Abstract
Insulin resistance (IR) is a hallmark of type 2 diabetes mellitus (T2DM). This study was performed to investigate the antidiabetic effect of Bacillus toyonensis SAU-19 and its possible mechanisms of action in mice with type 2 diabetes mellitus (T2DM). Thirty SPFKM mice were randomly assigned to three groups: control, diabetic model, and diabetes + Bacillus toyonensis SAU-19 group. After 35 days, blood was collected for biochemical analysis and liver tissue samples for histopathological analysis using H&E staining, qPCR, and ELISA. The results showed that the administration of B. toyonensis SAU-19 significantly improved the blood glucose, hepatic insulin resistance, and morphological changes of the liver characterized by significant improvement of dyslipidemia, glycogen synthesis, and antioxidant status (p < 0.05), indicating the strains’ ameliorating effects on hepatic insulin resistance in T2DM. In conclusion, the probiotic strain (B. toyonensis SAU-19) inhibits T2DM by reducing insulin resistance, improving antioxidant status, and downregulating genes related to glucose synthesis; hence, it may be used in treating diabetes and other metabolic disorders. This study provides the basis for further studies into the molecular mechanisms of B. toyonensis SAU-19 in treating T2DM.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic syndrome, chiefly associated with chronic hyperglycemia as a result of insulin resistance and inadequate insulin secretion [1]. T2DM is one of the major diseases that affect human health and life globally [2]. In the year 2014, it was estimated that about 422 million adults were suffering from diabetes [3]. Furthermore, in 2010, the. global frequency of adult diabetes was estimated as 285 million and was speculated to increase to about 439 million by 2030 [4]. The liver is the key organ involved in the preservation of glucose homeostasis as it controls the balance between gluconeogenesis and glycogen synthesis in the body [5]. Insulin resistance elevates gluconeogenesis and decrease glycogen synthesis in the liver, resulting in hyperglycemia [6]. The liver stores excessive lipids as fat droplets and the accumulation of these fat droplets may cause inflammation, insulin resistance, and diabetes [7]. Numerous studies have reported on the use of probiotics in treating diabetes and other metabolic diseases. In addition, since the effect and mechanisms of probiotics are species specific, there is a need to investigate specific probiotic strains and elucidate their efficiency for managing T2DM.
Bacillus toyonensis, a bacteria strain from the Bacillus cereus family, has been proven safe and is being used as a probiotic for many animals, such rabbits, pigs, chickens, and cattle [8,9]. Numerous studies have revealed the probiotic activities of this bacteria [10]. Bacillus toyonensis has been reported to improve feed conversion ratios and reduce post-weaning diarrhea and mortality in piglets [11] as well as reducing the activities of pathogenic bacteria in the gut [12]. However, studies on the probiotic bacteria’s antidiabetic properties have not been reported in the literature.
Therefore, in this study, we investigated the effect of the probiotic Bacillus toyonensis strain SAU-19, which was identified in our lab, on T2DM in mice. In our previous study, the Bacillus toyonensis strain SAU-19 isolated from Ageratina adenophora plant [13,14] showed tolerance to simulated gastrointestinal tract conditions, and improved the growth performance, antioxidant capacity, anti-inflammatory effects, and gut integrity in our animal experiment (unpublished), which provided a basis for the beneficial effects of Bacillus toyonensis SAU-19 in vivo. This study provides a theoretical basis for future use of the B. toyonensis strain SAU-19 in treating diabetes and other metabolic diseases.
2. Material and Methods
2.1. Sample Collection
Culture media were purchased from Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China and streptozotocin (STZ) was purchased from Solarbio solabao Beijing solabao Technology Co., Ltd., China. Mice, basal, and high-fat diet (Table 1) were purchased from the Chengdu Dashuo Experiment Animal Co., Ltd., Chengdu, China. Bacillus toyonensis SAU-19 (Accessory number: MW287198; Collection Preservation number: CCTCC NO: M 20211138) was obtained from the College of Veterinary Medicine (Professor Yanchun Hu’s lab), Sichuan Agricultural University, China.

Table 1.
Feed composition.
2.2. Preparation of Probiotic Bacteria Suspensions
B. toyonensis SAU-19 was transferred twice in LB broth and incubated anaerobically at 37 °C for 72 h. The bacterial cells were collected by centrifugation (3500× g, 5 min), and washed twice in 0.85% NaCl (Sigma), and then resuspended in 0.85% NaCl to a final concentration of 106 CFU/mL, and stored at 4 °C.
2.3. Experimental Animal and Design
Thirty male specific-pathogen-free Kun min mice (SPFKM) (5 weeks old; BW 25–30 g) were purchased from the Chengdu Dashuo Experiment Animal Co., Ltd., Chengdu, China. The animals were housed in an experimental animal house at Sichuan Agricultural University at a constant temperature (22 ± 2 °C) and humidity (65 ± 5%) under a 12-h light/12-h dark cycle with free access to food and water. This study was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University, Sichuan, China, under the permit number DKY-B2019603005.
The mice diabetes model was established by the administration of a high-fat diet (HFD) for 6 weeks and intraperitoneal injection of streptozotocin (STZ) solution (dissolved in a 0.01 M citrate buffer, pH 4.5 (Solarbio Science and Technology Co., Ltd., Beijing, China) at a dose of 35 mg/kg body weight for 3 consecutive days [15]. Then, 72 h after the injection, fasting blood glucose (FBG) was measured using a blood glucose meter (Bayer). The diabetes model was identified as successfully prepared in cases where random blood glucose level > 11.1 mmoL/L [1]. Mice injected with equivalent amounts of with equivalent amounts of precooling citrate buffer solution, pH 4.5 were used as controls (n = 10). The model mice were randomly divided into a diabetic group (DG) (n = 10) that was fed HFD + 1 mL 0.9% normal saline daily in drinking water and a diabetic + B. toyo SAU-19 group (DG + B. toyo SAU-19) (n = 10) that was fed HFD + 1 mL of 1 × 106 CFU mL−1 B. toyo SAU-19 in drinking water as well as a control group (C) (n = 10) fed a basal diet and 1 ml of 0.9% normal saline for 35 days. Feed and water intake was monitored and recorded daily throughout the experimental period. Feed and clean water were provided ad libitum. Water bottles were washed every week and fresh drinking water was placed in it for the next week’s administration. The bedding material (wood shavings) was also changed weekly. To administer the B. toyo SAU-19, new stocks were generated each week in LB, and their viability was monitored by serial dilution and viable cell count using LB agar, respectively.
2.4. Oral Glucose Tolerance Test
An oral glucose tolerance test (OGTT) was performed in the last week of B. toyo SAU-19 administration. Mice were fasted for 12 h and blood glucose was determined (time = 0 min). Then, mice were orally administered glucose (2 g kg−1 BW) and blood glucose levels were measured at 30, 60, 90, and 120 min.
2.5. Blood and Tissue Sample Collection
At the end of the experiment (week 12), mice were fasted for 12 h and anesthetized with sevoflurane. Blood samples were collected from the inferior vena cava and centrifuged at 4000× g for 10 min at 4 °C, and then the serum was collected and stored at −80 °C for further assays. Liver tissue samples were quickly removed, rinsed, and stored at −80 °C or fixed in 10% paraformaldehyde solution.
2.6. Biochemical Parameters
Liver glycogen and serum insulin were determined using ELISA kits (Jiangsu Jingmei biological Technology company limited, Jiangsu, China). Lipid profiles, including total cholesterol (TC), total triglyceride (TG), LDL-cholesterol (LDL-C), and HDL cholesterol (HDL-C), were determined by commercial kits (Jiangsu Jingmei biological Technology company limited, Jiangsu, China). Homeostatic model assessment of insulin resistance (HOMAIR), used to quantify insulin resistance, was calculated as: HOMA-IR = Fasting blood glucose (mmol L−1) × Fasting blood insulin (mU L−1)/22.5 [16]. The levels of glutathione (GSH), malondialdehyde (MDA), and superoxide dismutase (SOD) in mice serum and livers were also measured using commercial kits from Jiangsu Jingmei biological Technology company limited, Jiangsu, China.
2.7. Liver Histological Analysis
Livers fixed in 4% paraformaldehyde were embedded in paraffin and sectioned for 5 μm thick. Hematoxylinensin (H&E) staining was used for liver pathological evaluation. H&E staining kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). All kits were used according to the corresponding manufacturers’ instructions. Liver injury was numerically recorded following the method of Chen et al. [17].
2.8. Enzyme-Linked Immunosorbent Assay
Parts of the liver tissues were washed with PBS. Then, 0.1 g of the sample tissue was weighed and homogenized with 0.9 mL of ice-cold PBS in a glass homogenizer, and then the mixture was centrifuged (3000 rpm, 20 min) to obtain the supernatant. Furthermore, we determined the protein concentration in the supernatant using a Total Protein Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The supernatants were used to determine the concentrations of IL-1β, TNF-α, IL-4, and IL-10 using a commercial mice ELISA kit (Jiangsu Jingmei Biological Technology Co., Ltd., Jiangsu, China), respectively. The level of sensitivity of each kit was 0.1 pg/mL for each cytokine [18].
2.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Samples of liver tissues (30 mg/mouse) were snap-frozen with liquid N2 and then immediately ground into powder using a ceramic mortar. Total RNA from each sample was extracted using an Animal Total RNA Isolation Kit (Sagon Biotech, Shanghai, China) according to the manufacturer’s instructions. After confirming the isolated RNA concentration and purity using a NanoDrop One system (Thermo Fisher Scientific, Waltham, MA; OD260/280 ≈ 1.9–2.0), triplicate aliquots (each 1 µg) were removed, loaded into wells, and cDNA was prepared using a PrimeScrip RT reagent kit (Takara, Tokyo Japan). Thereafter, qRT-PCR was performed using a SYBR Premix ExTaq (Takara) and a CFX96 thermal cycler (BioRad, Hercules, CA, USA). The PCR conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C, 15 s for denaturation, 60 °C, 60 s for annealing at and 70 °C, 25 s for extension. Each qRT-PCR reaction was performed with volumes of 10 µL containing 5 µL of TB Green TM Premix (Takara), 1 µL of forward and reverse primers, 1 µL of cDNA, and 2 µL of DNase/RNase-Free Deionized Water (Tiangen, Beijing, China). The primers used to analyze the genes of interest were designed from NCBI genBank and are shown in Table 2. The relative gene expression in each sample was normalized to an internal control (β-actin); data analysis was performed using the 2−ΔΔCt method. All samples were evaluated in triplicate.

Table 2.
Primers used for the real-time PCR analysis.
2.10. Statistical Analysis
Statistical analysis of the data collected (from various independent experiments) was performed using GraphPad Prism 5.04 software (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 20 Statistical Analysis Software (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk Test was used to test the normality of the data. All experimental results are presented as mean ± SD, and statistical significance were determined by one-way analysis of variance (ANOVA) followed by the Tukey’s test. The values were significantly different at p < 0.05.
3. Results
3.1. Effects of B. toyonensis Strain SAU-19 on Growth Performance in HFD/STZ-Induced T2DM Mice
From the results, during the experimental trial, we observed a significant increase in feed and water intake in the DG group compared to the control (C) and B. toyo SAU-19 groups (Figure 1A,B, p < 0.05), typical of T2DM. However, there was no difference between the control (C) and B. toyo SAU-19 groups in feed and water intake (p > 0.05). Furthermore, we also observed a decrease in the weight gain in the DG group after 35 days compared to the control (C) and B. toyo SAU-19 groups even though feed intake was high in the DG group (Figure 1C, p < 0.05). No difference existed between the control (C) and B. toyo SAU-19 groups. Moreover, we observed a significant decrease in liver, kidney, and spleen weights of the DG group compared to the control (C) and B. toyo SAU-19 groups (Figure 1D, p < 0.05). The immune index scores for the DG group were significantly lower than that of the control (C) and B. toyo SAU-19 groups (Figure 1D, p < 0.05). There was no difference in the organ weights and immune index scores of the control (C) and B. toyo SAU-19 groups (p > 0.05). The immune index was calculated as: Immune index = spleen weight (g)/Body weight (g).
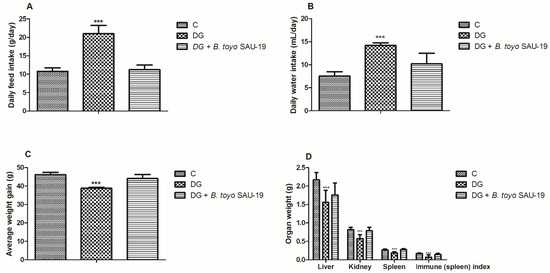
Figure 1.
Effects of Bacillus toyonensis SAU-19 on the growth performance in HFD/STZ-induced T2DM mice. (A) Daily feed intake (g/day). (B) Daily water intake (mL/day). (C) Average weight gain (g). (D) Organ weight (g) and immune (spleen) index. Values are shown as mean ± Sd. Bars with *** are statistically different (p < 0.05), DG compared with the control (C) and B. toyo SAU-19 groups (n = 8).
3.2. Effects of B. toyonensis Strain SAU-19 on Blood Glucose Levels and Oral Glucose Tolerance Test (OGTT) in HFD/STZ-Induced T2DM Mice
As shown in Figure 2A, the fasted blood glucose level of the DG group after the 35-day administration period was higher as compared to the control (C) and B. toyo SAU-19 groups (p < 0.05). However, there was no difference in the fasted blood glucose between the control (C) and B. toyo SAU-19 groups. Furthermore, the glucose area under the curve (AUC) for the OGTT value in DG mice was significantly larger than that of the control (C) (Figure 2B, p < 0.05). The glucose AUC was significantly lowered following oral administration of the B. toyonensis strain SAU-19 to mice compared to the DG group (p < 0.05), with no significant difference compared to the control (C) group (p > 0.05).
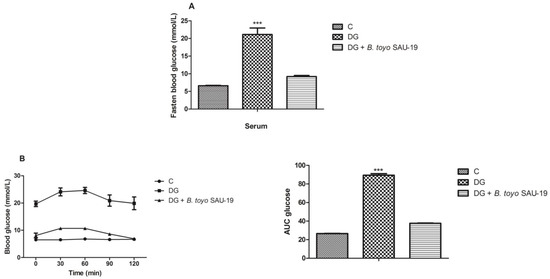
Figure 2.
Effects of Bacillus toyonensis SAU-19 on blood glucose tolerance in HFD/STZ-induced T2DM mice. (A) Fasted blood glucose (mmol/L). (B) Oral glucose test (mmol/L). Values are shown as mean ± Sd. Bars with *** are statistically different (p < 0.05), DG compared with the control (C) and B. toyo SAU-19 groups (n = 6).
3.3. Effects of B. toyonensis Strain SAU-19 on Biochemical Parameters in HFD/STZ-Induced T2DM Mice
As shown in Figure 3A–D, the levels of AST, ALT, fructosamine, and HOMA-IR in the diabetic (DG) group were significantly higher compared to the control (C) group (p < 0.05). However, administration of B. toyonensis SAU-19 significantly reduced the levels of AST, ALT, fructosamine, and HOMA-IR compared to the DG group mice (p < 0.05), but these parameters were not significantly different compared to the control (C) group.
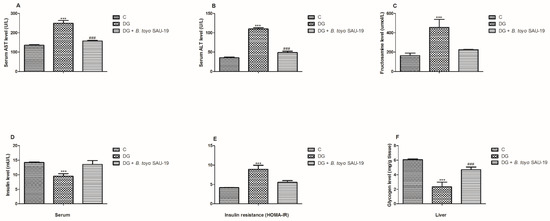
Figure 3.
Effects of Bacillus toyonensis SAU-19 on biochemical parameters in HFD/STZ-induced T2DM mice. (A) Levels of AST in blood serum (U/L). (B) Levels of ALT in blood serum (U/L). (C) Levels of fructosamine in serum (μmol/L). (D) Insulin level in the blood serum (mU/L). (E) Insulin resistance indicator. (F) Glycogen content in liver (mg/g tissue). Values are shown as mean ± Sd. *** p < 0.05, DG compared with the control (C) and B. toyo SAU-19 groups. ### p < 0.05, B. toyo SAU-19 compared with the control (C) group (n = 6). AST—Aspartate Aminotransferase, ALT—Alanine Aminotransferase.
The liver glycogen content and serum insulin levels were significantly lower in the DG group compared to the control (C) and B. toyo SAU-19 groups (Figure 3E, p > 0.05). In addition, the liver glycogen content was significantly lower in the B. toyo SAU-19 groups compared to the control (C) group (Figure 3E, p < 0.05). There was no difference in the insulin levels between the B. toyo SAU-19 groups and control (C) groups.
3.4. Effects of B. toyonensis Strain SAU-19 on Lipid Profiles in HFD/STZ-Induced T2DM Mice
The effects of B. toyonensis strain SAU-19 on the lipid profile are shown in Figure 4. TC, TG, and LDL-C levels in the diabetic group (DG) were higher compared to the control (C) group in both the serum and liver (Figure 4A–C, p < 0.05). However, these parameters were significantly attenuated in the B. toyo SAU-19 group (p < 0.05). Moreover, the TC levels in the B. toyo SAU-19 group were higher as compared to the control (C) group (Figure 4A, p < 0.05). There were no significant differences in the TG and LDL-C levels between the B. toyo SAU-19 group and control (C) group. The HDL-C levels in the diabetic group (DG) were reduced as compared to the B. toyo SAU-19 group and control (C) group (Figure 4D, p < 0.05); however, there were no significant differences in the HDL-C levels between the B. toyo SAU-19 group and control (C) group.
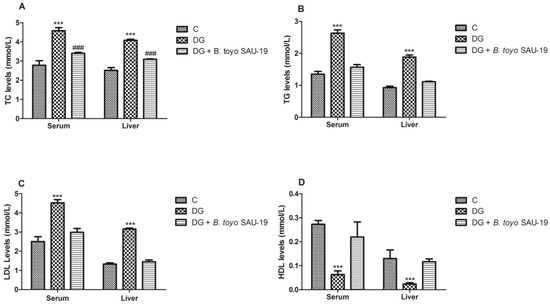
Figure 4.
Effects of Bacillus toyonensis SAU-19 on lipid profiles in HFD/STZ-induced T2DM mice. (A) Levels of TC in blood and liver serum (mmol/L). (B) Levels of TG in blood and liver serum (mmol/L). (C) Levels of LDL-C in blood and liver serum (mmol/L). (D) Levels of HDL-C in blood and liver serum (mmol/L). Values are shown as mean ± Sd. *** p < 0.05, DG compared with the control (C) and B. toyo SAU-19 groups. ### p < 0.05, B. toyo SAU-19 compared with the control (C) group (n = 6). TC—Total cholesterol, TG—Triglyceride, LDL-C—Low-density lipoprotein cholesterol, HDL-C—High-density lipoprotein cholesterol.
3.5. Effects of B. toyonensis Strain SAU-19 on Antioxidant Activity in HFD/STZ-Induced T2DM Mice
The variations in the antioxidant status of the mice livers are presented in Figure 5. As compared to the control (C) group, the oxidative stress parameter (MDA) was increased in the DG whereas the antioxidative stress components (SOD and GSH) in the DG group were greatly decreased (Figure 5A–C, p < 0.05). However, the administration of the B. toyonensis strain SAU-19 reverted these effects by increasing the levels of antioxidative stress components (SOD and GSH) and reducing the levels of the oxidative stress component MDA (p < 0.05). There was no significant difference in both the oxidative stress and antioxidant components between the B. toyo SAU-19 group and control (C) group (p > 0.05).
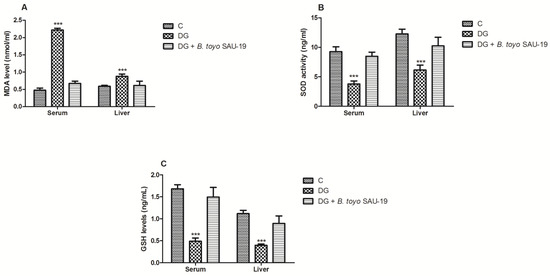
Figure 5.
Effects of Bacillus toyonensis SAU-19 on antioxidant activity in HFD/STZ-induced T2DM mice. (A) Levels of MDA in blood and liver serum (nmol/L). (B) Levels of SOD in blood and liver serum (ng/mL). (C) Levels of GSH in blood and liver serum (ng/mL). Values are shown as mean ± Sd. Bars with *** are statistically different (p < 0.05), DG compared with the control (C) and B. toyo SAU-19 groups (n = 6). MDA—malondialdehyde, SOD—superoxide dismutase, GSH—glutathione.
3.6. Effects of B. toyonensis Strain SAU-19 on Liver Histological Injury in HFD/STZ-Induced T2DM Mice
Histological analysis of the liver showed the well-ordered structure of the hepatic lobules in the control group’s liver sections, characterized by clear hepatic morphology and a centered nucleus. However, the diabetic mice group showed distortions in the hepatic lobule characterized by widespread degeneration, necrosis, and inflammation of the hepatocytes. These pathological disorders were noticeably improved by B. toyonensis SAU-19 feeding (Figure 6). Furthermore, SAU-19-fed mice had a significantly lower liver injury score compared to the HFD/STZ-fed mice; however, the injury scores of the SAU-19 group were significantly higher compared to the control group (Figure 6B, p < 0.05).
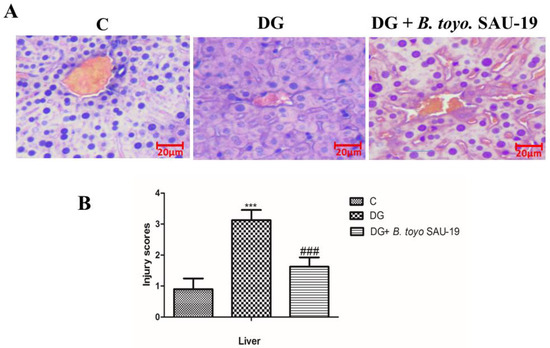
Figure 6.
Effects of Bacillus toyonensis SAU-19 on histology (×200) in HFD/STZ-induced T2DM mice. (A) Photograph of histopathological staining in treatment groups (Scale = 20 µm). (B) Histological necrosis injury scores of the liver section of mice. Values are shown as mean ± Sd. *** p < 0.05, DG compared with the control (C) and B. toyo SAU-19 groups. ### p < 0.05, B. toyo SAU-19 compared with the control (C) group C = Control, DG = Diabetic group, and DG + B. toyo SAU-19 (n = 5).
3.7. Effects of B. toyonensis Strain SAU-19 on Relative mRNA and Protein (ELISA) Expression of Genes Related to Inflammation in Liver Tissues of HFD/STZ-Induced T2DM Mice
As shown in Figure 7 and Figure 8, the mRNA and protein expression levels of proinflammatory cytokines (IL-1β and TNF-α) were significantly elevated whereas anti-inflammatory cytokines (IL-4 and IL-10) were reduced in the diabetic (DG) group compared to the control and SAU-19 groups (Figure 7A–D and Figure 8A–D, p < 0.05). However, the B. toyonensis strain SAU-19 reduced the expression levels of proinflammatory cytokines and increased the expression of anti-inflammatory cytokines compared to the DG group (p < 0.05). There was no significant difference between the B. toyonensis strain SAU-19 and control (C) (p > 0.05) in the mRNA expression of all cytokines and the protein expression of IL-1β, TNF-α, and IL-10; however, the protein levels (ELISA) of IL-4 in the SAU-19 group were significantly lower than the control.
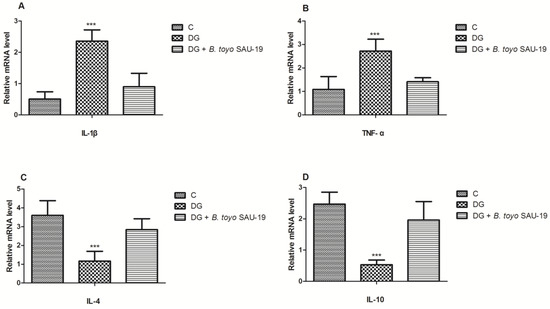
Figure 7.
Effects of Bacillus toyonensis SAU-19 on relative mRNA expression of pro- and anti-inflammation-related cytokines in HFD/STZ-induced T2DM mice. (A,B) mRNA expression levels of pro-inflammation cytokines. (C,D) mRNA expression of anti-inflammatory cytokines. Values are shown as mean ± Sd. Bars with *** are statistically different (p < 0.05). DG compared with the control (C) and B. toyo SAU-19 groups (n = 6).
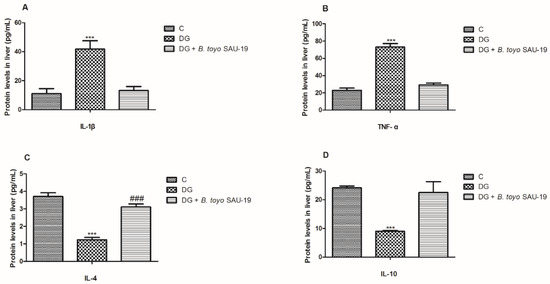
Figure 8.
Effects of Bacillus toyonensis SAU-19 on relative protein expression (ELISA) of pro- and anti-inflammation-related cytokines in HFD/STZ-induced T2DM mice. (A,B) Protein (ELISA) expression levels of pro-inflammation cytokines. (C,D) Protein (ELISA) expression of anti-inflammatory cytokines. Values are shown as mean ± Sd. *** p < 0.05, DG compared with the control (C) and B. toyo SAU-19 groups. ### p < 0.05, B. toyo SAU-19 compared with the control (C) group (n = 6).
3.8. Effects of B. toyonensis Strain SAU-19 on Relative mRNA Expression of Genes Related to Glucose and Glycogen Synthesis in the Liver Tissues of HFD/STZ-Induced T2DM Mice
As shown in Figure 9, the mRNA expression levels of genes related to glucose synthesis phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase) were elevated in the T2DM mice group whereas Forkhead Box O1 (FOXO1), Glucose Transporter 2 (GLUT2), Glycogen synthase (GS), and phosphofructokinase liver type (Pfkl) were significantly reduced in the diabetic group compared to the normal mice group, but the administration of the B. toyonensis strain SAU-19 reverted this effect by downregulating the expression levels of PEPCK and G6Pase and upregulating the expression of FOXO1, GLUT2, GS, and Pfkl (Figure 9A–F, p < 0.05) compared to the diabetes mice.
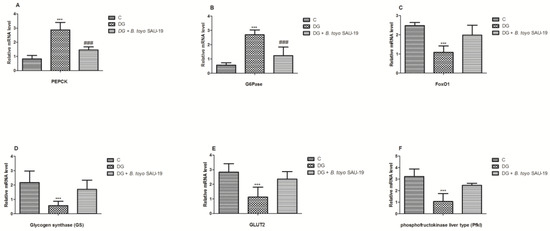
Figure 9.
Effects of Bacillus toyonensis SAU-19 on the relative mRNA expression of glucose and glycogen synthesis-related genes in HFD/STZ-induced T2DM mice. (A,B) mRNA expression levels of genes related to glucose synthesis. (C–F) mRNA expression of genes related to glycogen synthesis. Values are shown as mean ± Sd. *** p < 0.05, DG compared with the control (C) and B. toyo SAU-19 groups. ### p < 0.05, B. toyo SAU-19 compared with the control (C) group (n = 6).
4. Discussion
This study demonstrates the antidiabetic effect of B. toyonensis SAU-19 on mice with T2DM induced by HFD/STZ. We successfully modeled a T2DM mice model as basic characteristics of T2DM, such as weight loss, increased food and water consumption, and increase blood glucose, were evident in our study. Interestingly, the administration of B. toyonensis SAU-19 significantly improved hyperglycemia, insulin resistance, oxidative stress, and dyslipidemia. A study by Li et al. [19] reported that the body weights of HFD/STZ-induced diabetic mice were significantly lower than the control mice after the type 2 diabetes model was established. Similarly, in our study, we observed a decrease in the body weights of HFD/STZ-induced diabetic mice after the type 2 diabetes model was established. However, the feeding of B. toyonensis SAU-19 maintained the body weights compared to the type 2 diabetic mice. Kantas et al. [20] reported that Bacillus toyonensis could improve health and growth performance. Furthermore, we observed a reduction in the weights of the liver, kidney, and spleen in the diabetic group compared to the other treatment groups. This observation was consistent with the study by Zafar and Naeem-Ul-Hassan Naqvi [21], who reported a reduction in the body and organ weights of STZ-induced diabetes rats.
The immune organ weight and index are associated with immunity [22]. A study by Iftikhar et al. [23] reported that the weights of immune organs correlate with immune improvement; therefore, an improved immune organ weight signifies immunity improvement, whereas the opposite designates immunosuppression. From the results of our current study, we observed that the administration of Bacillus toyonensis SAU-19 increased the splenic weight and splenic organ index. Therefore, we concluded that Bacillus toyonensis SAU-19 stimulated the development of the spleen, hence enhancing immune performance in mice.
Recently, numerous probiotic strains have shown glucose-alleviating potential [24,25]. In our current study, B. toyonensis SAU-19 showed an effective antiglycemia activity via reduction and regulation of the glucose levels similarly to that in control mice throughout the experiment.
The liver is an important organ responsible for regulating glucose metabolism via assimilating excess blood glucose into glycogen [26]. The histopathological results obtained in this study revealed that Bacillus toyonensis SAU-19 did not cause any severe pathological changes in the liver tissues as compared to the diabetes group. Complications, such as hepatocyte structure disorder with extensive degeneration, congestion, and necrosis and inflammation, were observed in the liver of mice in the diabetes group. This was consistent with previous reports by Zeng et al. [1], who reported that probiotics Lactobacillus paracasei NL41 could prevent the pathological damage in the liver caused in HFD/STZ-induced mice.
Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) are markers of active liver inflammation and tissue damage [27]. Numerous studies have reported that diabetes causes a rise in the AST and ALT levels [28,29]. Similarly, in this study, we observed an increase in the levels of liver injury markers in the blood; however, the administration of B. toyonensis SAU-19 reduced the levels of these liver inflammation and damage markers. This result was consistent with the study by Mirmiranpour et al. [30], who reported that Lactobacillus acidophilus (probiotic) could reduce the levels of AST and ALT in type 2 diabetes patients. Furthermore, we also observed that liver glycogen levels were much higher in the B. toyo SAU-19 group, indicating that B. toyonensis SAU-19 reduced blood glucose by helping in the regulation and transport of high glucose loads from the blood to the liver to be converted into glycogen. This result was consistent with previous studies showing that Saccharomyces boulardii Tht 500101 significantly increased the storage of hepatic glycogen [31].
Insulin resistance is a pathophysiological disorder, which arises as a result of decreased insulin sensitivity in peripheral tissues [32]. In the present study, we observed that the glucose tolerance was unimpaired, and the increased levels of HOMA-IR were nullified in HFD/STZ-T2DM mice administrated B. toyonensis SAU-19, indicating that the administration of SAU-19 prevented or delayed the onset of T2DM by improving insulin resistance. This was consistent with the study by Balakumar et al. [33], who reported that the native probiotic strains MTCC 5690 and MTCC 5689 improve insulin resistance and type 2 diabetes.
Oxidative stress plays a crucial function in the onset of insulin resistance and T2DM [34]. Reactive oxygen species (ROS) activated by hyperglycemia and dyslipidemia induce injury in the liver [35,36]. Several probiotics have showed effective antioxidants activities [37]. In this study, supplementation of B. toyonensis SAU-19 significantly increased the activities of SOD and GSH but decreased the MDA activity. These results suggest that SAU-19 protected the body against oxidative damage, thus improving insulin resistance and reducing the injury to organs, such as the pancreas, liver, and kidney.
Systemic and subclinical inflammation is associated with type 2 diabetes mellitus [38,39]. The inflammatory process is characterized by increased levels of inflammatory factors, such as C-reactive protein (CRP) or high-sensitivity CRP (hs-CRP) and inflammatory cytokines [40]. In the hepatocytes, the process of inflammation causes the production of numerous acute-phase proteins, such as ferritin, which enhances insulin resistance [39]. The results from this study showed that the expression of proinflammatory cytokines (IL-1β and TNF-α) in the liver was elevated while the levels of anti-inflammatory cytokines (IL-4 and IL-10) were reduced in the diabetic group compared to the normal group; however, the administration of B. toyonensis SAU-19 reverted these effects. This result is consistent with the study by Liu et al. [41], who reported that Lactobacillus rhamnosus GG culture supernatant (LGGs) could reduce liver inflammation and injury in a high-fat/high-fructose diet and intermittent hypoxia exposure-induced metabolic dysfunction.
Gluconeogenesis and glycogenolysis are two major pathways for endogenous glucose production [42]. PEPCK and G6pase are two key enzymes of hepatic gluconeogenesis [43]. PEPCK and G6pase catalyzes the process of gluconeogenesis in the liver and thus is associated with glucose production [6]. Increased expression of PEPCK and G6pase in the liver has been linked with the onset of type 2 diabetes [44]. FOXO1 is a member of the forkhead family transcription factors, which directly binds to PEPCK and G6pase target DNA sequence to control their expression in the liver [45]. Studies have proved that the inhibition of FoxO1 decreases hepatic gluconeogenesis and improves glucose metabolism in animals with T2DM [46]. GS is the rate-limiting step for glycogen synthesis, and activation of GS by decreasing its phosphorylation results in increased glycogen synthesis [47]. GLUT2 is a bidirectional glucose transporter and a transmembrane carrier protein mostly found in the liver, kidney, and pancreas and is involved in supporting the passive movement of hexoses through the cell membranes [48]. The high expression levels of hepatic GLUT2 mRNA reported in this study were similar to those reported by Matsuzaka et al. [49] and Narasimhan et al. [50] in rats, Jung et al. [51] in mice, and Okamoto et al. [52] in the HepG2 cell line. The upregulation of GLUT2 expression in diabetes mice may increase the hepatic glucose output since it was suggested that GLUT2 transports glucose from the liver when the intracellular concentration of glucose exceeds its concentration in the plasma [53,54]. In cellular respiration, phosphofructokinase-1 (PFK-1) controls the oxidation of glucose [55]. An increase in the levels of PFK-1 has been reported to increase glucose metabolism [42,56]. The results from this study showed that the mRNA expression levels of PEPCK and G6pase genes were higher in the diabetic group as compared to the control group; however, the administration of B. toyonensis SAU-19 reduced the expression of these genes, indicating that B. toyonensis SAU-19 suppresses hepatic gluconeogenesis. This result was consistent with the study by Yadav et al. [57], who reported that Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897, and Lactobacillus fermentum MTCC: 5898 reduced the mRNA expression of PEPCK and g6pase. Furthermore, the expression of FOXO1, GS, GLUT2, and PFK-1 in the treatment group was elevated compared to the diabetes group. This indicated that B. toyonensis SAU-19 reduced T2DM by upregulating genes related to glycogen synthesis and excess glucose transport in the liver. This result was similar to the study by Kim et al. [58], who reported that Bifidobacterium lactis HY8101 upregulated the glycogen synthesis-related gene pp-1 and GLUT4 and downregulated the hepatic gluconeogenesis-regulated genes (PCK1 and G6PC) in diabetic mice.
This study reported on the antidiabetic activity of Bacillus toyonensis SAU-19; however, the molecular mechanisms involved in the treatment of T2DM were not fully reported. Therefore, we suggest that further studies should be conducted to elucidate the potential molecular mechanisms that the B. toyonensis strain SAU-19 uses to prevent or treat type 2 diabetes.
5. Conclusions
In conclusion, this study demonstrated that the B. toyonensis strain SAU-19 has an excellent antidiabetic effect in HFD/STZ-induced T2DM mice. The potential mechanism of this effect might be related to decreasing insulin resistance and oxidative stress, upregulating genes related to glycogen synthesis and glucose transport, and improving lipid profiles. Therefore, from the results, B. toyonensis SAU-19 could be used for the treatment of T2DM. However, further studies are still needed to clarify the detailed mechanisms of action by validating the efficacy of B. toyonensis SAU-19 through human clinical trials.
Author Contributions
S.K.O.: Conceptualization, data curation, Writing—Original, Writing—Review and Editing. L.X.: Conceptualization, Writing—Original Draft. J.W.: Software, Validation. Y.R.: Software, Validation. Z.R.: Writing—Software, Review and Editing, J.D.: Writing—Review and Editing and supervision, Y.H.: Writing—Review and Editing, Supervision and Funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Sichuan Province Science and Technology Support Program (Grant No. 2020YFS0337).
Institutional Review Board Statement
The animal experiment was approved by This study was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University, Sichuan, China, under the permit number DKY-B2019603005.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
I would like to thank all authors for their hard work in making this paper publishable. I also would like to extend my sincere gratitude to the teaching staffs of the College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, for their guidance and criticism in writing this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates for the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Jimenez, G.; Blanch, A.R.; Tamames, J.; Rossello-Mora, R. Complete genome sequence of Bacillus toyonensis BCT-7112T, the active ingredient of the feed additive preparation Toyocerin. Genome Announc. 2013, 1, 01080-13. [Google Scholar] [CrossRef] [Green Version]
- EFSA. FEEDAP panel (EFSA panel on additives and products or substances used in animal feed) scientific opinion on the safety and efficacy of Toyocerin (Bacillus toyonensis) as a feed additive for rabbits for fattening, chickens for fattening, weaned piglets, pigs for fattening, sows for reproduction, cattle for fattening, and calve s for rearing. EFSA J. 2014, 12, 17. [Google Scholar]
- Taras, D.; Vahjen, W.; Macha, M.; Simon, O. Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 2005, 59, 405–417. [Google Scholar] [CrossRef]
- Baum, B.; Liebler-Tenorio, E.M.; Enss, M.L.; Pohlenz, J.F.; Breves, G. Saccharomyces boulardii and Bacillus cereus var. toyoi influence the morphology and the mucins of the intestine of pigs. Z. Gastroenterol. 2002, 40, 277–284. [Google Scholar] [CrossRef]
- Simon, O. An interdisciplinary study on the mode of action of probiotics in pigs. J. Anim. Feed Sci. 2010, 19, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Okyere, S.K.; Mo, Q.; Pei, G.; Ren, Z.; Deng, J.; Hu, Y. Euptox A Induces G0 /GI arrest and apoptosis of hepatocyte via ROS, mitochondrial dysfunction and caspases-dependent pathways in vivo. J. Toxicol. Sci. 2020, 45, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoterapia 2021, 155, 105032. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Okyere, S.K.; Wen, J.; Xie, L.; Cui, Y.; Wang, S.; Wang, J.; Cao, S.; Shen, L.; Ma, X.; et al. An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions. Int. J. Mol. Sci. 2021, 22, 11581. [Google Scholar] [CrossRef]
- Nath, S.; Ghosh, S.K.; Choudhury, Y. A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. J. Pharmacol. Toxicol. Methods 2017, 84, 20–30. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, M.G.; Jiang, C.H.; Sheng, X.P.; Yang, H.M.; Liu, Y.; Yao, X.M.; Zhang, J.; Yin, Z.Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine 2020, 66, 153130. [Google Scholar] [CrossRef]
- Chen, R.; Ping, Z.; Chen, J.; Liang, L.; Lu, C. Mycelia Extract from Spent Ganoderma Lucidum Bagasse Substrate Ameliorates Carbon Tetrachloride-Induced Acute Liver Injury in Mice Through Inhibiting Oxidative Stress. Waste Biomass Valor 2021, 12, 3257–3269. [Google Scholar] [CrossRef]
- Cui, Y.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.; Wang, Y.; Deng, J.; Hu, Y. Ageratina adenophora Disrupts the Intestinal Structure and Immune Barrier Integrity in Rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef]
- Li, S.; Huang, Q.; Zhang, L.; Qiao, X.; Zhang, Y.; Tang, F.; Li, Z. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/ GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice. Eur. J. Pharmacol. 2019, 853, 1–10. [Google Scholar] [CrossRef]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Giavasis, I.; Bouki, P.; Tzika, E.D. A feed additive containing Bacillus toyonensis (Toyocerin (®)) protects against enteric pathogens in postweaning piglets. J. Appl. Microbiol. 2015, 118, 727–738. [Google Scholar] [CrossRef]
- Zafar, M.; Naeem-Ul-Hassan Naqvi, S. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Shu, G.; Kong, F.; Xu, D.; Yin, L.; He, C.; Lin, J.; Fu, H.; Wang, K.; Tian, Y.; Zhao, X. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci. Rep. 2020, 10, 12324. [Google Scholar] [CrossRef]
- Iftikhar, H.; Mahmood, M.S.; Arshad, M.I.; Akhtar, M.; Mahmood, F.; Rafique, A. Immune system dysfunction in broiler chickens experimentally inoculated with fowl adenovirus serotype-4 associated with inclusion body hepatitis hydropericardium syndrome. Turk. J. Vet. Anim. Sci. 2012, 36, 223–230. [Google Scholar]
- da Costa, W.K.A.; Brandão, L.R.; Martino, M.E.; Garcia, E.F.; Alves, A.F.; de Souza, E.L.; de Souza Aquino, J.; Saarela, M.; Leulier, F.; Vidal, H.; et al. Qualification of tropical fruit-derived Lactobacillus plantarum strains as potential probiotics acting on blood glucose and total cholesterol levels in Wistar rats. Food Res. Int. 2019, 124, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. 2020, 2020, 6108575. [Google Scholar] [CrossRef] [Green Version]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, L.; Shive, C.L.; Zebrowski, E.; Fuller, B.; Rife, K.; Hirsch, A.; Compan, A.; Moreland, A.; Falck-Ytter, Y.; Popkin, D.L.; et al. Soluble Markers of Immune Activation Differentially Normalize and Selectively Associate with Improvement in AST, ALT, Albumin, and Transient Elastography During IFN-Free HCV Therapy. Pathog. Immun. 2018, 3, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, L.; Zhang, G.; Varkaneh, H.K.; Rahmani, J.; Clark, C.; Ryan, P.M.; Abdulazeem, H.M.; Salehisahlabadi, A. The association of plasma levels of liver enzymes and risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Acta Diabetol. 2020, 57, 635–644. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef]
- Albuquerque, R.C.M.F.; Brandão, A.B.P.; De Abreu, I.C.M.E.; Ferreira, F.G.; Santos, L.B.; Moreira, L.N.; Taddei, C.R.; Aimbire, F.; Cunha, T.S. Saccharomyces boulardii Tht 500101 changes gut microbiota and ameliorates hyperglycaemia, dyslipidaemia, and liver inflammation in streptozotocin-diabetic mice. Benef. Microbes 2019, 10, 901–912. [Google Scholar] [CrossRef]
- Sampath, K.A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.S.; Zhu, C.J.; Yan, B.Y.; Yan, C.Y.; Ling, R. Stimulation of KLF14/PLK1 pathway by thrombin signaling potentiates endothelial dysfunction in Type 2 diabetes mellitus. Biomed. Pharmacother. 2018, 99, 859–866. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, Y.M.; Zeng, X.F.; Chen, H.Z. Circadian Clock and Sirtuins in Diabetic Lung: A Mechanistic Perspective. Front. Endocrinol. 2020, 11, 173. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia 2016, 59, 679–682. [Google Scholar] [CrossRef]
- Elimam, H.; Abdulla, A.M.; Taha, I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 800–804. [Google Scholar] [CrossRef]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 39–50. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Li, F.; Gu, Z.; Liu, M.; Shao, T.; Zhang, L.; Zhou, G.; Pan, C.; He, L.; et al. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J. Nutr. Biochem. 2020, 75, 108256. [Google Scholar] [CrossRef]
- Cui, X.; Qian, D.W.; Jiang, S.; Shang, E.X.; Zhu, Z.H.; Duan, J.A. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lv, Q.; Jia, S.; Chen, Y. Effects of flavonoid-rich Chinese bayberry (Morella rubra Sieb. et Zucc.) fruit extract on regulating glucose and lipid metabolism in diabetic KKA(y) mice. Food Funct. 2016, 7, 3130–3140. [Google Scholar] [CrossRef]
- Shi, C.X.; Zhao, M.X.; Shu, X.D.; Xiong, X.Q.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep. 2016, 6, 21924. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, F.G.; Song, W.; Yang, Y.L.; Liu, J.F.; Li, P.; Liu, B.L.; Qi, W. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Arch. Med. Res. 2018, 49, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Shin, H.M.; Jung, H.; Rhee, B.D.; Park, J.H. Comparison of pancreatic beta cells and alpha cells under hyperglycemia: Inverse coupling in pAkt-FoxO1. Diabetes Res. Clin. Pract. 2017, 131, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, A.J.; Birk, J.B.; Eilertsen, E.; Stuenaes, J.T.; Wojtaszewski, J.F.; Jensen, J. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles. Am. J. Physiol. Endocrinol. 2015, 308, E231–E240. [Google Scholar] [CrossRef] [Green Version]
- Hussar, P.; Karner, M.; Jarveots, T.; Pendovski, L.; Duritis, I.; Popovska-Percinic, F. Comparative study of glucose transporters GLUT-2 and GLUT-5 in ostriches gastrointestinal tract. Mac. Vet. Rev. 2016, 39, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; Tomita, S.; Sekiya, M.; et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes 2004, 53, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Tanaka, S.; Haga, Y. Enhanced GLUT2 gene expression in anoleic acid-induced in vitro fatty liver model. Hepatol, Res. 2002, 23, 138–144. [Google Scholar] [CrossRef]
- Mohammed, K.A.A.; Ahmed, H.M.S.; Sharaf, H.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Mehaya, F.M.; Abdel-Wahhab, M.A. Encapsulation of cinnamon oil in whey protein counteracts the disturbances in biochemical parameters, gene expression, and histological picture of the liver and pancreas of diabetic rats. Environ. Sci. Pollut. Res. 2020, 27, 2829–2843. [Google Scholar] [CrossRef]
- Song, J.; Gao, J.; Du, M.; Mao, X.Y. Casein lycomacropeptide hydrolysates ameliorate hepatic insulin resistance of C57BL/6J mice challenged with high-fat diet. J. Funct. 2018, 45, 190–198. [Google Scholar] [CrossRef]
- Lang, L.; Chemmalakuzhy, R.; Shay, C.; Teng, Y. PFKP Signaling at a Glance: An Emerging Mediator of Cancer Cell Metabolism. Adv. Exp. Med. Biol. 2019, 1134, 243–258. [Google Scholar]
- Bockus, L.B.; Matsuzaki, S.; Vadvalkar, S.S.; Young, Z.T.; Giorgione, J.R.; Newhardt, M.F.; Kinter, M.; Humphries, K.M. Cardiac Insulin Signaling Regulates Glycolysis Through Phosphofructokinase 2 Content and Activity. J. Am. Heart Assoc. 2017, 6, e007159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.; Dey, D.K.; Vij, R.; Meena, S.; Kapila, R.; Kapila, S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb. Pathog. 2018, 125, 454–462. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, C.S.; Choi, I.D.; Jeong, J.W.; Ku, H.K.; Ra, J.H.; Kim, T.Y.; Kim, G.B.; Sim, J.H.; Ahn, Y.T. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).