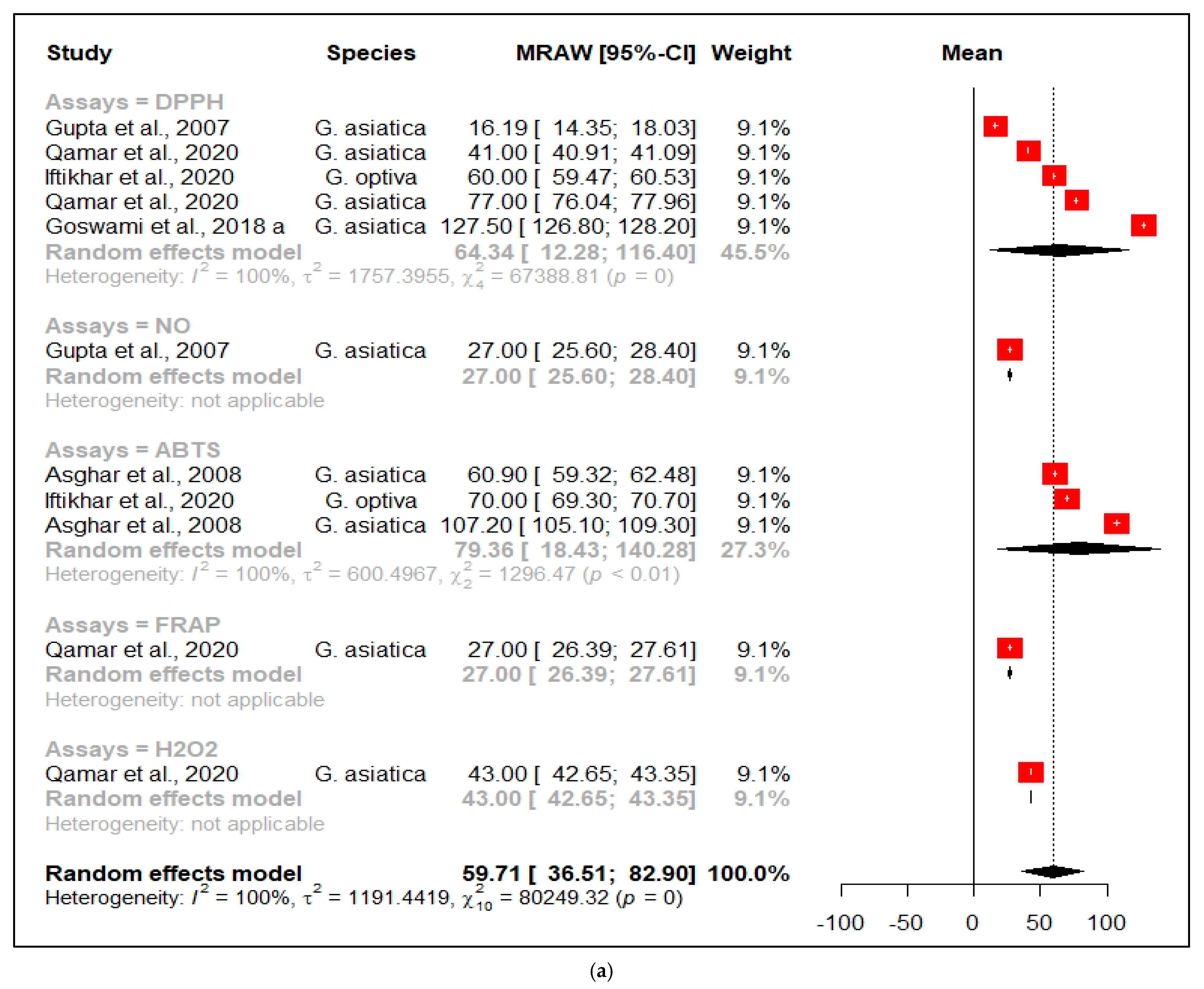
| Antioxidant Effects of Grewia species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Plant Part | Origin | Extraction Solvent | Activity | Assay | Activity of Extract | Std Dev | Positive Control | Activity | Std Dev | References |
| G. asiatica | Leaf | India | Acetone | Antioxidant | DPPH | 127.5 IC50 µg/mL | 0.8 | NG | NG | NG | [1] |
| G. asiatica | Fruit | Pakistan | 50% aqueous Methanol | Antioxidant | DPPH | 41 IC50 µg/mL | 0.1 | Ascorbic acid | 75.1 IC50 µg/mL | 0.01 | [24] |
| G. asiatica | Fruit | Pakistan | Methanol | Antioxidant | DPPH | 77 IC50 µg/mL | 1.1 | Ascorbic acid | 75.1% inhibition | 0.01 | [24] |
| G. asiatica | Leaf | India | Benzene | Antioxidant | DPPH | 16.19 IC50 µg/mL | 2.1 | Ascorbic acid | 78.1 IC50 µg/mL | 4.05 | [46] |
| G. optiva | Leaf | Pakistan | Water | Antioxidant | DPPH | 60 IC50 µg/mL | 0.6 | Ascorbic acid | 28 IC50 µg/mL | 0.40 | [27] |
| G. lasiocarpa | Stem | South Africa | Chloroform | Antioxidant | DPPH | >1000 IC50 µg/mL | 0.2 | NG | NG | NG | [15] |
| G. asiatica | Fruit | Pakistan | 80% aqueous Methanol | Antioxidant | DPPH | 85% inhibition | 1.5 | BHA | 89% inhibition | NG | [48] |
| G. asiatica | Fruit | India | Methanol | Antioxidant | DPPH | 84.8% inhibition | 0.9 | NG | NG | NG | [49] |
| G. asiatica | Fruit | Pakistan | Methanol | Antioxidant | DPPH | >60% inhibition | 0.7 | NG | NG | NG | [50] |
| G. flava | Peel | Botswana | Ethanol | Antioxidant | DPPH | 375 µmol GAE/g | 1.1 | NG | NG | NG | [52] |
| G. biocolor | Peel | Botswana | Ethanol | Antioxidant | DPPH | 165 µmol GAE/g | 0.2 | NG | NG | NG | [52] |
| G. asiatica | Leaf | India | Water | Antioxidant | NO | 1098 IC50 µg/mL | 0.9 | NG | NG | NG | [51] |
| G. asiatica | Leaf | India | Benzene | Antioxidant | NO | 27.0 IC50 µg/mL | 1.6 | Ascorbic acid | 20.5 IC50 µg/mL | 1.7 | [46] |
| G. asiatica | Seed | Pakistan | Ethyl acetate | Antioxidant | ABTS | 55.8 TEAC µmol/g | 0.3 | NG | NG | NG | [47] |
| G. asiatica | Peel | Pakistan | 70% aqueous acetone | Antioxidant | ABTS | 107.2 TEAC µmol/g | 2.4 | NG | NG | NG | [47] |
| G. asiatica | Pulp | Pakistan | 70% aqueous acetone | Antioxidant | ABTS | 60.9 TEAC µmol/g | 1.8 | NG | NG | NG | [47] |
| G. asiatica | Fruit | Pakistan | Methanol | Antioxidant | ABTS | % inhibition >60% | 0.7 | NG | NG | NG | [50] |
| G. optiva | Leaves | Pakistan | Water | Antioxidant | ABTS | 70 IC50 µg/ml | 0.8 | Ascorbic acid | 30 IC50 µg/mL | 0.30 | [27] |
| G. asiatica | Fruit | India | Methanol | Antioxidant | FRAP | 4.14 mg GAE/g | 1.1 | NG | NG | NG | [49] |
| G. asiatica | Fruit | Pakistan | 50% Aqueous methanol | Antioxidant | FRAP | 43 mg GAE/g | 0.6 | Ascorbic acid | 15.0 mg GAE/g | 0.01 | [24] |
| G. asiatica | Fruit | Pakistan | Methanol | Antioxidant | FRAP | 27 mg GAE/g | 0.7 | Ascorbic acid | 15.0 mg GAE/g | 0.01 | [24] |
| G. lasiocarpa | Stem | South Africa | Chloroform | Antioxidant | FRAP | >1000 IC50 µg/mL | 0.9 | NG | NG | NG | [15] |
| G. asiatica | Fruit | Pakistan | 50% Aqueous methanol | Antioxidant | H2O2 | 73% inhibition | 0.5 | Ascorbic acid | 79.1% inhibition | 0.02 | [24] |
| G. asiatica | Fruit | Pakistan | Methanol | Antioxidant | H2O2 | 43% inhibition | 0.4 | Ascorbic acid | 79.1% inhibition | 0.02 | [24] |
| Anticancer effects of Grewia species | |||||||||||
| Species | Plant Part | Origin | Extracting Solvent | Activity | Assays | Cancer Cell Line | Activity of Extract (IC50 ) | Reference Drug | Activity (IC50) | Std Dev | References |
| G. asiatica | Fruit | India | Water | Anticancer | MTT | HEp-2 | 50.31 µg/mL | Methotrexate | 0.98 µg/mL | NG | [55] |
| G. asiatica | Leaves | India | Aqueous | Anticancer | MTT | HEp-2 | 61.23 µg/mL | Methotrexate | 0.98 µg/mL | NG | [55] |
| G. asiatica | Fruit residue | India | Methanol | Anticancer | MTT | HEp-2 | >250 µg/mL | Not given | NG | NG | [30] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | HEp-2 | 80.41 µg/mL | Methotrexate | 0.82 µg/mL | NG | [24] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | HEp-2 | 80.41 µg/mL | Methotrexate | 0.82 µg/mL | NG | [24] |
| G. asiatica | Fruit | India | Aqueous | Anticancer | MTT | NCI-H522 | 59.03 µg/mL | Methotrexate | 0.96 µg/mL | NG | [55] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | NCI-H522 | Notable cytotoxicity | NG | NG | NG | [56] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | NCI-H522 | 73.01 µg/mL | Methotrexate | 0.91 µg/mL | 0.21 | [24] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | NCI-H522 | 73.01 µg/mL | Methotrexate | 0.91 µg/mL | 0.21 | [24] |
| G. asiatica | Fruit | India | Aqueous | Anticancer | MTT | MCF-7 | 58.65 µg/mL | Methotrexate | 0.98 µg/mL | 0.4 | [55] |
| G. asiatica | Leaves | India | Aqueous | Anticancer | MTT | MCF-7 | 50.37 µg/mL | Methotrexate | 0.98 µg/mL | 0.4 | [55] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | MCF-7 | Notable cytotoxicity | NG | NG | NG | [56] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | MCF-7 | 199.5 µg/mL | NG | NG | NG | [57] |
| G. asiatica | Fruit residue | India | Methanol | Anticancer | MTT | MCF-7 | 68.91 µg/mL | NG | NG | NG | [30] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | MCF-7 | 34.87 µg/mL | Methotrexate | 0.82 µg/mL | 0.1 | [24] |
| G. asiatica | Fruit | Pakistan | Aqueous methanol | Anticancer | MTT | MCF-7 | 34.87 µg/mL | Methotrexate | 0.82 µg/mL | 0.1 | [24] |
| G. lasiocarpa | Stem bark | South Africa | Chloroform | Anticancer | MTT | MCF-7 | >1000 µg/mL | NG | NG | NG | [15] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | Hela | 177.8 µg/mL | NG | NG | NG | [57] |
| G. asiatica | Fruit residue | India | Methanol | Anticancer | MTT | Hela | >100 µg/mL | NG | NG | NG | [30] |
| G. lasiocarpa | Stem bark | South Africa | Chloroform | Anticancer | MTT | Hela | >1000 µg/mL | NG | NG | NG | [15] |
| G. asiatica | Fruits | Pakistan | Methanol | Anticancer | MTT | Hela | 406.5 µg/mL | Methotrexate | 0.89 | 0.31 | [24] |
| G. asiatica | Fruits | Pakistan | Aqueous methanol | Anticancer | MTT | Hela | 282.4 µg/mL | Methotrexate | 0.89 | 0.31 | [24] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | K-562 | 54.90 µg/mL | NG | NG | NG | [57] |
| G. asiatica | Leaves | India | Methanol | Anticancer | MTT | HL-60 | 53.70 µg/mL | NG | NG | NG | [57] |
| G. lasiocarpa | Stem bark | South Africa | Chloroform | Anticancer | MTT | HEK293 | No Activity | NG | NG | NG | [15] |
| Anti-inflammatory properties of Grewia species | |||||||||||
| Species | Plant Part | Origin | Extracting Solvent | Activity | Assay | Negative Control (% Inhibition) | Activity of Extract (% Inhibition) | Std Dev | Positive Control (% Inhibition) | Std Dev | References |
| G. asiatica | Fruit | India | Water | Analgesic | Acetic acid induced writhing | none | 99.39 at 300 mg/kg | 0.21 | 99.18 at 400 mg/kg of aspirin | 0.4 | [58] |
| G. asiatica | Fruit | Pakistan | Methanol | Analgesic | Acetic acid induced writhing | none | % inhibition was 61.81 at 500 mg/kg | 0.54 | 75.1 at 10 mg/kg of indomethacin | 0.89 | [59] |
| G. asiatica | Fruit | Pakistan | Water | Analgesic | Acetic acid induced writhing | none | % inhibition was 55.34 at 500 mg/kg | 0.34 | 75.1 at 10 mg/kg of indomethacin | 0.89 | [59] |
| G. asiatica | Fruit | India | Water | Antipyretic | Hot plate method | Hot plate reaction time was 3.1 min | Hot plate reaction time was 7.4 min at 400 mg/kg | 1.07 | Hot plate reaction time was 2.12 min at 300 mg/kg of Aspirin | 0.42 | [58] |
| G. asiatica | Bark | India | Methanol | Analgesic | Hot plate method | Hot plate reaction time was 2.80 sec | Hot plate reaction time was 12.37 sec at 400 mg/kg | 1.42 | Hot plate reaction time was 13 sec at 300 mg/kg at 5 mg/kg of Pentazocine | 0.84 | [60] |
| G. asiatica | Bark | India | Methanol | Analgesic | Hot plate method | Hot plate reaction time was 2.80 sec | Hot plate reaction time was 12 sec at 400 mg/kg | 1.38 | Hot plate reaction time was 13 sec at 300 mg/kg at 5 mg/kg of Pentazocine | 0.84 | [60] |
| G. asiatica | Fruit | Pakistan | Methanol | Antipyretic | Breweris yeast induced pyrexia | Average temperature was 102 | Average temperature was 100.81 at 500 mg/kg | 0.19 | Average temperature was 98.6 at 150 mg/kg of paracetamol | 0.04 | [59] |
| G. asiatica | Fruit | Pakistan | Water | Antipyretic | Brewerís yeast induced pyrexia | Average temperature was 102 | Average temperature was 100.5 °C at 500 mg/kg | 0.12 | Average temperature was 98.6 °C at 150 mg/kg of paracetamol | 0.04 | [59] |
| G. asiatica | Bark | India | Methanol | Anti-inflammatory | Carrageenan- induced paw oedema | % inhibition was 0 | % inhibition was 59.14 at 400 mg/kg | 0.51 | % inhibition was 64.2 at 10 mg/kg of indomethacin | 0.38 | [60] |
| G. asiatica | Bark | India | Water | Anti-inflammatory | Carrageenan- induced paw oedema | % inhibition was 0 | % inhibition was 53.04 at 400 mg/kg | 0.39 | % inhibition was 64.2 at 10 mg/kg of indomethacin | 0.38 | [60] |
| G. asiatica | Fruit | India | Methanol | Anti-inflammatory | Carrageenan- induced paw oedema | [61] | |||||
| G. asiatica | Fruit | Pakistan | Methanol | Anti-inflammatory | Carrageenan- induced paw oedema | % inhibition was 0 | % inhibition was 36.12 at 500 mg/kg | 0.43 | % inhibition was 36.4 at 10 mg/kg of indomethacin | 0.03 | [59] |
| G. asiatica | Fruit | Pakistan | Water | Anti-inflammatory | Carrageenan- induced paw oedema | % inhibition was 0 | % inhibition was 32.44 at 500 mg/kg | 0.21 | % inhibition was 36.4 at 10 mg/kg of indomethacin | 0.03 | [59] |
| G. asiatica | Leaves | India | n-Hexane | Anti-inflammatory | Membrane protection | % inhibition was 0 | % inhibition was 80.91 at 600 µg/mL | NG | % inhibition was 21.1 at 600 µg/mL at 600 µg/mL of diclofenac potassium | NG | [62] |
| G. asiatica | Leaves | India | Methanol | Anti-inflammatory | Membrane protection | % inhibition was 0 | % inhibition was 2.5 at 600 µg/mL | NG | % inhibition was 21.1 at 600 µg/mL at 600 µg/mL of diclofenac potassium | NG | [62] |
| G. optiva | Leaves | India | n-Hexane | Anti-inflammatory | Membrane protection | % inhibition was 0 | % inhibition was 0 at 600 µg/mL | NG | % inhibition was 21.1 at 600 µg/mL at 600 µg/mL of diclofenac potassium | NG | [62] |
| G. optiva | Leaves | India | Methanol | Anti-inflammatory | Membrane protection | % inhibition was 0 | % inhibition was 3.00 at 600 µg/mL | NG | % inhibition was 21.1 at 600 µg/mL at 600 µg/mL of diclofenac potassium | NG | [62] |
| Antimicrobial activities of Grewia species | |||||||||||
| Species | Plant Part | origin | Extracting Solvent | Activity | Bacterial/Fungal Strain | Activity of Extract | Std Dev | Positive Control | Activity | Std Dev | References |
| G. asiatica | Leaves | Pakistan | Ethanol | Antibacterial | S. aureus | MIC was >1 mg/mL | NG | Amoxicillin | MIC was 20 mg/mL | 0.06 | [63] |
| G. asiatica | Fruit | Pakistan | Methanol | Antibacterial | S. aureus | MIC was 15.625 µg/mL | 0.11 | NG | NG | NG | [64] |
| G. asiatica | Bark Fruit | Pakistan | Ethanol | Antibacterial | S. aureus | Zone of inhibition 6.33 mm | 0.84 | Moxifloxacin | Zone of inhibition 30 mm | NG | [65] |
| G. asiatica | Leaves | Pakistan | Methanol | Antibacterial | S. aureus | Zone of inhibition 10.4 mm | 1.1 | Cefixime | Zone of inhibition 20 mm | 2.5 | [66] |
| G. asiatica | Leaves | Pakistan | Water | Antibacterial | S. aureus | Zone of inhibition 13.5 mm | 1.6 | Cefixime | Zone of inhibition 20 mm | 2.5 | [66] |
| G. optiva | Leaves | Pakistan | Water | Antibacterial | S. aureus | Zone of inhibition 9 mm | 0.99 | Cephradine | Zone of inhibition 24 mm | 1.20 | [27] |
| G. lasiocarpa | Stem | South Africa | Chloroform | Antibacterial | S. aureus | No activity observed | NG | Streptomycin | Zone of inhibition 12.3 mm | 2.31 | [15] |
| G. hirsuta | Leaves | India | 70% aqueous Methanol | Antibacterial | S. aureus | Zone of inhibition 19 mm | 0.47 | Ciprofloxacin | Zone of inhibition 22 mm | 2.16 | [67] |
| G. asiatica | Stem Bark | Pakistan | Ethanol | Antibacterial | S. typhi | Zone of inhibition 6.33 mm | 0.47 | Moxifloxacin | Zone of inhibition 19 mm | NG | [65] |
| G. asiatica | Leaves | Pakistan | Methanol | Antibacterial | S. typhi | Zone of inhibition 15.2 mm | 1.21 | Cefixime | Zone of inhibition 21.5 mm | 2.58 | [66] |
| G. asiatica | Leaves | Pakistan | Water | Antibacterial | S. typhi | No activity observed | NG | Cefixime | Zone of inhibition 21.5 mm | 2.58 | [66] |
| G. optiva | Leaves | Pakistan | Water | Antibacterial | S. typhi | Zone of inhibition 10 mm | 1.32 | Cephradine | Zone of inhibition 21 mm | 0.61 | [27] |
| G. lasiocarpa | Stem | South Africa | Chloroform | Antibacterial | S. typhi | No activity observed | NG | Gentamicin | Zone of inhibition 19.33 mm | 1.92 | [15] |
| G. asiatica | Leaves | Pakistan | Methanol | Antibacterial | E. coli | No activity observed | NG | Cefixime | No activity observed | NG | [66] |
| G. asiatica | Leaves | Pakistan | Water | Antibacterial | E. coli | No activity observed | NG | Cefixime | No activity observed | NG | [66] |
| G. optiva | Leaves | Pakistan | Water | Antibacterial | E. coli | Zone of inhibition 9 mm | 1.20 | Cephradine | Zone of inhibition 23 mm | 0.20 | [27] |
| G. lasiocarpa | Stem | South Africa | Chloroform | Antibacterial | E. coli | No activity observed | NG | Gentamicin | Zone of inhibition 18.3 mm | 1.68 | [15] |
| G. hirsuta | Leaves | India | 70% Aqueous methanol | Antibacterial | E. coli | Zone of inhibition 16 mm | 2.05 | Ciprofloxacin | Zone of inhibition 18 mm | 0.28 | [67] |
| G. optiva | Leaves | Pakistan | Water | Antibacterial | S. pneumoniae | Zone of inhibition 10 mm | 0.21 | Cephradine | Zone of inhibition 25 mm | 0.30 | [27] |
| G. asiatica | Bark Fruit | Pakistan | Ethanol | Antibacterial | Proteus vulgaris | Zone of inhibition 7.33 mm | 0.85 | Moxifloxacin | Zone of inhibition 16 mm | NG | [65] |
| G. asiatica | Leaves | Pakistan | Ethanol | Antifungal | Fusarium solani | MIC was >10 mg/mL | NG | Itraconazole | MIC was 12 mg/mL | 0.34 | [63] |
| G. asiatica | Fruit | Pakistan | Methanol | Antifungal | Aspergillus flavus | Zone of inhibition 35 mm | 0.50 | NG | NG | NG | [64] |
| G. asiatica | Fruit | Pakistan | Methanol | Antifungal | Aspergillus niger | Zone of inhibition 40 mm | 0.55 | NG | NG | NG | [64] |
| G. asiatica | Fruit | Pakistan | Methanol | Antifungal | Penicillium notatum | Zone of inhibition 35 mm | 0.90 | NG | NG | NG | [64] |
| G. asiatica | Leaves | India | Acetone | Antifungal | Aspergillus fumigates | Zone of inhibition 32 mm | 0.58 | NG | NG | NG | [51] |
| G. asiatica | Leaves | India | Acetone | Antifungal | Candida glabrata | Zone of inhibition 28 mm | 1.53 | NG | NG | NG | [51] |
| G. asiatica | Leaves | India | Acetone | Antifungal | Aspergillus niger | Zone of inhibition 25 mm | 0.58 | NG | NG | NG | [51] |
| G. asiatica | Leaves | Pakistan | Water | Antifungal | Rhizoctonia solani | 86% inhibition | 2 | NG | NG | NG | [68] |
| G. asiatica | Leaves | Pakistan | Water | Antifungal | Fusarium oxysporum | 62% inhibition | 1.5 | NG | NG | NG | [68] |
| G. asiatica | Leaves | Pakistan | Water | Antifungal | Macrophomina phaseolina | 81% inhibition | 4.1 | NG | NG | NG | [68] |
| Antidiabetic properties of the Grewia species | |||||||||||
| Species | Plant Part | Origin | Extraction Solvent | Assay | Negative Control | Std Dev | Positive Control | Std Dev | Activity of Extract | Std Dev | References |
| G. asiatica | Fruit | Egypt | Ethanol | Rats model | Serum glucose level was 150 | 10.9 | NG | NG | Serum glucose level was 105 at 200 mg/kg of extract | 10.4 | [69] |
| G. asiatica | Leaf | India | Ethanol | Rats model | Serum glucose level was 227.3 | 5.9 | Serum glucose level was 201 at Glibenclamide 10 mg/kg | 6.3 | Serum glucose level was 205 at 200 mg/kg of extract | 7.1 | [70] |
| G. asiatica | Bark | Bangladesh | Ethanol | Rats model | Serum glucose level was 14.9 | 3 | Serum glucose level was 5.9 at Metformin 150 mg/kg | 3 | Serum glucose level was 7.1 | 2.5 | [66] |
| G. asiatica | Leaf | Pakistan | Methanol | α-Amylase | NG | NG | 98% inhibition of α-amylase at Acarbose 0.1 µg/mL | NG | 80% inhibition at 500 µg/mL of extract | NG | [71] |
| G. asiatica | Leaf | Pakistan | Methanol | α-Glucosidase | NG | NG | 98% inhibition of α-glucosidase at Acarbose 0.1 µg/mL | NG | 80% inhibition at 500 µg/mL of extract | NG | [71] |
| G. asiatica | Fruit | India | Aqueous | α-Glucosidase | NG | NG | Acarbose exhibited IC50 0.006 µg/mL in α-glucosidase inhibition | NG | IC50 8.93 mg/mL | NG | [72] |
| G. asiatica | Fruit | India | Aqueous | α-Amylase | NG | NG | Acarbose exhibited IC50 0.83 µg/mL in α-amylase inhibition Inhibition | NG | IC50 0.41 mg/mL | NG | [72] |
| G. asiatica | Pomace | India | 20% Hydro-methanol | α-Amylase | NG | NG | IC50 0.39 μg/mL in α-amylase inhibition | NG | IC50 45.7 mg/mL | NG | [30] |
| G. asiatica | Pomace | India | 20% Hydro-acetone | α-Amylase | NG | NG | IC50 0.39 μg/mL in α-amylase inhibition | NG | IC50 85.2 mg/mL | NG | [30] |
| G. asiatica | Fruit | Pakistan | Methanol | Non-diabetic human model | NG | NG | NG | NG | 1.4% reduction in blood glucose level | NG | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, M.; Akhtar, S.; Ismail, T.; Wahid, M.; Barnard, R.T.; Esatbeyoglu, T.; Ziora, Z.M. The Chemical Composition and Health-Promoting Effects of the Grewia Species—A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4565. https://doi.org/10.3390/nu13124565
Qamar M, Akhtar S, Ismail T, Wahid M, Barnard RT, Esatbeyoglu T, Ziora ZM. The Chemical Composition and Health-Promoting Effects of the Grewia Species—A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(12):4565. https://doi.org/10.3390/nu13124565
Chicago/Turabian StyleQamar, Muhammad, Saeed Akhtar, Tariq Ismail, Muqeet Wahid, Ross T. Barnard, Tuba Esatbeyoglu, and Zyta M. Ziora. 2021. "The Chemical Composition and Health-Promoting Effects of the Grewia Species—A Systematic Review and Meta-Analysis" Nutrients 13, no. 12: 4565. https://doi.org/10.3390/nu13124565
APA StyleQamar, M., Akhtar, S., Ismail, T., Wahid, M., Barnard, R. T., Esatbeyoglu, T., & Ziora, Z. M. (2021). The Chemical Composition and Health-Promoting Effects of the Grewia Species—A Systematic Review and Meta-Analysis. Nutrients, 13(12), 4565. https://doi.org/10.3390/nu13124565









