Fermentable Oligo-, Di-, and Mono-Saccharides and Polyols (FODMAPs) Consumption and Irritable Bowel Syndrome in the French NutriNet-Santé Cohort
Abstract
1. Introduction
2. Materials and Methods
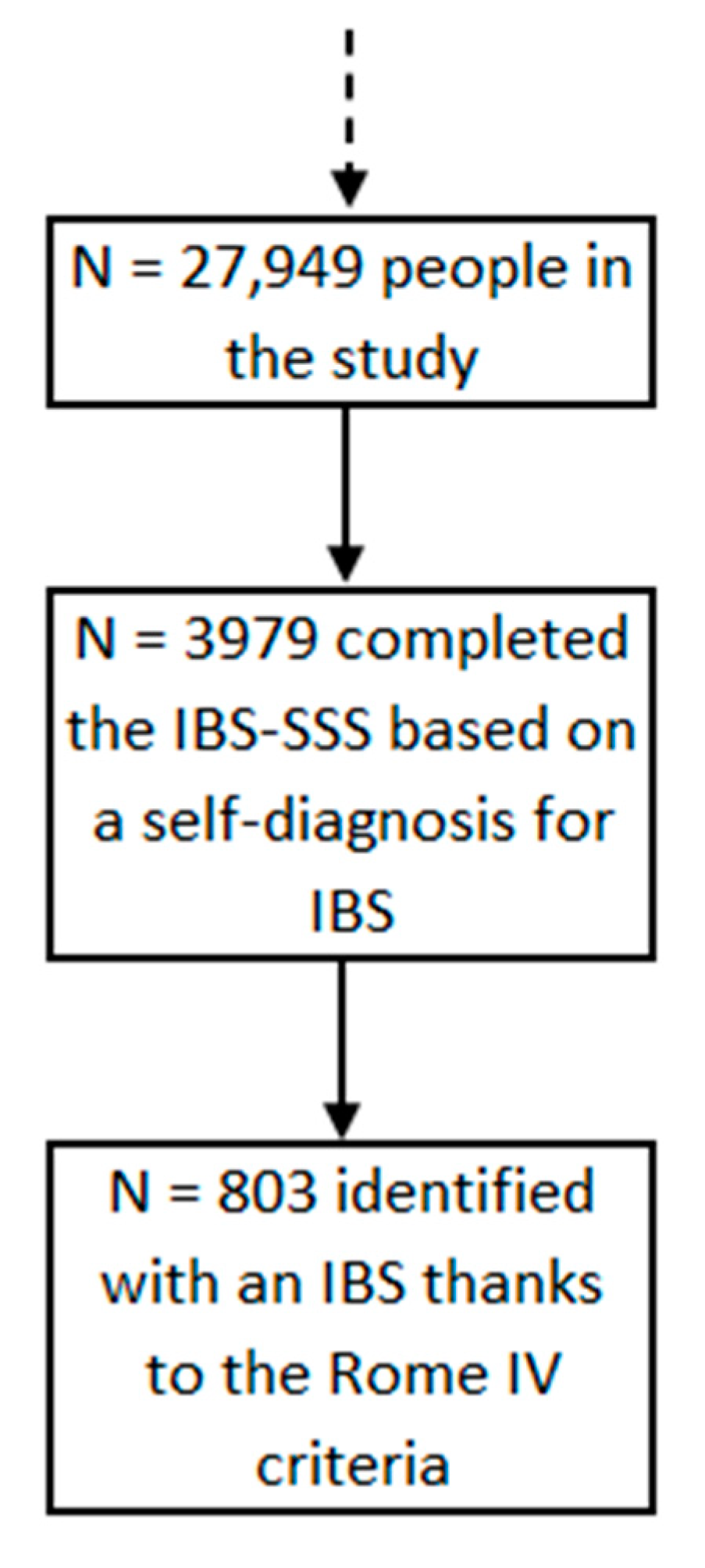
2.1. Population
2.2. Ethics
2.3. Data Collection
2.3.1. Irritable Bowel Syndrome
2.3.2. Diet
2.3.3. IBS Scoring System
2.3.4. Covariates
2.4. Statistical Analyses
3. Results
3.1. Population
3.2. Dietary Patterns and Proportion of IBS Cases in the Study Population
3.3. Association between FODMAP Consumption and IBS
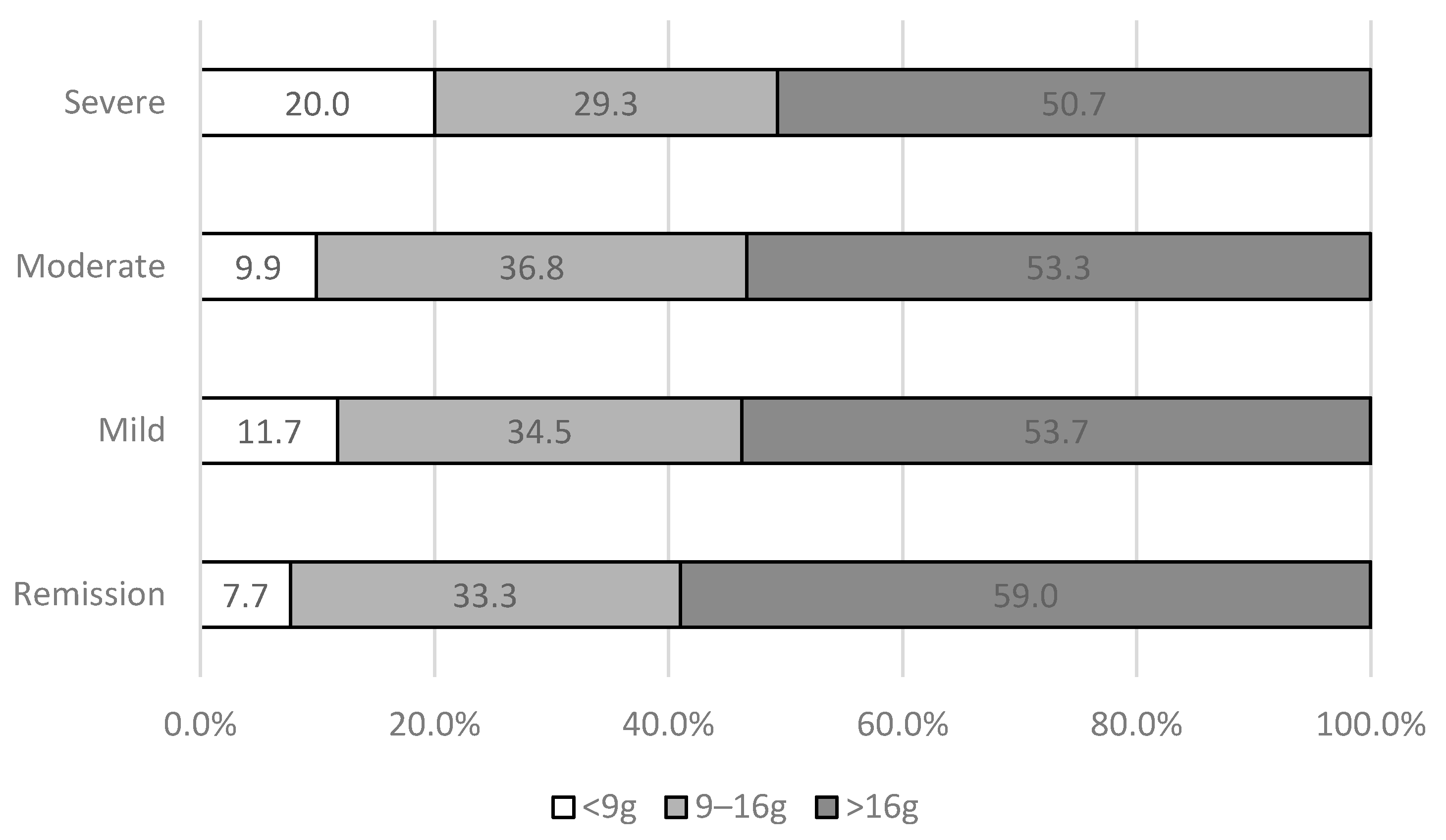
3.4. FODMAP Consumption and IBS Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, P.R. Food Intolerance in Functional Bowel Disorders: Food Intolerance. J. Gastroenterol. Hepatol. 2011, 26, 128–131. [Google Scholar] [CrossRef]
- Rumessen, J.J. Fructose and Related Food Carbohydrates. Sources, Intake, Absorption, and Clinical Implications. Scand. J. Gastroenterol. 1992, 27, 819–828. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary Triggers of Abdominal Symptoms in Patients with Irritable Bowel Syndrome: Randomized Placebo-Controlled Evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-Based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Hungin, A.P.S.; Whorwell, P.J.; Tack, J.; Mearin, F. The Prevalence, Patterns and Impact of Irritable Bowel Syndrome: An International Survey of 40,000 Subjects. Aliment. Pharmacol. Ther. 2003, 17, 643–650. [Google Scholar] [CrossRef]
- Corazziari, E. Definition and Epidemiology of Functional Gastrointestinal Disorders. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Dapoigny, M.; Bellanger, J.; Bonaz, B.; Bruley des Varannes, S.; Bueno, L.; Coffin, B.; Ducrotté, P.; Flourié, B.; Lémann, M.; Lepicard, A.; et al. Irritable Bowel Syndrome in France: A Common, Debilitating and Costly Disorder. Eur. J. Gastroenterol. Hepatol. 2004, 16, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Functional Gastrointestinal Disorders as a Public Health Problem. Neurogastroenterol. Motil. 2008, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K. The Low FODMAP Diet: Recent Advances in Understanding Its Mechanisms and Efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Lacy, B.E. The Science, Evidence, and Practice of Dietary Interventions in Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2015, 13, 1899–1906. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Eswaran, S. Low FODMAP in 2017: Lessons Learned from Clinical Trials and Mechanistic Studies. Neurogastroenterol. Motil. 2017, 29, e13055. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gibson, P.R. Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols (FODMAPs) and Nonallergic Food Intolerance: FODMAPs or Food Chemicals? Ther. Adv. Gastroenterol. 2012, 5, 261–268. [Google Scholar] [CrossRef]
- Ahmad, O.F.; Akbar, A. Dietary Treatment of Irritable Bowel Syndrome. Br. Med. Bull. 2015, 113, 83–90. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; Palma, G.D.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients. 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, L.R.; Lyngesen, M.; Bytzer, P. Systematic Review: Quality of Trials on the Symptomatic Effects of the Low FODMAP Diet for Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2017, 45, 1506–1513. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. Mechanisms and Efficacy of Dietary FODMAP Restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 256–266. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a Diet Low in FODMAPs Reduce Symptoms Associated with Functional Gastrointestinal Disorders? A Comprehensive Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Hercberg, S.; Castetbon, K.; Czernichow, S.; Malon, A.; Mejean, C.; Kesse, E.; Touvier, M.; Galan, P. The Nutrinet-Santé Study: A Web-Based Prospective Study on the Relationship between Nutrition and Health and Determinants of Dietary Patterns and Nutritional Status. BMC Public Health 2010, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Hercberg, S.; Deheeger, M.; Preziosi, P.; Suvimax. Portions Alimentaires: Manuel Photos pour L’Estimation des Quantités—Su.Vi.Max.; Polytechnica: Paris, France, 2012. [Google Scholar]
- Etude NutriNet-Santé. Table de Composition des Aliments; Economica: Paris, France, 2013; ISBN 978-2-7178-6537-0. [Google Scholar]
- Touvier, M.; Kesse-Guyot, E.; Méjean, C.; Pollet, C.; Malon, A.; Castetbon, K.; Hercberg, S. Comparison between an Interactive Web-Based Self-Administered 24 h Dietary Record and an Interview by a Dietitian for Large-Scale Epidemiological Studies. Br. J. Nutr. 2011, 105, 1055–1064. [Google Scholar] [CrossRef]
- Lassale, C.; Castetbon, K.; Laporte, F.; Camilleri, G.M.; Deschamps, V.; Vernay, M.; Faure, P.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Validation of a Web-Based, Self-Administered, Non-Consecutive-Day Dietary Record Tool against Urinary Biomarkers. Br. J. Nutr. 2015, 113, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Castetbon, K.; Laporte, F.; Deschamps, V.; Vernay, M.; Camilleri, G.M.; Faure, P.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Correlations between Fruit, Vegetables, Fish, Vitamins, and Fatty Acids Estimated by Web-Based Nonconsecutive Dietary Records and Respective Biomarkers of Nutritional Status. J. Acad. Nutr. Diet. 2016, 116, 427–438.e5. [Google Scholar] [CrossRef]
- Schneider, E.; Sabate, J.-M.; Bouchoucha, M.; Debras, C.; Touvier, M.; Hercberg, S.; Benamouzig, R.; Buscail, C.; Julia, C. FODMAP Consumption by Adults from the French Population-Based NutriNet-Santé Cohort. J. Nutr. 2021, 151, 3180–3186. [Google Scholar] [CrossRef]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of Dietary Short Chain Carbohydrates Alters the Pattern of Gas Production and Genesis of Symptoms in Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2019, 120, 535–547. [Google Scholar] [CrossRef]
- Black, A.E. Critical Evaluation of Energy Intake Using the Goldberg Cut-off for Energy Intake:Basal Metabolic Rate. A Practical Guide to Its Calculation, Use and Limitations. Int. J. Obes. 2000, 24, 1119. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The Irritable Bowel Severity Scoring System: A Simple Method of Monitoring Irritable Bowel Syndrome and Its Progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- The National Institute of Statistics and Economic Studies Définition—Unité de Consommation|Insee. Available online: https://www.insee.fr/fr/metadonnees/definition/c1802 (accessed on 21 May 2019).
- Vergnaud, A.-C.; Touvier, M.; Méjean, C.; Kesse-Guyot, E.; Pollet, C.; Malon, A.; Castetbon, K.; Hercberg, S. Agreement between Web-Based and Paper Versions of a Socio-Demographic Questionnaire in the NutriNet-Santé Study. Int. J. Public Health 2011, 56, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Méjean, C.; Kesse-Guyot, E.; Pollet, C.; Malon, A.; Castetbon, K.; Hercberg, S. Comparison between Web-Based and Paper Versions of a Self-Administered Anthropometric Questionnaire. Eur. J. Epidemiol. 2010, 25, 287–296. [Google Scholar] [CrossRef]
- Lassale, C.; Péneau, S.; Touvier, M.; Julia, C.; Galan, P.; Hercberg, S.; Kesse-Guyot, E. Validity of Web-Based Self-Reported Weight and Height: Results of the Nutrinet-Santé Study. J. Med. Internet Res. 2013, 15, e152. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Hercberg, S.; Benamouzig, R.; Julia, C. Western Dietary Pattern Is Associated with Irritable Bowel Syndrome in the French NutriNet Cohort. Nutrients 2017, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Allès, B.; Péneau, S.; Kesse-Guyot, E.; Baudry, J.; Hercberg, S.; Méjean, C. Food Choice Motives Including Sustainability during Purchasing Are Associated with a Healthy Dietary Pattern in French Adults. Nutr. J. 2017, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Analytics, Business Intelligence and Data Management. Available online: https://www.sas.com/en_us/home.html (accessed on 24 May 2019).
- Schafer, J.L.; Olsen, M.K. Multiple Imputation for Multivariate Missing-Data Problems: A Data Analyst’s Perspective. Multivar. Behav. Res. 1998, 33, 545–571. [Google Scholar] [CrossRef]
- Su, Y.-S.; Gelman, A.; Hill, J.; Yajima, M. Multiple Imputation with Diagnostics (Mi) in R: Opening Windows into the Black Box. J. Stat. Softw. 2011, 45, 1–31. [Google Scholar] [CrossRef]
- Schnabel, L.; Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R.; et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results from the French NutriNet-Santé Cohort. Am. J. Gastroenterol. 2018, 113, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Yiannakou, Y.; Houghton, L.A.; Ford, A.C. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-Reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin. Gastroenterol. Hepatol. 2019, 18, 392–398. [Google Scholar] [CrossRef]
- Van den Houte, K.; Carbone, F.; Pannemans, J.; Corsetti, M.; Fischler, B.; Piessevaux, H.; Tack, J. Prevalence and Impact of Self-Reported Irritable Bowel Symptoms in the General Population. United Eur. Gastroenterol. J. 2019, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef]
- Liljebo, T.; Störsrud, S.; Andreasson, A. Presence of Fermentable Oligo-, Di-, Monosaccharides, and Polyols (FODMAPs) in Commonly Eaten Foods: Extension of a Database to Indicate Dietary FODMAP Content and Calculation of Intake in the General Population from Food Diary Data. BMC Nutr. 2020, 6, 47. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate Modelling of Faecal Bacterial Profiles of Patients with IBS Predicts Responsiveness to a Diet Low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133.e2. [Google Scholar] [CrossRef]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Role of FODMAPs in Patients with Irritable Bowel Syndrome. Nutr. Clin. Pract. 2015, 30, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived Food Intolerance in Subjects with Irritable Bowel Syndrome—Etiology, Prevalence and Consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Halpert, A.; Dalton, C.B.; Palsson, O.; Morris, C.; Hu, Y.; Bangdiwala, S.; Hankins, J.; Norton, N.; Drossman, D. What Patients Know about Irritable Bowel Syndrome (IBS) and What They Would like to Know. National Survey on Patient Educational Needs in IBS and Development and Validation of the Patient Educational Needs Questionnaire (PEQ). Am. J. Gastroenterol. 2007, 102, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Ferch, C.; Shaw, M.; Chey, W.D. Use of Dietary Management in Irritable Bowel Syndrome: Results of a Survey of Over 1500 United States Gastroenterologists. J. Neurogastroenterol. Motil. 2018, 24, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Varney, J.; Malakar, S.; Muir, J.G. Food Components and Irritable Bowel Syndrome. Gastroenterology 2015, 148, 1158–1174.e4. [Google Scholar] [CrossRef]
- Halpert, A.; Dalton, C.B.; Palsson, O.; Morris, C.; Hu, Y.; Bangdiwala, S.; Hankins, J.; Norton, N.; Drossman, D.A. Patient Educational Media Preferences for Information about Irritable Bowel Syndrome (IBS). Dig. Dis. Sci. 2008, 53, 3184–3190. [Google Scholar] [CrossRef]
- Melchior, C.; Fremaux, S.; Jouët, P.; Macaigne, G.; Raynaud, J.-J.; Facon, S.; Iglicki, F.; Taes, Y.; Sabate, J.-M. Perceived Gastrointestinal Symptoms and Association with Meals in a French Cohort of Patients With Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2021, 27, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Duboc, H.; Dior, M.; Coffin, B. Le syndrome de l’intestin irritable: Nouvelles pistes physiopathologiques et conséquences pratiques. La Revue de Médecine Interne 2016, 37, 536–543. [Google Scholar] [CrossRef]
- Austin, G.L.; Dalton, C.B.; Hu, Y.; Morris, C.B.; Hankins, J.; Weinland, S.R.; Westman, E.C.; Yancy, W.S.; Drossman, D.A. A Very Low-Carbohydrate Diet Improves Symptoms and Quality of Life in Diarrhea-Predominant Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2009, 7, 706–708.e1. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential Effects of FODMAPs (Fermentable Oligo-, Di-, Mono-Saccharides and Polyols) on Small and Large Intestinal Contents in Healthy Subjects Shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef]
- Gibson, P.R.; Varney, J.E.; Muir, J.G. Diet Therapy for Irritable Bowel Syndrome: Is a Diet Low in FODMAPS Really Similar in Efficacy to Traditional Dietary Advice? Gastroenterology 2016, 150, 1046–1047. [Google Scholar] [CrossRef][Green Version]
- BMJ Publishing Group Does a Low FODMAP Diet Help IBS? DTB 2015, 53, 93–96. [CrossRef][Green Version]
- Camilleri, M.; Acosta, A. Re: Halmos et al, A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Braverman, D.; Stankiewicz, H. Carbohydrate Malabsorption and the Effect of Dietary Restriction on Symptoms of Irritable Bowel Syndrome and Functional Bowel Complaints. Isr. Med. Assoc. J. 2000, 2, 583–587. [Google Scholar]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Öhman, L.; Simrén, M. New Insights into the Pathogenesis and Pathophysiology of Irritable Bowel Syndrome. Dig. Liver Dis. 2007, 39, 201–215. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.L.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.C.; et al. Development and Validation of the Rome IV Diagnostic Questionnaire for Adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef]
- Vork, L.; Weerts, Z.Z.R.M.; Mujagic, Z.; Kruimel, J.W.; Hesselink, M.A.M.; Muris, J.W.M.; Keszthelyi, D.; Jonkers, D.M.A.E.; Masclee, A.A.M. Rome III vs Rome IV Criteria for Irritable Bowel Syndrome: A Comparison of Clinical Characteristics in a Large Cohort Study. Neurogastroenterol. Motil. 2018, 30, e13189. [Google Scholar] [CrossRef]
- Bai, T.; Xia, J.; Jiang, Y.; Cao, H.; Zhao, Y.; Zhang, L.; Wang, H.; Song, J.; Hou, X. Comparison of the Rome IV and Rome III Criteria for IBS Diagnosis: A Cross-Sectional Survey. J. Gastroenterol. Hepatol. 2017, 32, 1018–1025. [Google Scholar] [CrossRef]
- Aziz, I.; Törnblom, H.; Palsson, O.S.; Whitehead, W.E.; Simrén, M. How the Change in IBS Criteria from Rome III to Rome IV Impacts on Clinical Characteristics and Key Pathophysiological Factors. Am. J. Gastroenterol. 2018, 113, 1017–1025. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of Participants | All | IBS (Yes) | IBS (No) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| N = 27,949 | N = 1295 (4.63%) | N = 26,654 (95.37%) | ||||||
| n | % | n | % | n | % | |||
| Age (years) | mean (SD) | 47.8 | (14.1) | 43.4 | (14.5) | 48.0 | (14.1) | <0.0001 |
| Energy (kcal) | mean (SD) | 1903 | (495) | 1859 | (488) | 1905 | (496) | <0.0001 |
| Sex | Men | 6871 | 24.6 | 149 | 11.5 | 6722 | 25.2 | <0.0001 |
| Women | 21,078 | 75.4 | 1146 | 88.5 | 19,932 | 74.8 | ||
| Marital status | Single/divorced/widowed | 7565 | 27.1 | 412 | 31.9 | 7153 | 26.9 | <0.0001 |
| Married/cohabiting | 20,327 | 72.7 | 881 | 68.1 | 19,446 | 73.1 | ||
| Educational level | No diploma or primary school | 712 | 2.57 | 28 | 2.18 | 684 | 2.59 | 0.26 |
| Secondary school | 8573 | 30.9 | 371 | 28.9 | 8202 | 31.0 | ||
| Undergraduate | 8372 | 30.2 | 407 | 31.7 | 7965 | 30.1 | ||
| Graduate | 10,059 | 36.3 | 479 | 37.3 | 9580 | 36.2 | ||
| Income per consumption unit (€/month) | Unwilling to answer | 2034 | 7.50 | 78 | 6.27 | 1956 | 7.56 | <0.0001 |
| <1110 | 3101 | 11.4 | 198 | 15.9 | 2903 | 11.2 | ||
| 1110–2330 | 17,932 | 66.2 | 821 | 65.9 | 17,111 | 66.2 | ||
| >2330 | 4040 | 14.9 | 148 | 11.9 | 3892 | 15.0 | ||
| Residence | Rural area | 6051 | 22.1 | 255 | 20.2 | 5796 | 22.2 | 0.10 |
| Urban area | 21,350 | 77.9 | 1007 | 79.8 | 20,343 | 77.8 | ||
| Smoking status | Current smoker | 3366 | 12.1 | 204 | 15.8 | 3162 | 11.9 | 0.0001 |
| Former | 10,070 | 36.1 | 437 | 33.8 | 9633 | 36.2 | ||
| Never | 14,458 | 51.8 | 652 | 50.4 | 13,806 | 51.9 | ||
| BMI (kg/m2) | <18.5 | 1300 | 4.66 | 90 | 6.96 | 1210 | 4.55 | <0.0001 |
| 18.5–25 | 17,932 | 64.3 | 784 | 60.6 | 17,148 | 64.4 | ||
| 25–30 | 6355 | 22.8 | 276 | 21.3 | 6079 | 22.8 | ||
| ≥30 | 2319 | 8.31 | 144 | 11.1 | 2175 | 8.17 | ||
| IPAQ | Intense | 8693 | 35.0 | 372 | 32.5 | 8321 | 35.1 | 0.16 |
| Moderate | 10,705 | 43.1 | 508 | 44.3 | 10,197 | 43.0 | ||
| Low | 5433 | 21.9 | 266 | 23.2 | 5167 | 21.8 | ||
| Global | IBS (Yes) | IBS (No) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N = 27,949 | N = 1295 (4.6%) | N = 26,654 (95.4%) | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Energy (kcal) | 1903 | 495 | 1859 | 488 | 1906 | 496 | <0.0001 |
| Total FODMAPs (g/day) | 19.4 | 9.5 | 18.4 | 9.6 | 19.5 | 9.5 | <0.0001 |
| Excess fructose | 4.62 | 3.92 | 4.18 | 3.96 | 4.64 | 3.91 | <0.0001 |
| Lactose | 10.3 | 7.5 | 10.1 | 7.3 | 10.3 | 7.5 | <0.0001 |
| GOS | 0.39 | 0.37 | 0.36 | 0.38 | 0.39 | 0.37 | <0.0001 |
| Fructans | 2.28 | 1.64 | 2.10 | 1.49 | 2.22 | 1.64 | <0.0001 |
| Polyols | 1.92 | 2.05 | 1.71 | 2.00 | 1.93 | 2.06 | <0.0001 |
| Continuous | Classes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤9 g | 9–16 g | >16 g | |||||||
| N = 2792 | N = 8865 | N = 16,292 | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | OR | 95% CI | p-Trend | ||
| IBS | |||||||||
| Model 1 | 0.88 | [0.83–0.94] | <0.0001 | Ref | 0.90 | [0.75–1.09] | 0.77 | [0.64–0.92] | 0.0005 |
| Model 2 | 0.92 | [0.86–0.98] | 0.0066 | Ref | 0.93 | [0.77–1.13] | 0.82 | [0.69–0.99] | 0.0110 |
| Model 3 | 0.88 | [0.82–0.95] | 0.0011 | Ref | 0.99 | [0.80–1.22] | 0.82 | [0.67–1.01] | 0.0086 |
| IBS-SSS | n | % | |
|---|---|---|---|
| Remission | 0–75 | 78 | 9.7 |
| Mild | 75–175 | 281 | 35.0 |
| Moderate | 175–300 | 304 | 37.9 |
| Severe | >300 | 140 | 17.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, E.; Sabaté, J.-M.; Bouchoucha, M.; Hercberg, S.; Touvier, M.; Benamouzig, R.; Julia, C.; Buscail, C. Fermentable Oligo-, Di-, and Mono-Saccharides and Polyols (FODMAPs) Consumption and Irritable Bowel Syndrome in the French NutriNet-Santé Cohort. Nutrients 2021, 13, 4513. https://doi.org/10.3390/nu13124513
Schneider E, Sabaté J-M, Bouchoucha M, Hercberg S, Touvier M, Benamouzig R, Julia C, Buscail C. Fermentable Oligo-, Di-, and Mono-Saccharides and Polyols (FODMAPs) Consumption and Irritable Bowel Syndrome in the French NutriNet-Santé Cohort. Nutrients. 2021; 13(12):4513. https://doi.org/10.3390/nu13124513
Chicago/Turabian StyleSchneider, Elodie, Jean-Marc Sabaté, Michel Bouchoucha, Serge Hercberg, Mathilde Touvier, Robert Benamouzig, Chantal Julia, and Camille Buscail. 2021. "Fermentable Oligo-, Di-, and Mono-Saccharides and Polyols (FODMAPs) Consumption and Irritable Bowel Syndrome in the French NutriNet-Santé Cohort" Nutrients 13, no. 12: 4513. https://doi.org/10.3390/nu13124513
APA StyleSchneider, E., Sabaté, J.-M., Bouchoucha, M., Hercberg, S., Touvier, M., Benamouzig, R., Julia, C., & Buscail, C. (2021). Fermentable Oligo-, Di-, and Mono-Saccharides and Polyols (FODMAPs) Consumption and Irritable Bowel Syndrome in the French NutriNet-Santé Cohort. Nutrients, 13(12), 4513. https://doi.org/10.3390/nu13124513







