Abstract
Inclusion of bovine-derived milk fat globule membrane (bMFGM) or bMFGM components in infant formulas (IFs) may support healthy brain development. This double-blind, prospective trial evaluated growth, tolerance, and iron status in infants receiving added bMFGM and modified protein, iron, and arachidonic acid (ARA) concentrations in IF. Healthy term infants were randomized to: control (marketed, routine cow’s milk-based IF/100 kcal: 2.1 g protein, 1.8 mg iron, 34 mg ARA) or INV-MFGM (investigational cow’s milk-based IF/100 kcal: 1.9 g protein, 1.2 mg iron, 25 mg ARA and whey protein-lipid concentrate, 5 g/L (source of bMFGM)). Anthropometrics, stool characteristics, fussiness, and gassiness through day 365 and blood markers of iron status at day 365 were evaluated. The primary outcome was rate of weight gain from 14–120 days of age. Of 373 infants enrolled (control: 191, INV-MFGM: 182), 275 completed the study (control: 141; INV-MFGM: 134). No group differences in growth rate (g/day) from day 14–120 or study discontinuation were detected. Few group differences in growth or parent-reported fussiness, gassiness, or stool characteristics were detected. No group differences were detected in hemoglobin, hematocrit, or incidence of anemia. In healthy term infants, bMFGM and modified protein, iron, and ARA concentrations in a cow’s milk-based IF were well-tolerated, associated with adequate growth throughout the first year of life, and supported normal iron status at one year of age.
1. Introduction
Milk fat globule membrane (MFGM) is a complex protein-phospholipid trilayer (2–6% of fat globule) that surrounds each fat droplet secreted into milk and is highly conserved across mammalian species [1,2,3,4]. MFGM is present in both human and bovine milk and therefore has a long history of safe use in infants and young children. However, currently marketed infant formulas typically have been designed to approximate human milk fatty acid composition using vegetable oils. Added bovine-derived MFGM (bMFGM) in infant formula may better approximate the composition of complex human milk lipids because human and bovine milk are highly homologous [5,6]. For example, total phospholipids and certain phospholipid species (including phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidylcholine) have been measured at comparable concentrations in bovine and human milk [7,8]. Furthermore, bMFGM ingredients (specifically Lacprodan® MFGM-10, Arla Foods Ingredients P/S, Denmark) added to infant formulas have been clinically demonstrated to support normal growth and tolerance [9,10,11,12,13]. Furthermore, a growing number of studies has associated the addition of dietary bMFGM with beneficial effects on neurodevelopment [11,12,14], behavior [15], and digestive or immune health [10,16].
Protein and iron must also be considered in designing an infant formula. Expert recommendations support the use of infant formulas that have protein to fall more in line with human milk [17,18]. The ability to reformulate protein concentration closer to human milk and minimums identified by expert recommendations and regulatory provisions, while maintaining the protein quality of the infant formula, is highly dependent on protein source. Compared to human milk, total protein in infant formula has traditionally been higher to ensure sufficient protein quality to support normal growth. However, increasing evidence suggests infant formulas that have lower protein concentrations, closer to human milk, support adequate growth and potentially benefit later growth outcomes [11,19,20,21,22,23,24,25]. For iron, an important functional component of many proteins and an essential mineral for brain development, human milk content is relatively low (~0.35 mg/L) and reflected by the dietary iron requirement (0.27 mg/day) through to 6 months of age [26]. Iron fortification of infant formula is necessary to lower anemia risk in the first year of life. Most commercial formulas currently available in the United States are fortified at 12 mg iron/L (~2 mg/100 kcal, considered safe for intended use by the American Academy of Pediatrics (AAP) [27]), although the safety and nutritional suitability for lower iron, particularly for infants 0–6 months may be indicated [17,18,28,29].
Human milk also provides the long chain polyunsaturated fatty acids (LCPUFAs) docosahexaenoic acid (DHA) and arachidonic acid (ARA). Based on early human milk composition data, infant formulas that have DHA and ARA at ~0.3% and ~0.6% of total fatty acids are associated with visual and cognitive development in term infants [30,31,32,33,34]. However, a more recent comprehensive analysis of worldwide human milk composition data reported mean DHA at 0.32% and ARA at 0.47% of total fatty acids [35], and a growing body of data supports the use of infant formulas that have lower ARA content. We have previously demonstrated LCPUFA concentrations that correspond to these updated means (ARA at 25 mg/100 kcal and DHA at 17 mg/100 kcal) in infant formula support normal growth and tolerance [12,36,37,38], and red blood cell DHA has been demonstrated as equivalent to previously studied concentrations, suggesting equivalent availability for central nervous system development [38].
Regulations for growth monitoring studies have been established to ensure that new infant formulas support normal physical growth [39,40]. Consequently, the present study was designed to evaluate growth and tolerance in healthy term infants receiving formula that had an added bMFGM ingredient (5 g/L whey protein-lipid concentrate) and modified total protein (1.9 g/100 kcal, approaching the minimum identified in expert recommendations and regulatory reference ranges [17,18,39,40,41,42]), iron (1.2 mg/100 kcal), and arachidonic acid (ARA; 25 mg/100 kcal) through 365 days of age compared to a marketed, routine cow’s milk-based (2.1 g/100 kcal protein, 1.8 mg/100 kcal iron, and 34 mg/100 kcal ARA) that had no added bMFGM.
2. Materials and Methods
2.1. Study Objectives
Growth, tolerance, and adverse events were assessed from 14 to approximately 365 days of age. The rate of weight gain from 14–120 days of age was the primary variable to establish that the new formula provides adequate growth compared to a control formula. The hypothesis to be tested was that rate of weight gain using the control formula would be less than or equal to the new formula. Rejection of the hypothesis with a mean difference exceeding a clinically relevant amount (3 g/day, as outlined in American Academy of Pediatrics [AAP] guidance [43]) would indicate inadequate growth using the new formula. Failure to reject with post-study confirmation of adequate power would allow concluding that the new formula does provide adequate growth. Because the AAP recommends universal screening for iron deficiency anemia at 9–12 months of age [27], a key secondary objective was to evaluate blood markers of iron status at 365 days of age.
2.2. Study Design and Participants
In this multicenter, double-blind, randomized, controlled, parallel-group, prospective trial, participants were enrolled between July 2015 and November 2015 at 22 clinical sites in the United States (clinicaltrials.gov: NCT02481531). Mothers who had decided to exclusively provide infant formula were screened for study eligibility. Parents or guardians provided written informed consent prior to enrollment.
Participants were 10–14 days of age at randomization. Eligible infants were singleton births at 37–42 weeks gestational age with birth weight ≥2500 g and solely receiving formula at least 24 h prior to randomization. Exclusion criteria included: diagnosis of anemia at any time after birth or current use of iron or iron-containing supplements; history of underlying disease or congenital malformation likely to interfere with normal growth and development or participant evaluation; feeding difficulties or formula intolerance; weight at randomization <98% of birth weight; large for gestational age, born from a mother diabetic at childbirth; and immunodeficiency. Study visits corresponded to 14 (−4 days; enrollment), 30 (±3), 42 (±3), 60 (±3), 90 (±3), 120 (+5), 180 (±7), 275 (±7), and 365 (±7) days of age.
2.3. Randomization and Study Group Allocation
The study sponsor created a computer-generated, sex-stratified randomization schedule provided in sealed, opaque, consecutively numbered envelopes for each study site. Study formula was assigned by opening the next sequential envelope from the appropriate set at the study site. Participants were randomly assigned to receive a control (previously marketed Enfamil®) or investigational formula (INV-MFGM) from day 14 up to day 365 (Mead Johnson Nutrition, Evansville, IN, USA; Table 1). The source of bovine MFGM used in this study was a commercially available ingredient (whey protein-lipid concentrate added at 5 g/L; Lacprodan MFGM-10, Arla Foods Ingredients P/S, Denmark) (as reviewed [44]). Study formulas, each designated by two unique codes known only to the sponsor, were dispensed to parents at each study visit prior to study completion or withdrawal. Blinding for a participant could be broken by study sponsor personnel in the event of a medical emergency. In this study, it was not necessary to break the study code prematurely. Participants received exclusive study formula feeding through day 120. Participants who continued through day 365 were considered to complete the study even if study formula consumption discontinued or decreased to fewer than 2 feedings/day after day 180 (6 months old).

Table 1.
Nutrient composition per 100 kcal (20 Calories/fl oz).
2.4. Study Objectives and Outcomes
The objective was to evaluate growth and tolerance in healthy, term infants. Birth anthropometric measures (body weight, length, and head circumference) were obtained from participant birth records. At all study sites, anthropometrics were recorded at days 14, 30, 42, 60, 90, 120, 180, 275, and 365 using the following standardized procedures. At each study visit, body weight was measured on a study-designated, calibrated pediatric balance (nearest g or oz); body length was measured (nearest ½ cm or ¼ in) using a recumbent pediatric stadiometer (Ellard Instrumentation, Monroe, WA, USA); and head circumference was measured (nearest ½ cm or ¼ inch) using a flexible, non-stretchable tape (infant head tape measure, Hopkins Medical Supply, Baltimore, MD, USA) provided by the study sponsor. Parents completed a baseline recall of tolerance (fussiness and gassiness) and stool characteristics (frequency and consistency) at study enrollment and 24 h recall of study formula intake, tolerance (fussiness and gassiness), and stool characteristics (frequency and consistency) at subsequent study visits. Responses were scaled for amount of gas (none = 0, slight = 1, moderate = 2, excessive = 3); fussiness (not fussy = 0, slightly = 1, moderately = 2, very = 3, extremely fussy = 4); and stool consistency (hard = 1, formed = 2, soft = 3, unformed = 4, or seedy, watery = 5). Adverse events (AEs) were coded according to specific event and the body system involved.
To assess iron status, whole blood (approximately 3 mL) was drawn via venipuncture at day 365. The following group of blood markers was selected and prioritized as follows: hemoglobin (Hb) and hematocrit (Hct); serum ferritin (SF); C-reactive protein (CRP). Per 2010 AAP guidance, a combination of blood markers is needed to accurately assess the overall iron status of a child [27]. In addition to defining iron deficiency, the AAP policy statement defines iron sufficiency as a state in which there is sufficient iron to maintain normal physiologic functions. Both Hb concentration (g/dL) and Hct, which represent the packed volume of red blood cells, are commonly used in clinical practice as determinants of anemia. Relative body iron stores are measured using SF (μg/L), but also may be elevated in a state of chronic inflammation; therefore, simultaneous measure of CRP (μg/L) is used to rule out inflammation. The Clinical Laboratory Improvement Amendment (CLIA) legal regulations are applicable to facilities that test human specimens for health assessments [45], and were passed by the United States Congress in 1988 to establish standards for “…laboratory testing to ensure the accuracy, reliability and timeliness of test results regardless of where or by whom the test was performed” [46]. In the current study, all testing facilities used to assess blood samples were CLIA-certified commercial central laboratories or institutional core laboratories using standard CLIA accepted methods of analysis (specifically, automated cell counting for Hb and Hct, immunoassay for SF, and turbidimetry for CRP). Consequently, all blood testing in the current study was performed by routine, standardized methods for clinical samples and methodology and reference ranges were comparable between testing facilities.
2.5. Statistical Methods
The sample size was chosen to detect a clinically relevant difference of 3 g/day in weight gain from day 14–120 (80% power; one-tailed). Assuming a standard deviation of 6.5 g/day for male and 5.5 g/day for female participants, 59 males and 43 females per study group were required to complete through day 120. Allowing for a 35% dropout rate, approximately 315 participants were targeted for enrollment. Analysis of variance (ANOVA) was used to assess growth rates in five pre-specified time intervals: from days 14 to 30, 42, 60, 90, or 120, calculated for each participant by linear regression of weight on age. Mean weight growth rates by study group and sex were compared using one-tailed tests as outlined in AAP guidelines [43].
Secondary outcomes included markers of iron status and inflammation at day 365 and anthropometrics, tolerance measures, and medically confirmed AEs through day 365. Per protocol, CRP above the laboratory reference (marker of inflammation) was analyzed (Fisher’s exact); Hb, Hct, and SF (markers of iron status) were, (1) analyzed (Kruskal–Wallis) and (2) classified relative to laboratory reference and analyzed (Cochran–Mantel-Haenszel (CMH)). For both analyses, participants with SF, but no CRP value, were included; SF was excluded when CRP was above the laboratory reference. Achieved weight, length, and head circumference; length and head circumference growth rates; formula intake; and stool frequency were analyzed by ANOVA. Stool consistency, fussiness, and gas were analyzed by CMH; AEs were analyzed by Fisher’s exact test. Post-hoc analysis compared Hb values using 2010 AAP guidelines for diagnosis and prevention of iron deficiency and iron deficiency anemia. With the exception of one-tailed tests for comparison of mean weight growth rates, all other tests were two-tailed (α = 0.05). All analyses were conducted using SAS version 9.2 (Cary, NC, USA).
3. Results
3.1. Participants
A total of 373 participants were enrolled and randomized (control: n = 191; INV-MFGM: n = 182); 275 completed the study (control: n = 141; INV-MFGM: n = 134). Participants who were randomized but consumed no study formula (Control: n = 1) were excluded from analyses (Figure 1). Sex, race, and ethnic distribution and birth anthropometric measures were similar between groups (Table 2). No differences in body weight, length, or head circumference were observed by sex among groups at study enrollment.
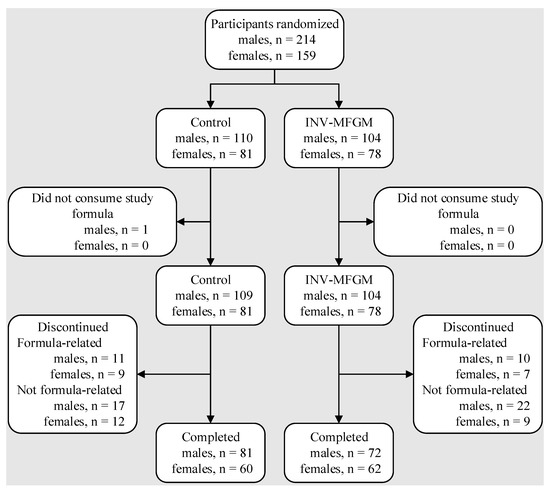
Figure 1.
Flow of study participants.

Table 2.
Infant characteristics at birth and study entry.
3.2. Growth
Growth rates were analyzed from 14–120 days of age. As outlined in AAP guidelines, rate of weight gain is used as the most important parameter in clinical evaluation of IFs, with differences of >3 g/day over a 3–4 month period considered clinically significant [43]. No statistically significant group differences in the primary outcome, weight growth rate from day 14–120, were detected by sex (Table 3). No statistically significant group differences were detected for weight, length, or head circumference growth rates by sex for any measured range. In addition, no statistically significant differences were observed for mean achieved weight, length, or head circumference at any measured time point up to day 365 with the exception of mean achieved weight in female infants at day 365 (control, n = 60; 9892 ± 140, INV-MFGM, n = 62; 9468 ± 138; p = 0.034). Mean achieved weight on the WHO weight-for-age standard growth chart [47] for males (Figure 2) and females (Figure 3) in the control group remained between 25th–75th percentiles of growth through day 180 and tracked near the 75th percentile through day 365. Females in the INV-MFGM group tracked similarly through day 180 and remained between 50th–75th percentile through day 365.

Table 3.
Weight, length, and head circumference growth rates from 14 days to 30, 42, 60, 90, and 120 days of age a.
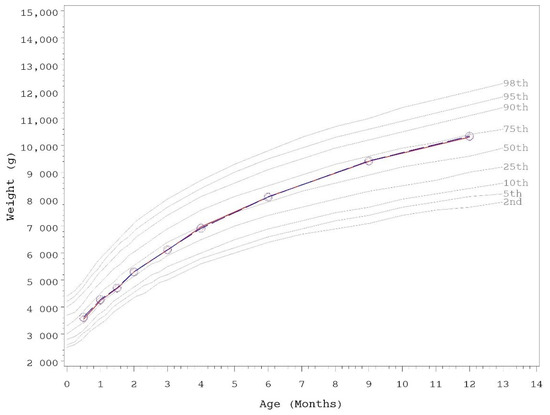
Figure 2.
Mean achieved weight for male participants with World Health Organization (WHO) reference percentiles (2nd to 98th) through 12 months (days 14 to 365) of age. Control, red diamonds; INV-MFGM, blue circles. No statistically significant group differences by two-tailed ANOVA test.
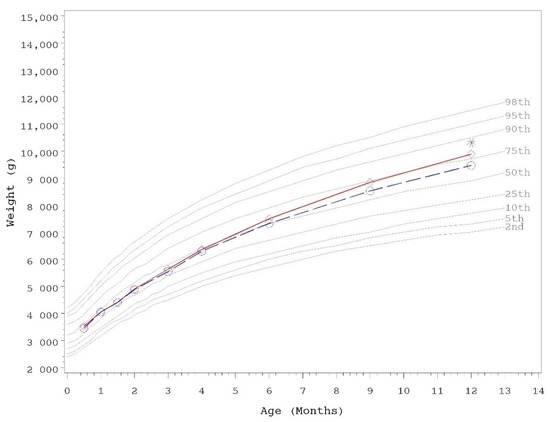
Figure 3.
Mean achieved weight for female participants with World Health Organization (WHO) reference percentiles (2nd to 98th) through 12 months (days 14 to 365) of age. Control, red diamonds; INV-MFGM, blue circles. * Denotes significant group difference, p < 0.05, two-tailed ANOVA test.
3.3. Tolerance
There were no statistically significant group differences in parent-reported mean study formula intake (fluid oz/day) by sex at any time point assessed or in mean duration (days) of study formula intake (Table 4). After day 180, mean reported study formula intake began to generally decline for all participants as expected, as parents and caregivers likely begin to offer complementary foods to infants at approximately 4–6 months of age. Parent-reported gassiness and fussiness were similar among groups at all time points (data not shown). Using 24 h recall, the amount of gas most commonly reported was “slight amount” or “moderate amount” up to 180 days of age, and “none at all” or “slight amount” by days 275 and 365. Fussiness was most often characterized as “slightly fussy” or “not fussy”. No significant group differences in stool frequency or consistency were detected at any time point assessed (Table 5), with the exception of stool consistency at day 90. By category, the primary differences at this time point were fewer infants with soft and more with unformed or seedy stool consistency in the INV-MFGM compared to the control group.

Table 4.
Study formula intake (fluid oz/day) at days 30, 42, 60, 90, 120, 180, 275, and 365 *.

Table 5.
Stool characteristics at days 14, 30, 42, 60, 90, 120, 180, 275, and 365.
In the overall study population (all participants up to day 365), no statistically significant group differences were detected for study formula discontinuation, either related (control: 20, 11%; INV-MFGM: 17, 9%) or not related to study formula (control: 57, 30%; INV-MFGM: 56, 31%). For formula-related discontinuation, formula intolerance determined by the study investigator was the most common reason (control: 20; INV-MFGM: 15); fussiness (control: 9; INV-MFGM: 7) and gas (control: 8; INV-MFGM: 5) were the most common symptoms. Parental decision was the most common reason for discontinuation not related to study formula (control: 22; INV-MFGM: 24). No group difference was detected in the number of participants for whom at least one medically-confirmed AE was reported (control: 176, 93%; INV-MFGM: 166; 91%). No statistically significant group differences were detected in the incidence of medically confirmed AEs by system: body as a whole; cardiovascular; endocrine; eyes, ear, nose and throat; gastrointestinal (GI); metabolic and nutrition; musculoskeletal; nervous system; skin; respiratory; and urogenital. Within the skin system, group incidence of medically-confirmed eczema was similar (control: 34, 18%; INV-MFGM: 32, 18%; p = 1.000). Within the eyes, ears, nose, and throat system, nasal/tear duct obstruction incidence was significantly different (control: 18, 9%; INV-MFGM: 6, 3%; p = 0.019). Within the GI system, gas incidence was significantly lower for INV-MFGM (9, 5%) versus control (24, 13%; p = 0.010). Within the “feeding problem” category, AE incidence was low but statistically significant (control: 0, 0%; INV-MFGM: 7, 4%; p = 0.006); assorted AEs included feeding difficulty/intolerance, including that associated with beginning complementary foods (mild, 5) and newborn feeding problems (mild, 1; moderate, 1). No group differences were detected in the incidence of AEs associated with allergic manifestations or infection. For 30 participants (control: 17, 9%; INV-MFGM: 13, 7%) who experienced serious adverse events, all were assessed as unrelated to study formulas by study physicians, with the exception of one infant (INV-MFGM) considered intolerant to study formula (later diagnosed with esophageal reflux) and one infant (INV-MFGM) diagnosed with cow milk protein allergy after study enrollment.
3.4. Iron Status
Blood markers of iron status evaluated at day 365 (control: 141; INV-MFGM: 127) were a key secondary outcome (Table 6). No significant differences were detected between study formula groups in actual Hb (g/dL) or Hct (%) or relative to laboratory references, and the majority of participants fell within reference ranges for Hb (control: 88%; INV-MFGM: 91%) and Hct (control: 87%; INV-MFGM: 87%). SF was significantly higher (p = 0.048) for control versus the INV-MFGM group. However, no significant group differences were detected relative to laboratory references and the majority of participants fell within SF reference ranges (control: 84%; INV-MFGM: 90%). Few participants were diagnosed with anemia during the study period (recorded as individual AEs) (control: 5, 3%; INV-MFGM: 1, 1%). By post-hoc analysis, no significant group difference was detected using the 2010 AAP Hb ≥ 11.0 g/dL cutoff for anemia evaluation (control: 91%; INV-MFGM: 87%).

Table 6.
Hemoglobin, hematocrit, and serum ferritin at day 365.
No significant group difference in C-reactive protein relative to the laboratory reference was detected with most participants falling below the upper reference ranges (control: 95%; INV-MFGM: 92%) (Table 7).

Table 7.
C-reactive protein relative to laboratory references at day 365.
3.5. C-reactive Protein
No significant group difference in C-reactive protein relative to the laboratory reference was detected with most participants falling below the upper reference ranges (control: 95%; INV-MFGM: 92%) (Table 7).
4. Discussion
In healthy infants, the addition of a bMFGM ingredient and iron at 1.2 mg/100 kcal, protein at 1.9 g/100 kcal, and ARA at 25 mg/100 kcal in a routine cow’s milk-based infant formula was well-tolerated and associated with adequate growth throughout the first year of life and supported normal iron status at one year of age. Safety and potential benefits of adding a bMFGM ingredient (in particular MFGM-10) or its components in infant formula [9,10,11,13,14,48] or complementary food for infants and young children [16] have been previously demonstrated. Comparable modifications in concentrations of protein [49], iron [50], and ARA [38] have also been tested separately and shown to support adequate growth and be well tolerated. We also recently demonstrated an accelerated neurodevelopmental profile by 12 months of age in infants receiving the same added bMFGM ingredient and bovine lactoferrin in infant formula at concentrations similar to human milk through 12 months of age [12]. The current study is the first large pediatric nutrition trial designed to evaluate an added bMFGM ingredient (MFGM-10) in addition to modified iron, protein, and ARA concentrations.
In the current study, acceptance and tolerance of study formulas were good. No differences in overall study discontinuation or study discontinuation due to study formula were detected. No significant group differences were detected in fussiness, gassiness, or mean stool frequency at any measured time point. Few group differences from day 30 to 365 were reported for stool consistency. In one previous clinical study of infants receiving formula with added bMFGM, post-hoc analysis suggested that eczema incidence was low, but increased in the group receiving added bMFGM in formula [9]. In the present trial, there were no significant group differences in eczema incidence or the overall incidence of AEs within the skin system. Previous studies using the same source of bMFGM (MFGM-10) in formula demonstrated no association between bMFGM and increased risk of eczema through 6 or 18 months of age [11,12]. Overall, the present study adds to the growing body of evidence that the addition of MFGM-10 to formulas for infants is well-tolerated.
In the current study, adequate growth exhibited by infants receiving the investigational formula demonstrates nutritional suitability of a formula protein blend at a protein concentration (1.9 g/100 kcal) that approaches the minimum identified in expert recommendations and regulatory reference ranges [17,18,39,40,41,42], and includes added MFGM-10. Previous studies have demonstrated formula protein blends with added bMFGM and similar total protein concentration (~1.9–2.0 g/100 kcal) support adequate growth in infants [9,11]. In infants receiving MFGM-10, a different lipid-rich bMFGM ingredient, or standard formula (no added bMFGM) with similar total protein, no significant group differences in weight gain or growth were demonstrated and weight Z-scores indicated normal growth [9]. Growth was also similar in infants receiving formulas that had added MFGM-10 and adapted energy (60 kcal/100 mL) vs. a control with no added bMFGM and higher energy (66 kcal/100 mL), although mean formula intake was higher for the former group [11]. Demonstration of adequate growth in the present study is evidence of adequate protein quality of formula that has a blend of bovine-derived protein (including added MFGM-10) at 1.9 g/100 kcal.
Recent expert recommendations have supported the conclusion that lower iron content is sufficient to support healthy growth and iron fortification at 1.2 mg/100 kcal, which complies with regulatory provisions [39,40,41,51] and aligns with expert recommendations applicable to formulas designed for infants 0–12 months of age [18,28,29,52,53,54]. In the current study, no significant group differences in Hb or Hct at day 365 were detected, and the majority of participants fell within laboratory reference ranges. Consistent with higher dietary iron intake, serum ferritin was significantly higher in participants receiving the control versus investigational formula, but fell within or above laboratory reference ranges in all but six study participants: three (control) had been considered iron deficient and received medically prescribed iron supplements; three (control, 1; INV-MFGM, 2) were classified iron deficient based on laboratory references but iron sufficient based on 2010 AAP cutoffs. In addition, there were no group differences in physiologically relevant outcomes (including anemia, iron deficiency).
A key strength of this study included the randomized, double-blind, controlled design. In addition, because no single measure can characterize iron status, a combination of blood markers recommended by the AAP and commonly used in clinical practice was assessed. In addition, the day 365 study timepoint when iron status was evaluated corresponded with the AAP recommendation for universal screening for iron deficiency at 12 months of age [27]. Although the current study did not include a reference group of infants exclusively receiving human milk for comparison, in accordance with AAP guidance [43], the investigational formula was compared to a previously marketed formula demonstrated to support adequate growth in infants. In addition, WHO reference standards, which are representative of typical growth in breastfed infants, were used to plot current growth data. Finally, although study formula intake was recorded in the current study through 365 days of age, participants were exclusively receiving study feeding through 120 days of age only. After this age, infants may begin complementary feeding, which will also impact growth and iron status. Collecting complementary feeding in addition to study formula intake could provide a more complete dietary recall throughout the first year of life and may be warranted in future infant nutrition studies.
5. Conclusions
Overall, intact cow’s milk protein infant formula with the addition of a bMFGM ingredient and iron at 1.2 mg/100 kcal, protein at 1.9 g/100 kcal, and ARA at 25 mg/100 kcal was well-tolerated and associated with age-appropriate growth throughout the first year of life. Blood markers and physiological outcomes associated with iron status were within normal ranges in infants receiving added bMFGM in formula and modified protein, iron, and ARA. Consequently, this study demonstrated that added bMFGM and modified protein, iron, and ARA concentration in a routine cow’s milk infant formula were safe, well-tolerated, and associated with adequate growth throughout the first year of life and supported normal iron status at one year of age.
Author Contributions
Conceptualization, S.S.W.; study design, S.S.W., C.L.H., H.E.L., A.C.P. and J.L.W.; data collection, J.H. and M.Y.; formal analysis, C.L.H.; writing—original draft preparation, J.L.W.; writing—review and editing, S.S.W., J.H., M.Y., C.L.H., H.E.L., A.C.P. and J.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the study sponsor, Reckitt|MJN, Evansville, IN.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki (including October 1996 amendment). The research protocol (protocol #3387-1) and informed consent forms were approved by Schulman Associates Institutional Review Board (Research Triangle Park, NC) (date of approval: 04-29-2015, IRB #201501268). The study complied with good clinical practices.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors and study sponsor encourage and support the responsible and ethical sharing of data from clinical trials. De-identified participant data from the final research dataset used in the published manuscript may only be shared under the terms of a Data Use Agreement. Requests may be directed to: steven.wu2@rb.com.
Acknowledgments
We thank Suzanne Stolz (previously employed by Reckitt|Mead Johnson Nutrition Institute (MJNI)) for study management.
Conflicts of Interest
The authors J.H. and M.Y. were provided funding in order to independently enroll study participants. C.L.H., E.L., J.L.W., A.C.P. and S.S.W. are employees of Reckitt|MJNI and had a role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
American Academy of Pediatrics, AAP; arachidonic acid, ARA; C-reactive protein, CRP; docosahexaenoic acid, DHA; hematocrit, Hct; hemoglobin, Hb; long chain polyunsaturated fatty acids, LCPUFAs; milk fat globule membrane, MFGM; serum ferritin, SF; World Health Organization, WHO.
References
- Heid, H.W.; Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Briard-Bion, V.; Menard, O.; Rousseau, F.; Pradel, P.; Besle, J.M. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. J. Agric. Food Chem. 2008, 56, 5226–5236. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Gragson, D.; Cabral, C.; Jimenez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, C.; Menard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [Green Version]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Cichello, F.; Ragonese, C.; Donato, P.; Cacciola, F.; Dugo, P.; Mondello, L. Profiling and quantifying polar lipids in milk by hydrophilic interaction liquid chromatography coupled with evaporative light-scattering and mass spectrometry detection. Anal. Bioanal. Chem. 2013, 405, 4617–4626. [Google Scholar] [CrossRef]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clin. Med. Insights Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Timby, N.; Hernell, O.; Vaarala, O.; Melin, M.; Lönnerdal, B.; Domellöf, M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 384–389. [Google Scholar] [CrossRef]
- Timby, N.; Domellof, E.; Hernell, O.; Lonnerdal, B.; Domellof, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Stenlund, H.; Lönnerdal, B.; Hernell, O. Feeding infants formula with probiotics or milk fat globule membrane: A double-blind, randomized controlled trial. Front. Pediatr. 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lonnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabulsi, J.; Capeding, R.; Lebumfacil, J.; Ramanujam, K.; Feng, P.; McSweeney, S.; Harris, B.; DeRusso, P. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur. J. Clin. Nutr. 2011, 65, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Totzauer, M.; Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Verduci, E.; ReDionigi, A.; Hoyos, J.; Langhendries, J.P.; Gruszfeld, D.; Socha, P.; et al. Effect of lower versus higher protein content in infant formula through the first year on body composition from 1 to 6 Years: Follow-up of a randomized clinical trial. Obesity 2018, 26, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Yan, J.; Bylsma, L.C.; Northington, R.S.; Grathwohl, D.; Steenhout, P.; Erdmann, P.; Spivey-Krobath, E.; Haschke, F. Growth of infants consuming whey-predominant term infant formulas with a protein content of 1.8 g/100 kcal: A multicenter pooled analysis of individual participant data. Am. J. Clin. Nutr. 2016, 104, 1083–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkeson, P.M.K.; Axelsson, I.E.M.; Räihä, N.C.R. Growth and nutrient intake in three- to twelve-month-old infants fed human milk or formulas with varying protein concentrations. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 151153. [Google Scholar] [CrossRef]
- Kouwenhoven, S.M.P.; Antl, N.; Finken, M.J.J.; Twisk, J.W.R.; van der Beek, E.M.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; Schierbeek, H.; Holdt, L.M.; van Goudoever, J.B.; et al. A modified low-protein infant formula supports adequate growth in healthy, term infants: A randomized, double-blind, equivalence trial. Am. J. Clin. Nutr. 2020, 111, 962–974. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes for vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001; p. 800. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.D.; Greer, F.R. Clinical Report—Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiten, D.J.; Talbot, J.M.; Waters, J.H. LSRO Report: Assessment of nutrient requirements for infant formulas. J. Nutr. 1998, 128, 2059S–2237S. [Google Scholar]
- Hernell, O.; Fewtrell, M.S.; Georgieff, M.K.; Krebs, N.F.; Lonnerdal, B. Summary of current recommendations on iron provision and monitoring of iron status for breastfed and formula-fed infants in resource-rich and resource-constrained countries. J. Pediatr. 2015, 167, S40–S47. [Google Scholar] [CrossRef]
- Birch, E.E.; Hoffman, D.R.; Uauy, R.; Birch, D.G.; Prestidge, C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr. Res. 1998, 44, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef]
- Birch, E.E.; Hoffman, D.R.; Castañeda, Y.S.; Fawcett, S.L.; Birch, D.G.; Uauy, R.D. A randomized controlled trial of long-chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. Am. J. Clin. Nutr. 2002, 75, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, D.R.; Birch, E.E.; Castañeda, Y.S.; Fawcett, S.L.; Wheaton, D.H.; Birch, D.G.; Uauy, R. Visual function in breast-fed term infants weaned to formula with or without long-chain polyunsaturates at 4 to 6 months: A randomized clinical trial. J. Pediatr. 2003, 142, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Castaneda, Y.S.; Wheaton, D.H.; Birch, D.G.; Uauy, R.D.; Hoffman, D.R. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am. J. Clin. Nutr. 2005, 81, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Johnston, W.H.; Ashley, C.; Yeiser, M.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Wittke, A.; Cooper, T.R. Growth and tolerance of formula with lactoferrin in infants through one year of age: Double-blind, randomized, controlled trial. BMC Pediatr. 2015, 15, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, C.; Johnston, W.H.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Berseth, C.L. Growth and tolerance of infants fed formula supplemented with polydextrose (PDX) and/or galactooligosaccharides (GOS): Double-blind, randomized, controlled trial. Nutr. J. 2012, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, D.R.; Harris, C.L.; Wampler, J.L.; Patterson, A.C.; Berseth, C.L. Growth, tolerance, and DHA and ARA status of healthy term infants receiving formula with two different ARA concentrations: Double-blind, randomized, controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2019, 146, 19–27. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. Title 21 Food and Drugs §106 Infant Formula Requirements Pertaining to Current Good Manufacturing Practice, Quality Control Procedures, Quality Factors, Recordsand Reports, and Notifications; US Congress: Silver Spring, MD, USA, 2014. Available online: https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-B/part-106 (accessed on 12 December 2021).
- US Food and Drug Administration. Title 21 Food and Drugs §107 Infant Formula: General Provisions, Labeling, Exempt Infant Formulas, Nutrient Requirements, and Infant Formula Recalls; US Congress: Silver Spring, MD, USA, 1985. Available online: https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-B/part-107 (accessed on 12 December 2021).
- Food and Agriculture Organization of the United Nations. World Health Organization. CXS 72-1981 Standard for infant formula and formulas for special medical purposes intended for infants. In Codex Alimentarius International Food Standards; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2007; pp. 1–18. [Google Scholar]
- European Union Commission. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No. 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. O. J. 2016, 59, 1–29. [Google Scholar]
- AAP Task Force on Clinical Testing of Infant Formulas, Committee on Nutrition (Finberg, L.; Bell, E.F.; Cooke, R.J.; Fomon, S.J.; Kleinman, R.E.; Pencharz, P.B.; Reynolds, J.W.; Schanler, R.J.; Forbes, A.L.). Report: Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants; U.S. Food and Drug Administration: Silver Spring, MD, USA; Center for Food Safety and Applied Nutrition: College Park, MD, USA, 1988; pp. 1–16. Available online: https://wayback.archive-it.org/7993/20170722090324/https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm170649.htm (accessed on 12 December 2021).
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jimenez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) and Centers for Medicare & Medicaid Services (CMS), US Dept of Health and Human Services (HHS). Medicare, Medicaid and CLIA Programs; Regulations Implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Fed. Regist. 1992, 57, 7002–7186. [Google Scholar]
- Centers for Medicare & Medicaid Services. How to Obtain a CLIA Certificate; Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2019. [Google Scholar]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; pp. 1–312. [Google Scholar]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Chen, C.L. Effects of formula protein level and ratio on infant growth, plasma amino acids and serum trace elements. I. Cow’s milk formula. Acta Paediatr. Scand. 1990, 79, 257–265. [Google Scholar] [CrossRef]
- Bradley, C.K.; Hillman, L.; Sherman, A.R.; Leedy, D.; Cordano, A. Evaluation of two iron-fortified, milk-based formulas during infancy. Pediatrics 1993, 91, 908–914. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Infant formulae and follow-on formulae and amending Directive 1999/21/EC. 2006. Regulation (EC) No 2006/141. O. J. 2006, 49, 1–33. [Google Scholar]
- Committee on Nutrition. Iron fortification of infant formulas. Pediatrics 1999, 104, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Food. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-On Formulae (Adopted on 4 April 2003); European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Koletzko, B.; Bhutta, Z.A.; Cai, W.; Cruchet, S.; El Guindi, M.; Fuchs, G.J.; Goddard, E.A.; van Goudoever, J.B.; Quak, S.H.; Kulkarni, B.; et al. Compositional requirements of follow-up formula for use in infancy: Recommendations of an international expert group coordinated by the Early Nutrition Academy. Ann. Nutr. Metab. 2013, 62, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).