The Relationship between Macronutrient Distribution and Type 2 Diabetes in Asian Indians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Macronutrient Distribution
2.4. Statistical Analysis
3. Results
3.1. Participant Demographics
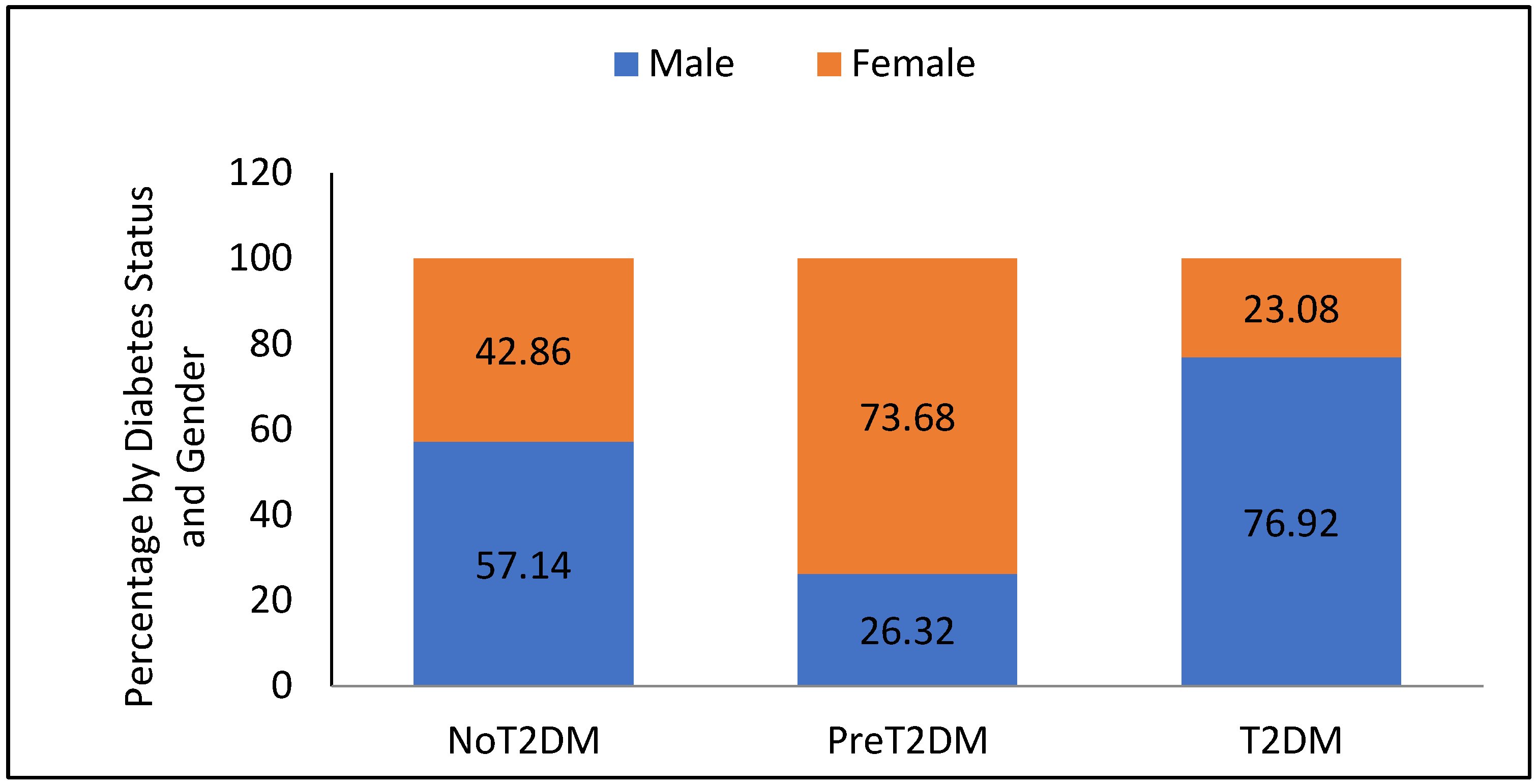
3.2. Diabetes Status
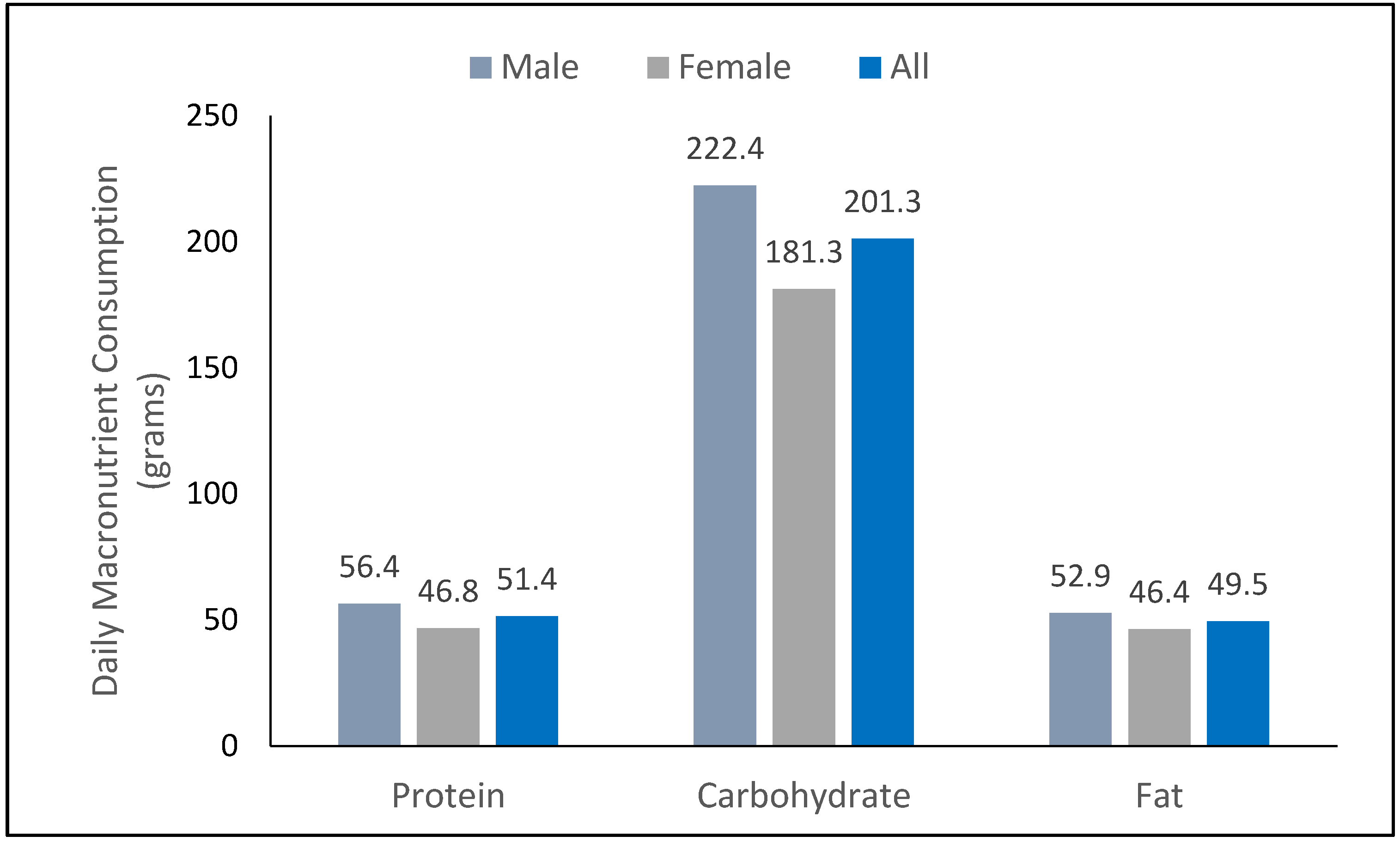
3.3. Participant Diets
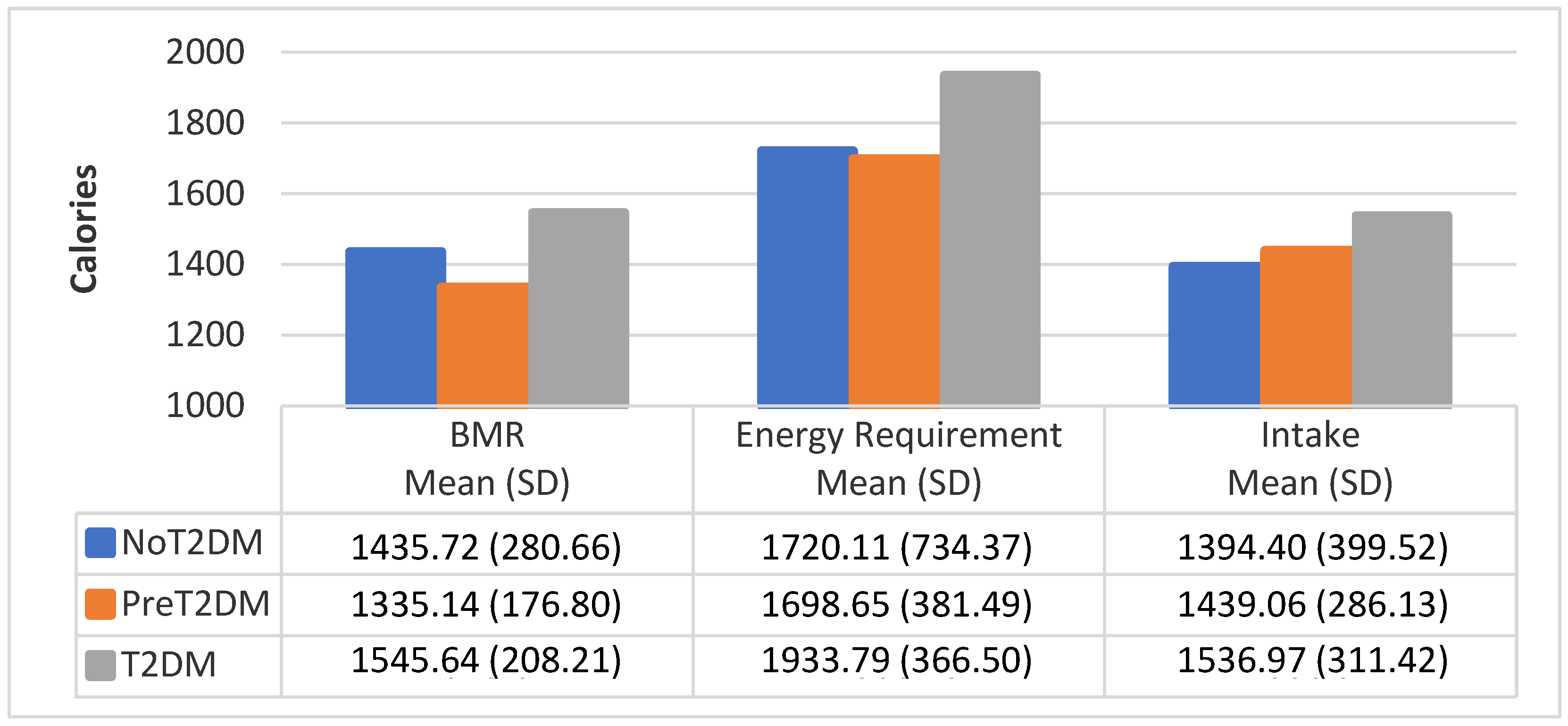
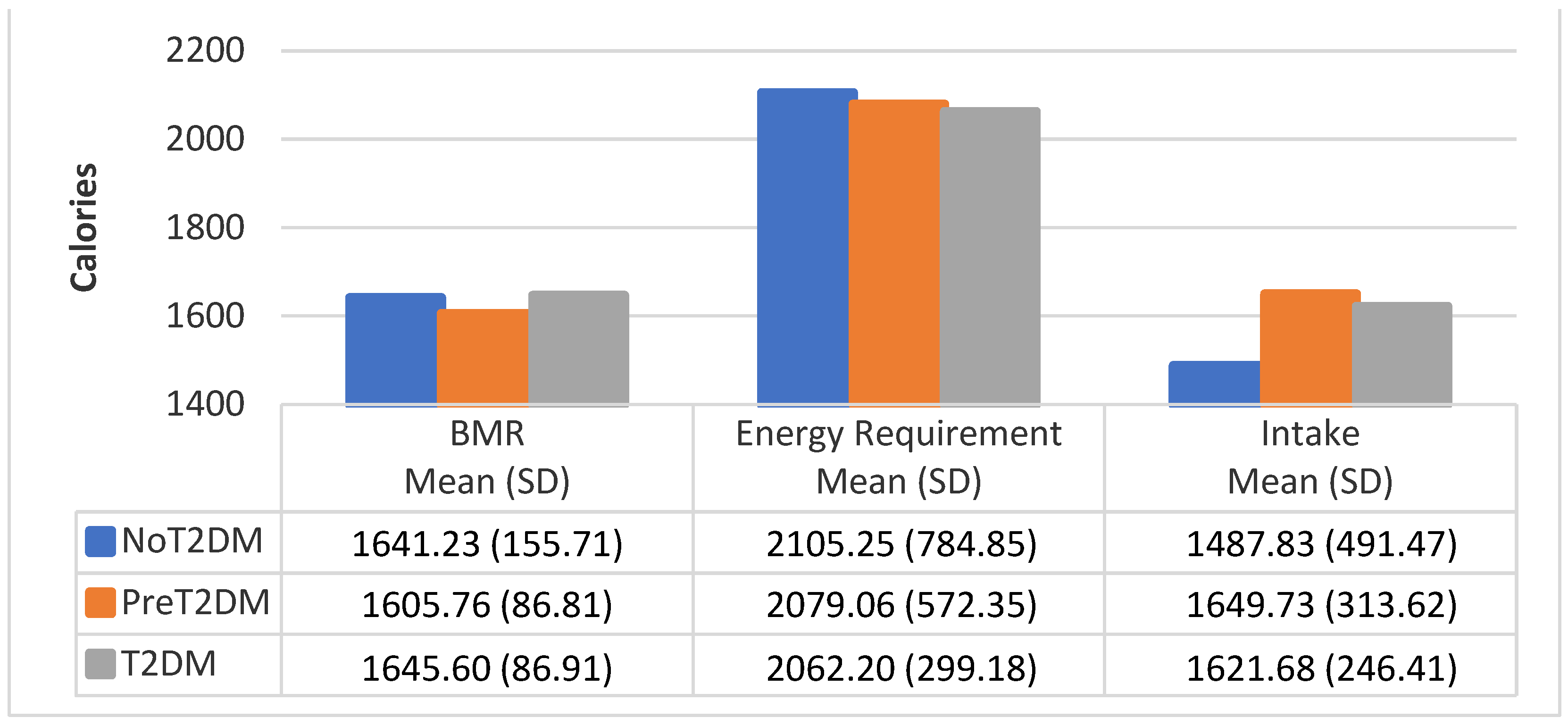
3.4. BMR by Diabetes Status
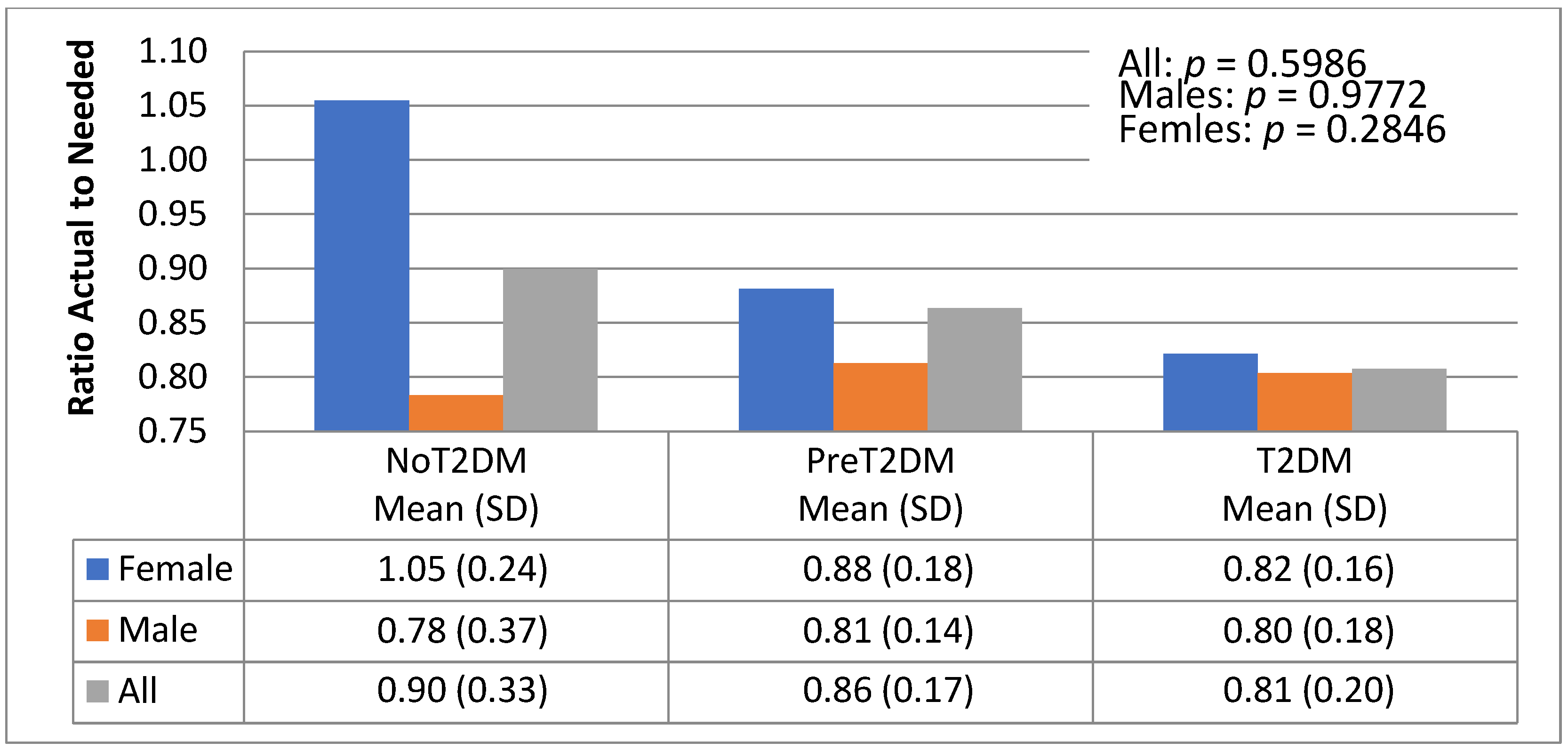
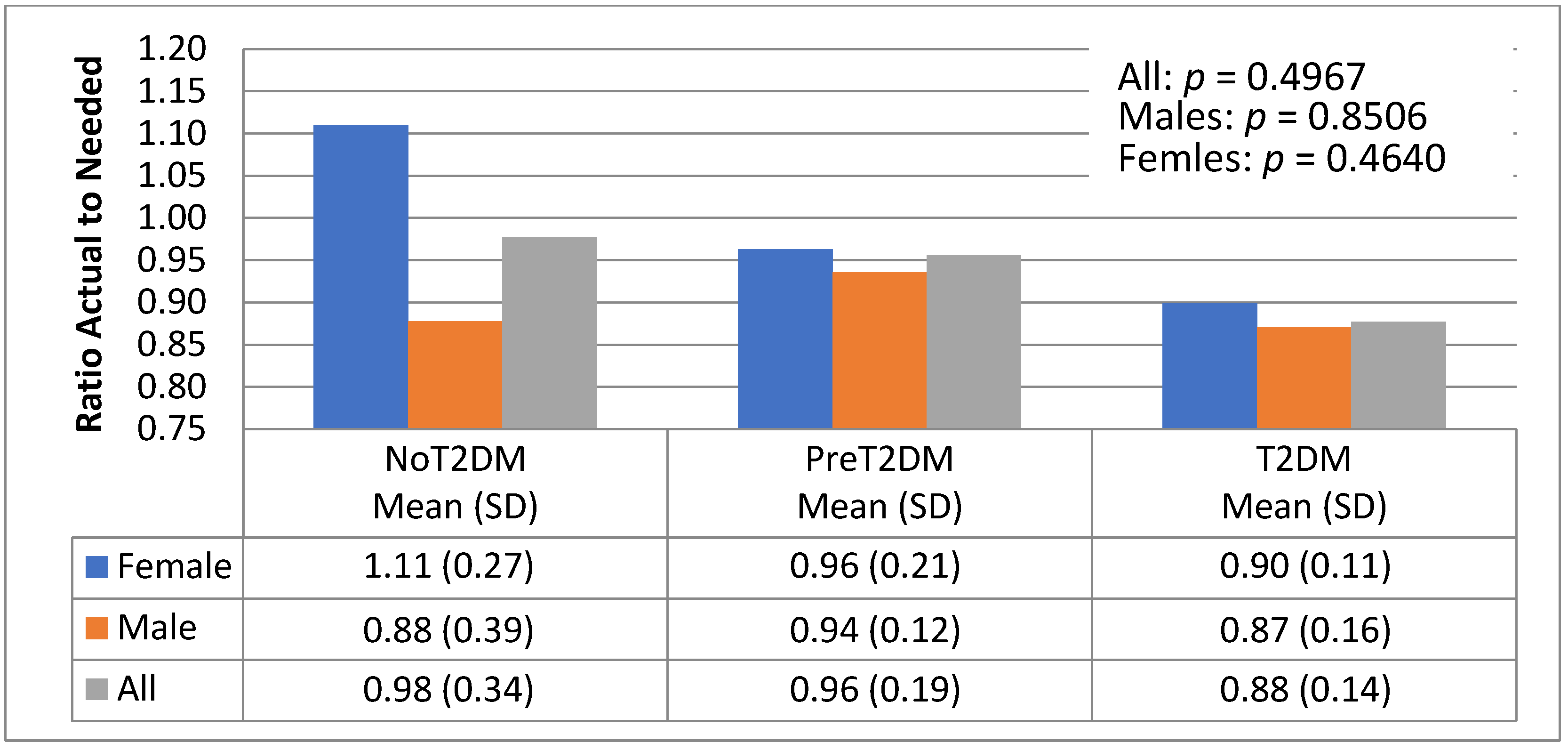
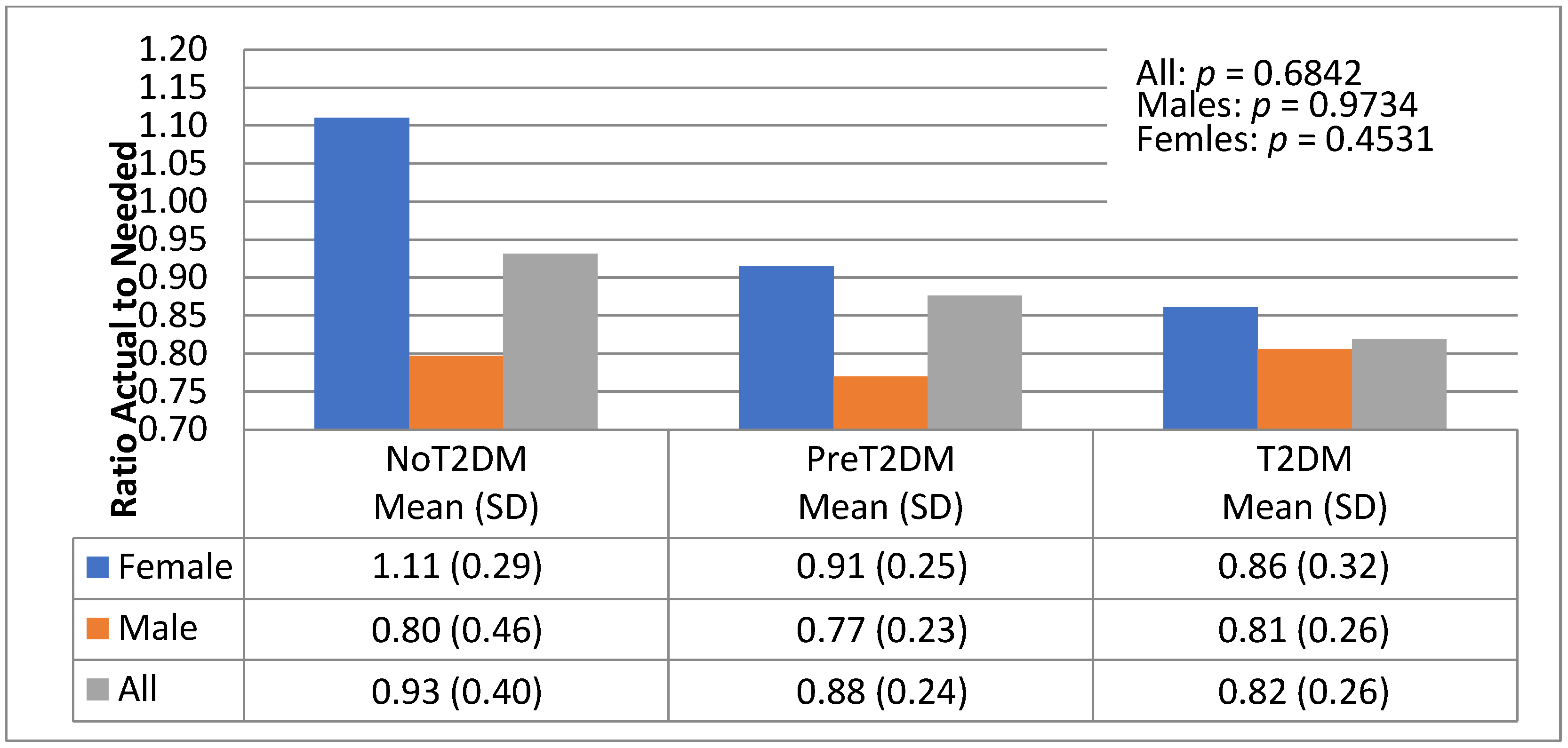
3.5. Diet Quantity—Macronutrient Distributions—Actual to Needed Calorie Ratios
3.6. Diet Quality
3.7. Predicting Diabetes Status
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, M.B.; Oza-Frank, R.; Staimez, L.R.; Ali, M.K.; Venkat Narayan, K.M. Type 2 Diabetes in Asians: Prevalence, Risk Factors, and Effectiveness of Behavioral Intervention at Individual and Population Levels. Annu. Rev. Nutr. 2012, 32, 417–439. [Google Scholar] [CrossRef]
- International Diabetes Federation, I.D.F. IDF Diabetes Atlas. 2015. Cited 2016; Sseventh. Available online: www.diabetesatlas.org (accessed on 17 April 2017).
- Venkataraman, R.; Nanda, N.C.; Baweja, G.; Parikh, N.; Bhatia, V. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am. J. Cardiol. 2004, 94, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Misra, A.; Misra, R.; Goel, K.; Bhatt, S.P.; Rastogi, K.; Vikram, N.K.; Gulati, S. High Prevalence of Abdominal, Intra-Abdominal and Subcutaneous Adiposity and Clustering of Risk Factors among Urban Asian Indians in North India. PLoS ONE 2011, 6, e24362. [Google Scholar] [CrossRef]
- Joshi, S.R. Diabetes Care in India. Ann. Glob. Health 2016, 81, 830–838. [Google Scholar] [CrossRef]
- Bhopal, R.S. A four-stage model explaining the higher risk of Type 2 diabetes mellitus in South Asians compared with European populations. Diabet. Med. 2013, 30, 35–42. [Google Scholar] [CrossRef]
- Staimez, L.R.W.; Mary, B.; Ranjani, H.; Ali, M.K.; Echouffo-Tcheugui, J.B.; Phillips, L.S.; Mohan, V.; Narayan, K.M.V. Evidence of Reduced B-Cell Function in Asian Indians With Mild Dysglycemia. Diabetes Care 2013, 36, 2772–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovegrove, J.A. CVD risk in South Asians: The importance of defining adiposity and influence of dietary polyunsaturated fat. Proc. Nutr. Soc. 2007, 66, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas 2010, 65, 315–319. [Google Scholar] [CrossRef]
- Misra, A.; Vikram, N.K. Insulin Resistance Syndrome (Metabolic Syndrome) and Obesity in Asian Indians: Evidence and Implications. Nutrition 2004, 20, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Vikram, N.K.; Pandey, R.M.; Misra, A.; Sharma, R.; Devi, J.R.; Khanna, N. Non-obese (body mass index < 25 kg/m2) Asian Indians with normal waist circumference have high cardiovascular risk. Nutrition 2003, 19, 503–509. [Google Scholar] [CrossRef]
- Misra, A.; Singhal, N.; Sivakumar, B.; Bhagat, N.; Jaiswal, A.; Khurana, L. Nutrition transition in India: Secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J. Diabetes 2011, 3, 278–292. [Google Scholar] [CrossRef]
- Holmboe-Ottesen, G.; Wandel, M. Changes in dietary habits after migration and consequences for health: A focus on South Asians in Europe. Food Nutr. Res. 2012, 56, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hou, X.; Lu, J.; Zhou, J.; Lu, F.; Kan, K.; Tang, J.; Bao, Y.; Jia, W. Assessment of the Performance of A1CNow1 and Development of an Error Grid Analysis Graph for Comparative Hemoglobin A1c Measurements. Diabetes Technol. Ther. 2014, 16, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Hirst, J.A.; McLellan, J.; Price, C.P.; English, E.; Feakins, B.; Stevens, R.J.; Farmer, A.J. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2017, 55, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Walicka, M.; Jozwiak, J.; Rzeszotarski, J.; Zonenberg, A.; Masierek, M.; Bijos, P.; Franek, E. Diagnostic Accuracy of Glycated Haemoglobin and Average Glucose Values in Type 2 Diabetes Mellitus Treated wtih Premixed Insulin. Diabetes Ther. 2019, 10, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin Şahingöz Erdal, N.I.; Murat, K.; Nursel, K. Hemoglobin A1c Measurement Using Point of Care Testing. Istanb. Med. J. 2020, 21, 37–41. [Google Scholar] [CrossRef]
- Mattewal, A.; Aldasouqi, S.; Solomon, D.; Gossain, V.; Koller, A. A1cNow® InView™: A New Simple Method for Office-Based Glycohemoglobin Measurement. J. Diabetes Sci. Technol. 2007, 1, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.T.; Koning Ld Kanaya, A.M. Higher Protein Intake Is Associated with Diabetes Risk in South Asian Indians: The Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) Study. J. Am. Coll. Nutr. 2010, 29, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelemen, L.E.; Anand, S.S.; Vuksan, V.; Yi, Q.; Teo, K.K.; Devanesen, S.; Yusuf, S. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J. Am. Diet. Assoc. 2003, 103, 1178–1184. [Google Scholar] [CrossRef]
- Joseph, M.; Gupta, R.D.; Prema, L.; Inbakumari, M.; Thomas, N. Are Predictive Equations for Estimating Resting Energy Expenditure Accurate in Asian Indian Male Weightlifters? Indian J. Endocrinol. Metab. 2017, 21, 515–519. [Google Scholar]
- Song, L.; Venkataraman, K.; Gluckman, P.; Chong, Y.S.; Chee, M.-W.L.; Khoo, C.M.; Leow, M.-K.S.; Lee, Y.S.; Tai, E.S.; Khoo, E.Y.H. Smaller size of high metabolic rate organs explains lower resting energy expenditure in Asian-Indian than Chinese men. Int. J. Obes. 2016, 40, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Varte, L.R.; Pal, M. Predictive equation for basal metabolic rate of young Indian soldiers. Asian J. Med Sci. 2016, 7, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Hasson, R.E.; Howe Cheryl, A.; Jones Bryce, L.; Jones Freedson Patty, S. Accuracy of four resting metabolic rate prediction equations: Effects of sex, body mass index, age, and race/ethnicity. J. Sci. Med. Sport 2011, 14, 344–351. [Google Scholar] [CrossRef]
- Miller, S.; Milliron, B.-J.; Woolf, K. Common Prediction Equations Overestimate Measured Resting Metabolic Rate in Young Hispanic Women. Top. Clin. Nutr. 2013, 28, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. J. Am. Diet. Assoc. 2005, 105, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Venkataraman, K.; Gluckman, P.; Seng, C.Y.; Meng, K.C.; Khoo, E.Y.; Leow, M.K.; Seng, L.Y.; Shyong, T.E. Validation of prediction equations for resting energy expenditure in Singaporean Chinese men. Obes. Res. Clin. Pract. 2015, 8, 283–290. [Google Scholar] [CrossRef]
- Noakes, M.; Foster Paul, R.; Keogh Jennifer, B.; James Anthony, P.; Mamo John, C.; Clifton Peter, M. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr. Metab. 2006, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Georg, H. Comparison of the long-term effects of high-fat v. low-fat diet consumption on cardiometabolic risk factors in subjects with abnormal glucose metabolism: A systematic review and meta-analysis. Br. J. Nutr. 2014, 111, 2047–2058. [Google Scholar] [CrossRef] [Green Version]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sara Castro-Barquero, A.M.R.-L.; Maria Sierra-Pérez, R.E.; Rosa, C. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- National Institutes of Medicine, F.a.N.B. Dietary Reference Intakes (DRI) for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Lawton, J.; Ahmad, N.; Hanna, L.; Douglas, M.; Bains, H.; Hallowell, N. ‘We should change ourselves, but we can’t’: Accounts of food and eating practices amongst British Pakistanis and Indians with type 2 diabetes. Ethn. Health 2008, 13, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Venn, B.J. Macronutrients and Human Health for the 21st Century. Nutrients 2020, 12, 2363. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fu, Y.; Tian, Y.; Gou, W.; Miao, Z.; Yang, M.; Ordovás, J.M.; Zheng, J.S. Individual Postprandial Glycemic Responses to Diet in n-of-1 Trials: Westlake N-of-1 Trials for Macronutrient Intake (WE-MACNUTR). J. Nutr. 2021, 151, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Edward JMJoy, R.G.; Sutapa, A.; Lukasz, A.; Liza, B.; Sanjay, K.; Jennie IMacdiarmid, A.H.; Alan, D.D. Dietary patterns and non-communicable disease risk in Indian adults: Secondary analysis of Indian Migration Study data. Public Health Nutr. 2017, 20, 1963–1972. [Google Scholar]


| Variable | IOM Males | IOM Females | Female (n = 20) | Male (n = 19) |
|---|---|---|---|---|
| Protein as Percent of Total Kilocalories | 10–35% | 10–35% | 14.1% | 14.0% |
| Carbohydrates as Percent of Total Kilocalories | 45–65% | 45–65% | 54.5% | 56.0% |
| Fat as Percent of Total Kilocalories | 20–35% | 20–35% | 31.0% | 29.3% |
| Total Fiber | 30–38 g | 21–25 g | 16.1 g | 19.0 g |
| Soluble Fiber | NA | NA | 7.3g | 8.6g |
| Insoluble Fiber | NA | NA | 7.4g | 9.5g |
| Sugar | 25% | 25% | 0.76% | 0.87% |
| Monounsaturated Fat | NA | NA | 1.4% | 1.3% |
| Polyunsaturated Fat | NA | NA | 0.77% | 0.69% |
| n-6 (linoleic acid) | 5–10% | 5–10% | NA | NA |
| n-3 (α-linolenic acid) | 0.6–1.2% | 0.6–1.2% | NA | NA |
| Saturated Fat | Minimal | Minimal | 12.7g (0.94%) | 14.8g (0.91%) |
| Trans Fat | Minimal | Minimal | 0.20g (0.01%) | 0.19g (0.01%) |
| Cholesterol | Minimal | Minimal | 68.9g (5.35%) | 106.4g (6.63%) |
| BMR Method | No-Diabetes | Pre-Diabetes | Diabetes |
|---|---|---|---|
| All | (n = 7) | (n = 19) | (n = 13) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Owen | 1435.73 (280.7) | 1335.14 (176.8) | 1545.64 (208.2) |
| Harris-Benedict | 1359.18 (300.2) | 1291.30 (140.0) | 1411.39 (186.9) |
| Mifflin St. Jeor | 1286.67 (307.4) | 1192.23 (193.9) | 1381.08 (203.2) |
| WHO | 1253.27 (177.0) | 1276.27 (106.9) | 1362.19 (116.2) |
| Tanita Scale | 1347.50 (303.0) | 1252.87 (183.2) | 1455.81 (234.7) |
| Females | (n = 3) | (n = 14) | (n = 3) |
| Owen | 1161.72 (53.5) | 1238.49 (52.3) | 1209.09 (72.5) |
| Harris-Benedict | 1188.51 (157.0) | 1238.88 (96.7) | 1198.90 (107.4) |
| Mifflin St. Jeor | 1049.64 (193.2) | 1103.50 (126.8) | 1086.58 (129.0) |
| WHO | 1147.99 (110.2) | 1248.83 (99.2) | 1247.90 (136.7) |
| Tanita Scale | 1101.83 (111.8) | 1164.61 (94.3) | 1094.50 (119.0) |
| Males | (n = 4) | (n = 5) | (n = 10) |
| Owen | 1641.23 (155.7) | 1605.76 (86.8) | 1646.60 (86.9) |
| Harris-Benedict | 1487.18 (.335.9) | 1438.10 (145.7) | 1475.14 (156.3) |
| Mifflin St. Jeor | 1464.45 (256.6) | 1440.68 (110.2) | 1469.43 (117.5) |
| WHO | 1332.23 (187.5) | 1353.08 (96.8) | 1396.47 (90.4) |
| Tanita Scale | 1531.75 (263.9) | 1500.00 (136.1) | 1564.20 (117.1) |
| Variable | DF | Parameter Estimate | Standard Error | t Value | Pr > |t| | Standardized Estimate |
|---|---|---|---|---|---|---|
| Intercept | 1 | 8.24 | 2.74 | 3.01 | 0.01 * | 0.00 |
| Ratio of Actual to Needed Kilocalories from Protein | 1 | 9.68 | 3.75 | 2.58 | 0.02 * | 2.51 |
| Ratio of Actual to Needed Kilocalories from Carbohydrates | 1 | −4.60 | 1.68 | −2.73 | 0.01 * | −1.35 |
| Ratio of Actual to Needed Kilocalories from Fat | 1 | −2.18 | 1.02 | −2.14 | 0.04 * | −0.86 |
| Proportion of Trans Fat (of Total Fat) | 1 | −39.61 | 19.41 | −2.04 | 0.05 | −0.32 |
| Proportion of Cholesterol (of Total Fat) | 1 | −0.19 | 0.10 | −1.91 | 0.07 | −0.33 |
| Proportion of Soluble Fiber (of Total Carbohydrates) | 1 | −61.04 | 29.21 | −2.09 | 0.04 * | −0.75 |
| Proportion of Insoluble Fiber (of Total Carbohydrates) | 1 | 60.82 | 22.42 | 2.71 | 0.01 * | 0.91 |
| Percent Protein (of Total Kilocalories) | 1 | −0.45 | 0.18 | −2.5 | 0.02 * | −1.43 |
| Variable | DF | Parameter Estimate | Standard Error | t Value | Pr > |t| | Standardized Estimate |
|---|---|---|---|---|---|---|
| Intercept | 1 | 14.41 | 3.39 | 4.25 | <<0.01 * | 0 |
| Ratio of Actual to Needed Kilocalories from Protein | 1 | 15.50 | 4.73 | 3.28 | <0.01 * | 2.74 |
| Ratio of Actual to Needed Kilocalories from Carbohydrates | 1 | −6.54 | 2.10 | −3.12 | <0.01 * | −1.31 |
| Ratio of Actual to Needed Kilocalories from Fat | 1 | −3.37 | 1.31 | −2.57 | 0.01 * | −0.90 |
| Proportion of Trans Fat (of Total Fat) | 1 | −61.91 | 25.17 | −2.46 | 0.02 * | −0.34 |
| Proportion of Soluble Fiber (of Total Carbohydrates) | 1 | −57.59 | 34.42 | −1.67 | 0.10 | −0.49 |
| Proportion of Insoluble Fiber (of Total Carbohydrates) | 1 | 75.27 | 27.56 | 2.73 | 0.01 * | 0.77 |
| Percent Protein (of Total Kilocalories) | 1 | −0.64 | 0.23 | −2.75 | 0.01 * | −1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandya, A.; Mehta, M.; Sankavaram, K. The Relationship between Macronutrient Distribution and Type 2 Diabetes in Asian Indians. Nutrients 2021, 13, 4406. https://doi.org/10.3390/nu13124406
Pandya A, Mehta M, Sankavaram K. The Relationship between Macronutrient Distribution and Type 2 Diabetes in Asian Indians. Nutrients. 2021; 13(12):4406. https://doi.org/10.3390/nu13124406
Chicago/Turabian StylePandya, Amisha, Mira Mehta, and Kavitha Sankavaram. 2021. "The Relationship between Macronutrient Distribution and Type 2 Diabetes in Asian Indians" Nutrients 13, no. 12: 4406. https://doi.org/10.3390/nu13124406
APA StylePandya, A., Mehta, M., & Sankavaram, K. (2021). The Relationship between Macronutrient Distribution and Type 2 Diabetes in Asian Indians. Nutrients, 13(12), 4406. https://doi.org/10.3390/nu13124406






