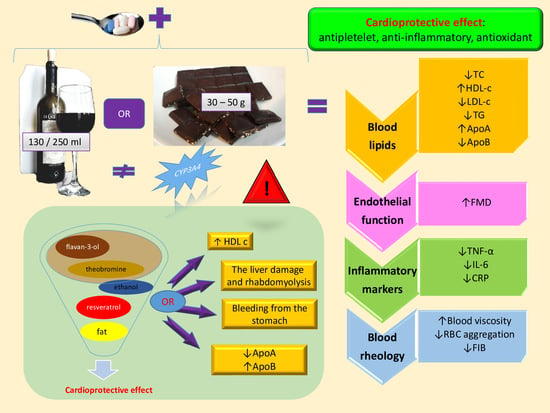
Cardiovascular Effects of Chocolate and Wine—Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Atherosclerosis
3.1. Chocolate
3.1.1. Cardioprotective Properties of Chocolate
Epidemiologic Studies
Clinical Trials
3.2. Wine
3.2.1. Phytochemical Structure of Wine
3.2.2. Cardioprotective Effects of Wine
Epidemiological Studies
Clinical Trials
4. Food–Drug Interaction
- (1)
- a statin as the drug of choice to improve prognosis in individuals with TG >200 mg/dL;
- (2)
- patients with TG 135–499 mg/dL—irrespective of statins, administration of omega-3 fatty acids in a dose of 2 × 2 g/day is recommended;
- (3)
- in primary prevention, reasonable LDL-cholesterol control TG >200 mg/dL fenofibrate or benzafibrate, together with statins;
- (4)
- high cardiovascular risk groups, with reasonable LDL-cholesterol control with TG > 200 mg/dL, fenofibrate or benzafibrate together with a statin [116].
4.1. Chocolate–Drug Interaction
4.2. Wine–Drug Interaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borén, J.; John Chapman, M.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teissedre, P.L.; Stockley, C.; Boban, M.; Gambert, P.; Alba, M.O.; Flesh, M.; Ruf, J.C. The effects of wine consumption on cardiovascular disease and associated risk factors: A narrative review. Oeno One 2018, 50, 67–79. [Google Scholar] [CrossRef]
- Bittner, V. The New 2019 AHA/ACC Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2020, 2402–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokose, C.; McCormick, N.; Rai, S.K.; Lu, N.; Curhan, G.; Schwarzfuchs, D.; Shai, I.; Choi, H.K. Effects of low-fat, mediterranean, or low-carbohydrate weight loss diets on serum urate and cardiometabolic risk factors: A secondary analysis of the dietary intervention randomized controlled trial (direct). Diabetes Care 2020, 43, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Martínez-González, M.A.; Barbería-Latasa, M.; Bes-Rastrollo, M.; Gea, A. Mediterranean diet, alcohol-drinking pattern and their combined effect on all-cause mortality: The Seguimiento Universidad de Navarra (SUN) cohort. Eur. J. Nutr. 2021, 60, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J.; Guercio, V.; Tavani, A. The Mediterranean Diet and Cardiovascular Disease: Gaps in the Evidence and Research Challenges. Cardiol. Rev. 2019, 27, 127–130. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayón-Orea, C.; Razquin, C.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; Martínez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients With Metabolic Syndrome Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA 2019, 322, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Bonsignore, G.; Patrone, M.; Ranzato, E. Mediterranean Diet Polyphenols: Anthocyanins and their Implications for Health. Mini-Rev. Med. Chem. 2021, 21, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Bazal, P.; Gea, A.; Martínez-González, M.A.; Salas-Salvadó, J.; Asensio, E.M.; Muñoz-Bravo, C.; Fiol, M.; Muñoz, M.A.; Lapetra, J.; Serra-Majem, L.L.; et al. Mediterranean alcohol-drinking pattern, low to moderate alcohol intake and risk of atrial fibrillation in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: Cardiometabolic benefits. Endocrine 2017, 56, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, M.; Odegaard, A.O.; Schmidt, M.I.; Duncan, B.B.; de Oliveira Cardoso, L.; Matos, S.M.; Maria del Carmen, B.M.; Barreto, S.M.; Pereira, M.A. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Navarro, J.C.; Oki, A.M.; Antoniazzi, L.; Bonfim, M.A.C.; Hong, V.; De Almeida Gaspar, M.C.; Sandrim, V.C.; Nogueira, A.; Aparicio, H.J.; Benjamin, E.J.; et al. Consumption of animal-based and processed food associated with cardiovascular risk factors and subclinical atherosclerosis biomarkers in men. Rev. Assoc. Med. Bras. 2019, 65, 43–50. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s phenolic compounds and health: A pythagorean view. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Zatońska, K.; Psikus, P.; Basiak-Rasała, A.; Stępnicka, Z.; Wołyniec, M.; Wojtyła, A.; Szuba, A.; Połtyn-Zaradna, K. Patterns of alcohol consumption in the pure poland cohort study and their relationship with health problems. Int. J. Environ. Res. Public Health 2021, 18, 4185. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; O’Neill, D.; Bell, S.; Stamatakis, E.; Britton, A. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease: Original data and meta-analysis of 48,423 men and women. BMC Med. 2021, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef]
- Ho, Y.-L.; Nguyen, X.-M.T.; Yan, J.Q.; Vassy, J.L.; Gagnon, D.R.; Gaziano, J.M.; Wilson, P.W.; Cho, K.; Djoussé, L. Chocolate consumption and risk of coronary artery disease: The Million Veteran Program on behalf of the VA Million Veteran Program. Am. J. Clin. Nutr. 2021, 113, 1137–1144. [Google Scholar] [PubMed]
- Garcia, J.P.; Santana, A.; Baruqui, D.L.; Suraci, N. The cardiovascular effects of chocolate. Rev. Cardiovasc. Med. 2018, 19, 123–127. [Google Scholar] [CrossRef]
- Gammone, M.A.; Efthymakis, K.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Riccioni, G.; Orazio, N.D. Impact of chocolate on the cardiovascular health. Front. Biosci. 2018, 23, 852–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, J.M.; Libman, R.B.; Wang, J.J.; Sanelli, P.; Filippi, C.G.; Gribko, M.; Pacia, S.V.; Kuzniecky, R.I.; Najjar, S.; Azhar, S. Cerebrovascular Complications of COVID-19. Stroke 2020, 51, e227–e231. [Google Scholar] [CrossRef]
- Chang, W.-T.; Toh, H.S.; Liao, C.-T.; Yu, W.-L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 2019, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Cheruiyot, I.; Aggarwal, S.; Wong, J.; Lippi, G.; Lavie, C.J.; Henry, B.M.; Sanchis-Gomar, F. Association of Cardiovascular Disease With Coronavirus Disease 2019 (COVID-19) Severity: A Meta-Analysis. Curr. Probl. Cardiol. 2020, 45, 100617. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, A.; Georgiadou, E.; Reinhard, I.; Müller, A.; Lemenager, T.; Kiefer, F.; Hillemacher, T. The Effects of the Lockdown during the COVID-19 Pandemic on Alcohol and Tobacco Consumption Behavior in Germany. Eur. Addict. Res. 2021, 27, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Saguner, A.M.; An, J.; Ning, Y.; Day, J.D.; Ding, L.; Waintraub, X.; Wang, J. Cardiovascular disease during the covid-19 pandemic: Think ahead, protect hearts, reduce mortality. Cardiol. J. 2020, 27, 616–624. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Visioli, F. Covid-19 and the subsequent lockdown modified dietary habits of almost half the population in an Italian sample. Foods 2020, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ratnasabapathya, R.; Gardiner, J. Carbohydrate Craving- not everything is sweet. Curr. Opin. Clin. Nutr. Metab. 2018, 20, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Y.; Sun, X.Z.; Wang, B.Y.; Zhao, Y.; Liu, D.C.; Zhang, D.D.; Liu, X.J.; Zhang, R.Y.; Sun, H.H.; et al. Chocolate consumption and risk of cardiovascular diseases: A meta-Analysis of prospective studies. Heart 2019, 105, 49–55. [Google Scholar] [CrossRef]
- Morze, J.; Schwedhelm, C.; Bencic, A.; Hoffmann, G.; Boeing, H.; Przybylowicz, K.; Schwingshackl, L. Chocolate and risk of chronic disease: A systematic review and dose-response meta-analysis. Eur. J. Nutr. 2020, 59, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.Y.C.; Lim, X.Y.; Yeo, J.H.H.; Lee, S.W.H.; Lai, N.M. The Health Effects of Chocolate and Cocoa: A Systematic Review. Nutrients 2021, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Jessen, G.; Momi, S.; Höltje, H.D.; Gresele, P.; Cerletti, C.; De Gaetano, G. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level: Functional and modelling studies. Thromb. Haemost. 2009, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Alakhali, K.M.; Vigneshwaran, E.; Shaik, M.A.A. Effect of food and antacid on simvastatin bioavailability on healthy adult volunteers. J. Health Res. Rev. 2018, 5, 26–32. [Google Scholar] [CrossRef]
- Zubair, M.H.; Zubair, M.H.; Zubair, M.N.; Zubair, M.M.; Aftab, T.; Asad, F. Augmentation of anti-platelet effects of aspirin by chocolate. J. Pak. Med. Assoc. 2011, 61, 304–307. [Google Scholar]
- Collyer, T.C.; Gray, D.J.; Sandhu, R.; Berridge, J.; Lyons, G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography® Platelet MappingTM. Br. J. Anaesth. 2009, 102, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions—A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 600–609. [Google Scholar]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, I.; Li, A.; Manson, J.A.E.; Sesso, H.D.; Wang, L.; Liu, S. Cocoa flavanol intake and biomarkers for cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2016, 146, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folsom, A.R.; Chambless, L.E.; Ballantyne, C.M.; Coresh, J.; Heiss, G.; Wu, K.K.; Boerwinkle, E.; Mosley, T.H.; Sorlie, P.; Diao, G.; et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: The atherosclerosis risk in communities study. Arch. Intern. Med. 2006, 166, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodondi, N.; Marques-Vidal, P.; Butler, J.; Sutton-Tyrrell, K.; Cornuz, J.; Satterfield, S.; Harris, T.; Bauer, D.C.; Ferrucci, L.; Vittinghoff, E.; et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am. J. Epidemiol. 2010, 171, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Hear. Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Agell, M.; Sacanella, E.; Tobias, E.; Monagas, M.; Antúnez, E.; Zamora-Ros, R.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Fernández-Solá, J.; Nicolás, J.M.; et al. Inflammatory markers of atherosclerosis are decreased after moderate consumption of cava (sparkling wine) in men with low cardiovascular risk. J. Nutr. 2007, 137, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.S. The relationships between alcohol, wine and cardiovascular diseases—A review. Nutr. Aging 2016, 3, 55–88. [Google Scholar] [CrossRef] [Green Version]
- Roth, I.; Casas, R.; Ribó-Coll, M.; Doménech, M.; Lamuela-Raventós, R.M.; Estruch, R. Acute consumption of Andalusian aged wine and gin decreases the expression of genes related to atherosclerosis in men with high cardiovascular risk: Randomized intervention trial. Clin. Nutr. 2019, 38, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Teixeira, A.M.N.; Luzia, L.A.; de Souza, S.J.; de Almeida Petrilli, A.; De Moraes Pontilho, P.; de Souza, J.M.P.; Segurado, A.A.C.; Efraim, P.; De Melo Picone, C.; De Carvalho Rondo, P.H.; et al. The impact of dark chocolate intake on arterial elasticity in individuals with HIV/AIDS undergoing ART: A randomized, double-blind, crossover trial. Food Funct. 2017, 8, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Halib, H.; Ismail, A.; Mohd Yusof, B.N.; Osakabe, N.; Daud, Z.A.M. Effects of cocoa polyphenols and dark chocolate on obese adults: A scoping review. Nutrients 2020, 12, 3695. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillén, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial (American Journal of Clinical Nutrition (2012) 95, (326–334)). Am. J. Clin. Nutr. 2012, 95, 1506. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Estruch, R.; Sacanella, E. Influence of bioactive nutrients on the atherosclerotic process: A review. Nutrients 2018, 10, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debreceni, B.; Debreceni, L. Role of vitamins in cardiovascular health and disease. Res. Reports Clin. Cardiol. 2014, 5, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Imhof, A.; Blagieva, R.; Marx, N.; Koenig, W. Drinking modulates monocyte migration in healthy subjects: A randomised intervention study of water, ethanol, red wine and beer with or without alcohol. Diabetes Vasc. Dis. Res. 2008, 5, 48–53. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of fruits and vegetables with major cardiometabolic risk factors, markers of oxidation, and inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, P.J.; Dhatariya, K.; Sampson, M.; Kroon, P.A.; Potter, J.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostertag, L.M.; Philo, M.; Colquhoun, I.J.; Tapp, H.S.; Saha, S.; Duthie, G.G.; Kemsley, E.K.; De Roos, B.; Kroon, P.A.; Le Gall, G. Acute Consumption of Flavan-3-ol-Enriched Dark Chocolate Affects Human Endogenous Metabolism. J. Proteome Res. 2017, 16, 2516–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, potential therapeutic interest in joint disorders: A critical narrative review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, C.S.; Loke, Y.K.; Welch, A.A.; Luben, R.N.; Lentjes, M.A.H.; Boekholdt, S.M.; Pfister, R.; Mamas, M.A.; Wareham, N.J.; Khaw, K.T.; et al. Habitual chocolate consumption and the risk of incident heart failure among healthy men and women. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 722–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Demurtas, J.; Celotto, S.; Caruso, M.G.; Maggi, S.; Bolzetta, F.; Firth, J.; Smith, L.; Schofield, P.; Koyanagi, A.; et al. Is chocolate consumption associated with health outcomes? An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2019, 38, 1101–1108. [Google Scholar] [CrossRef]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-response relationship between cocoa flavanols and human endothelial function: A systematic review and meta-analysis of randomized trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, D.; Rosi, A.; Tassotti, M.; Antonini, M.; Dall’Asta, M.; Bresciani, L.; Fantuzzi, F.; Spigoni, V.; Domínguez-Perles, R.; Angelino, D.; et al. Effect of coffee and cocoa-based confectionery containing coffee on markers of cardiometabolic health: Results from the pocket-4-life project. Eur. J. Nutr. 2021, 60, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Mangels, D.R.; Mohler, E.R. Catechins as potential mediators of cardiovascular health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Regecova, V.; Jurkovicova, J.; Babjakova, J.; Bernatova, I. The Effect of a Single Dose of Dark Chocolate on Cardiovascular Parameters and Their Reactivity to Mental Stress. J. Am. Coll. Nutr. 2020, 39, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Salvatori, T.; Nucci, D.; Villarini, M.; Moretti, M. Can chocolate consumption reduce cardio-cerebrovascular risk? A systematic review and meta-analysis. Nutrition 2018, 46, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, M.; Samoggia, A. Chocolate consumption and purchasing behaviour review: Research issues and insights for future research. Sustainability 2020, 12, 5586. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Wang, S.; Zhu, F. Chemical and biological properties of cocoa beans affected by processing: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Corbi, G.; Righetti, S.; Sears, B.; Olarte, H.H.; Grassi, D.; Scapagnini, G. Cardioprotection by cocoa polyphenols and ω-3 fatty acids: A disease-prevention perspective on aging-associated cardiovascular risk. J. Med. Food 2018, 21, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, “food of the gods”: History, science, and human health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Food Composition Tables; PZWL: Warsaw, Poland, 2020. [Google Scholar]
- MELOCW; Bandeira, M.D.; Maciel, L.F.; Bispo, E.D.; SOUZACO; Soares, S.E. Chemical composition and fatty acids profile of chocolates produced with different cocoa (Theobroma cacao L.) cultivars. Food Sci. Technol. 2020, 40, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Kühn, J.; Schröter, A.; Hartmann, B.M.; Stangl, G.I. Cocoa and chocolate are sources of vitamin D 2. Food Chem. 2018, 269, 318–320. [Google Scholar] [CrossRef]
- Okamoto, T.; Kobayashi, R.; Natsume, M.; Nakazato, K. Habitual cocoa intake reduces arterial stiffness in postmenopausal women regardless of intake frequency: A randomized parallel-group study. Clin. Interv. Aging 2016, 11, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.P.J.J.; Mensink, R.P.; Smolders, L.; Bakeroot, V.; Plat, J. Theobromine Does Not Affect Fasting and Postprandial HDL Cholesterol Efflux Capacity, While It Decreases Fasting miR-92a Levels in Humans. Mol. Nutr. Food Res. 2018, 62, 1800027. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Danese, E.; Salvagno, G.L.; Lippi, G. Short-term effect of dark chocolate consumption on routine haemostasis testing. Int. J. Food Sci. Nutr. 2017, 68, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Dong, J.Y.; Iso, H.; Yamagishi, K.; Sawada, N.; Tsugane, S. Chocolate consumption and risk of stroke among men and women: A large population-based, prospective cohort study. Atherosclerosis 2017, 260, 8–12. [Google Scholar] [CrossRef]
- Latif, R.; Majeed, F. Association between chocolate consumption frequency and heart rate variability indices. Explore 2020, 16, 372–375. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Erne, P.; Zimmermann, B.; Chrubasik-Hausmann, S. The Impact of Cocoa Flavanols on Cardiovascular Health. Phyther. Res. 2016, 30, 1641–1657. [Google Scholar] [CrossRef]
- Steinhaus, D.A.; Mostofsky, E.; Levitan, E.B.; Dorans, K.S.; Håkansson, N.; Wolk, A.; Mittleman, M.A. Chocolate intake and incidence of heart failure: Findings from the Cohort of Swedish Men. Am. Heart J. 2017, 183, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Mostofsky, E.; Berg Johansen, M.; Tjønneland, A.; Chahal, H.S.; Mittleman, M.A.; Overvad, K. Chocolate intake and risk of clinically apparent atrial fibrillation: The Danish Diet, Cancer, and Health Study. Heart 2017, 103, 1163–1167. [Google Scholar] [CrossRef]
- Larsson, S.C.; Drca, N.; Jensen-Urstad, M.; Wolk, A. Chocolate consumption and risk of atrial fibrillation: Two cohort studies and a meta-analysis. Am. Heart J. 2018, 195, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Smith, L.; Armstrong, N.C.; Yakkundi, A.; Barnett, Y.; Butler, L.; McDermott, D.T.; Koyanagi, A.; Shin, J., II; Meyer, J.; et al. Alcohol use and mental health during COVID-19 lockdown: A cross-sectional study in a sample of UK adults. Drug Alcohol Depend. 2021, 219, 108488. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Fulgoni, V.L.; Nicklas, T.A. Candy consumption was not associated with body weight measures, risk factors for cardiovascular disease, or metabolic syndrome in US adults: NHANES 1999–2004. Nutr. Res. 2011, 31, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Koppensteiner, R.; Steiner, S.; Niessner, A.; Goliasch, G.; Gschwandtner, M.; Hoke, M. Dark chocolate and vascular function in patients with peripheral artery disease: A randomized, controlled cross-over trial. Clin. Hemorheol. Microcirc. 2015, 59, 145–153. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Dearborn, P.; Robbins, M. Habitual chocolate intake and type 2 diabetes mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective observations. Appetite 2017, 108, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Chareonrungrueangchai, K.; Wongkawinwoot, K.; Anothaisintawee, T.; Reutrakul, S. Dietary factors and risks of cardiovascular diseases: An umbrella review. Nutrients 2020, 12, 1088. [Google Scholar] [CrossRef] [Green Version]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Li, X.; Jin, Y.; Lu, J. Chocolate consumption and risk of coronary heart disease, stroke, and diabetes: A meta-analysis of prospective studies. Nutrients 2017, 9, 688. [Google Scholar] [CrossRef] [Green Version]
- Mellor, D.D.; Georgousopoulou, E.N.; Naumovski, N. Cocoa and chocolate, their clinical benefits: Insights in study design. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2017, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Vieira Humia, B.; Santos, K.S.; Mendonça Barbosa, A.; Sawata, M.; Da Costa Mendonça, M.; Ferreira Padilha, F.; Cosmi, F.; Di Giulio, P.; Masson, S.; Finzi, A.; et al. The relationships between alcohol, wine and cardiovascular diseases—A review. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 55–88. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: A prospective cohort study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A.S. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Kuebler, U.; Arpagaus, A.; Meister, R.E.; von Känel, R.; Huber, S.; Ehlert, U.; Wirtz, P.H. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: A randomized controlled trial. Brain. Behav. Immun. 2016, 57, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C.H. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football athletes: A randomized controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef]
- Lee, Y.; Berryman, C.E.; West, S.G.; Chen, C.Y.O.; Blumberg, J.B.; Lapsley, K.G.; Preston, A.G.; Fleming, J.A.; Kris-Etherton, P.M.; Kris-Etherton, P.M.; et al. Effects of Dark Chocolate and Almonds on Cardiovascular Risk Factors in Overweight and Obese Individuals: A Randomized Controlled-Feeding Trial. J. Am. Heart Assoc. 2017, 6, e005162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, T.; Bergqvist, J.; Vieira, C.; Grüner Sveälv, B.; Castanheira, J.; Conde, J. Randomized study of the effects of cocoa-rich chocolate on the ventricle–arterial coupling and vascular function of young, healthy adults. Nutrition 2019, 63–64, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Barrios, M.; Orozco, L.C.; Stashenko, E.E. Cocoa ingestion protects plasma lipids in healthy males against ex vivo oxidative conditions: A randomized clinical trial. Clin. Nutr. ESPEN 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Loffredo, L.; Baratta, F.; Ludovica, P.; Battaglia, S.; Carnevale, R.; Nocella, C.; Novo, M.; Pannitteri, G.; Ceci, F.; Angelico, F.; et al. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 143–149. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Quifer-Rada, P.; de Alvarenga, J.F.R.; Pérez-Fernández, S.; Tresserra-Rimbau, A.; Lamuela-Raventos, R.M. Changing to a low-polyphenol diet alters vascular biomarkers in healthy men after only two weeks. Nutrients 2018, 10, 1766. [Google Scholar] [CrossRef] [Green Version]
- Bertolami, A.; Botelho, P.B.; Macedo, L.F.L.; Abdalla, D.S.P.; Faludi, A.A.; Galasso, M.; Castro, I. Pleiotropic effects of plant sterols compared with ezetimibe on atherosclerosis biomarkers in type 2 diabetic patients treated with statins. Atherosclerosis 2014, 235, e74. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, S.; Alexander, B.; Santi, R.L.; Liprandi, A.S.; Baranchuk, A. What’s in wine? A clinician’s perspective. Trends Cardiovasc. Med. 2019, 29, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Haseeb, S.; Aquistapache, F.; Grosso, P.; Alexander, B.; Hopman, W.; Santi, R.L.; Baranchuk, A. Alcohol consumption and cardiovascular health: A nationwide survey of Uruguayan cardiologists. Alcohol 2019, 79, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or beer? Comparison, changes and improvement of polyphenolic compounds during technological phases. Molecules 2020, 25, 4960. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. OIV State of the World Vitivinicultural Sector in 2019; International Organisation of Vine and Wine: Paris, France, 2020; pp. 1–15. [Google Scholar]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Fuhrmann, J.C.; Van Dorsten, F.A.; Rein, D.; Peters, S.; Van Velzen, E.J.J.; Hollebrands, B.; Draijer, R.; Van Duynhoven, J.; Garczarek, U. Impact of short-term intake of red wine and grape polyphenol extract on the human metabolome. J. Agric. Food Chem. 2012, 60, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Fernandez, M.L. The Relevance of Dietary Polyphenols in Cardiovascular Protection. Curr. Pharm. Des. 2017, 23, 244–2452. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.L.; Rooney, C.; McCall, D.; Young, I.S.; McCance, D.; McKinley, M.C.; Woodside, J.V.; McKeown, P.P. Beneficial effect of a polyphenol-rich diet on cardiovascular risk: A randomised control trial. Heart 2016, 102, 1371–1379. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.P.; Oliveira, A.C.; Mendes-Junior, L.G.; França, K.C.; Nakao, L.S.; Schini-Kerth, V.B.; Medeiros, I.A. Cardiovascular effects induced by northeastern Brazilian red wine: Role of nitric oxide and redox sensitive pathways. J. Funct. Foods 2016, 22, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [Green Version]
- Bennemann, G.D.; de Assis, C.F.; Moreira, G.C.R.C.; de Lima, L.H.; Carvalho, K.K.; Torres, Y.R.; Botelho, R.V. Mineral analysis, anthocyanins and phenolic compounds in wine residues flour. BIO Web Conf. 2016, 7, 04007. [Google Scholar] [CrossRef] [Green Version]
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; De La Torre, R. Generation of the antioxidant hydroxytyrosol from tyrosol present in beer and red wine in a randomized clinical trial. Nutrients 2019, 11, 2241. [Google Scholar] [CrossRef] [Green Version]
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Badea, M.; Colombo, F.; Orgiu, F.; Frigerio, G.; Pastor, R.F.; Restani, P. Antioxidant activity of wine assessed by different in vitro methods. BIO Web Conf. 2017, 9, 4008. [Google Scholar] [CrossRef]
- Tverdal, A.; Magnus, P.; Selmer, R.; Thelle, D. Consumption of alcohol and cardiovascular disease mortality: A 16 year follow-up of 115,592 Norwegian men and women aged 40–44 years. Eur. J. Epidemiol. 2017, 32, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, X.; Bao, L.; Tarantino, G. Effects of wine on blood pressure, glucose parameters, and lipid profile in type 2 diabetes mellitus: A meta-analysis of randomized interventional trials (PRISMA Compliant). Medicine 2019, 98, e15771. [Google Scholar] [CrossRef]
- Révész, D.; Bours, M.J.L.; Wegdam, J.A.; Keulen, E.T.P.; Breukink, S.O.; Slooter, G.D.; Vogelaar, F.J.; Weijenberg, M.P.; Mols, F. Associations between alcohol consumption and anxiety, depression, and health-related quality of life in colorectal cancer survivors. J. Cancer Surviv. 2021, 1–10. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V.; Santos-Buelga, C.; Feliciano, A.S. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Leeuwenburgh, C.; Guralnik, J.M.; Tian, L.; Sufit, R.; Zhao, L.; Criqui, M.H.; Kibbe, M.R.; Stein, J.H.; Lloyd-Jones, D.; et al. Effect of resveratrol onwalking performance in older people with peripheral artery disease the restore randomized clinical trial. JAMA Cardiol. 2017, 2, 902–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism. 2018, 83, 102–119. [Google Scholar] [CrossRef]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and beer within a moderate alcohol intake is associated with higher levels of HDL-c and adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. Advice on lifestyle changes (Diet, Red Wine and Physical Activity) does not affect internal carotid and middle cerebral artery blood flow velocity in patients with carotid arteriosclerosis in a randomized controlled trial. Cerebrovasc. Dis. 2014, 37, 368–375. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brncic, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadeniz, M.; Akçay, Y.D.; Yildirim, H.K.; Yilmaz, C.; Sözmen, E.Y. Effect of red wine consumption on serum oxidation and adiponectin levels in overweight and healthy individuals. Pol. J. Food Nutr. Sci. 2014, 64, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; Rago, L.; De Curtis, A.; Persichillo, M.; Bracone, F.; Olivieri, M.; Cerletti, C.; Donati, M.B.; et al. Moderate Alcohol Consumption Is Associated With Lower Risk for Heart Failure But Not Atrial Fibrillation. JACC Hear. Fail. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Da Luz, P.L.; Favarato, D.; Moriguchi, E.H.; De Carli, W.; Bruscato, N.; Mochiduky, R.I.; Schwartzman, P.; Rochitte, C.E.; Laurindo, F.R. Red wine consumption, coronary calcification, and long-term clinical evolution. Braz. J. Med. Biol. Res. 2018, 51, 1–5. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Kouli, G.M.; Magriplis, E.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Beer, wine consumption, and 10-year CVD incidence: The ATTICA study. Eur. J. Clin. Nutr. 2019, 73, 1015–1023. [Google Scholar] [CrossRef]
- Park, H.; Kim, K. Association of alcohol consumption with lipid profile in hypertensive men. Alcohol Alcohol. 2012, 47, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Attard, R.; Dingli, P.; Doggen, C.J.M.M.; Cassar, K.; Farrugia, R.; Bezzina Wettinger, S. The impact of frequency, pattern, intensity, and type of alcohol consumption, and its combined effect with smoking on inflammation, lipid profile, and the risk of myocardial infarction. J. Public Health 2021, 29, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Ruf, J.C. Alcohol, wine and platelet function. Biol. Res. 2004, 37, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Levantesi, G.; Marfisi, R.; Mozaffarian, D.; Franzosi, M.G.; Maggioni, A.; Nicolosi, G.L.; Schweiger, C.; Silletta, M.; Tavazzi, L.; Tognoni, G.; et al. Wine consumption and risk of cardiovascular events after myocardial infarction: Results from the GISSI-Prevenzione trial. Int. J. Cardiol. 2013, 163, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.D.; McQueenie, R.; Nicholl, B.I.; Field, R.; Hanlon, P.; Gallacher, K.I.; Mair, F.S.; Lewsey, J. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: A prospective cohort study. BMC Med. 2021, 19, 8. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: A 2-year randomized, controlled trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: Results from a randomized controlled trial. Nutr. J. 2013, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; McElduff, P.; Linneberg, A.; Salomaa, V.; Männistö, S.; Moitry, M.; Ferrières, J.; Dallongeville, J.; et al. Alcohol Intake and Total Mortality in 142,960 Individuals from the MORGAM Project: A population-based study. Addiction 2021. [Google Scholar] [CrossRef]
- De La Torre, R.; Corella, D.; Castañer, O.; Martínez-González, M.A.; Salas-Salvador, J.; Vila, J.; Estruch, R.; Sorli, J.V.; Arós, F.; Fiol, M.; et al. Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: Virgin olive oil, wine, and catechol-methylathion. Am. J. Clin. Nutr. 2017, 105, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, Ö.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; Skala, T. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Taborsky. Bratislava Med. J. 2017, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Chen, Y.H.; Tsai, H.Y.; Chen, J.S.; Wu, T.C.; Lin, F.Y.; Sata, M.; Chen, J.W.; Lin, S.J. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef]
- Do Rosario, V.A.; Schoenaker, D.A.J.M.J.M.; Kent, K.; Weston-Green, K.; Charlton, K. Association between flavonoid intake and risk of hypertension in two cohorts of Australian women: A longitudinal study. Eur. J. Nutr. 2020, 60, 2507–2519. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Vanden Daele, L.; Van de Voorde, J. Protective effect of resveratrol and quercetin on in vitro-induced diabetic mouse corpus cavernosum. Cardiovasc. Diabetol. 2016, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Carnevale, R.; Pignatelli, P.; Pastori, D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxidants Redox Signal. 2017, 27, 1083–1124. [Google Scholar] [CrossRef] [PubMed]
- Guilford, J.M.; Pezzuto, J.M. Wine and health: A review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Wotherspoon, A.; Elshahat, S.; McAlinden, N.; Dean, K.; Young, I.S.; Sharpe, P.C.; Blankenburg, S.; Patterson, C.C.; McKinley, M.C.; Evans, A.; et al. Effect of Moderate Red Wine versus Vodka Consumption on Inflammatory Markers Related to Cardiovascular Disease Risk: A Randomized Crossover Study. J. Am. Coll. Nutr. 2020, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Cioccoloni, G.; Salimei, P.S.; Ceravolo, I.; De Lorenzo, A.; Gratteri, S. Alcoholic beverage and meal choices for the prevention of noncommunicable diseases: A randomized nutrigenomic trial. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health—New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Zilkens, R.R.; Hodgson, J.M.; Beilin, L.J.; Puddey, I.B. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: A randomized intervention. J. Hypertens. 2016, 34, 421–428. [Google Scholar] [CrossRef]
- Tousoulis, D.; Ntarladimas, I.; Antoniades, C.; Vasiliadou, C.; Tentolouris, C.; Papageorgiou, N.; Latsios, G.; Stefanadis, C. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 2008, 27, 594–600. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Bae, J.-S.; Oh, A.-R.; Cha, J.-Y. Regulation of Cholesterol Metabolism in Liver: Link to NAFLD and Impact of n-3 PUFAs. J. Lifestyle Med. 2013, 3, 19–25. [Google Scholar]
- Wang, H.; Blumberg, J.B.; Chen, C.Y.O.; Choi, S.W.; Corcoran, M.P.; Harris, S.S.; Jacques, P.F.; Kristo, A.S.; Lai, C.Q.; Lamon-Fava, S.; et al. Dietary modulators of statin efficacy in cardiovascular disease and cognition. Mol. Aspects Med. 2014, 38, 1–53. [Google Scholar] [CrossRef]
- Antal, E.J.; Hendershot, P.E.; Batts, D.H. Linezolid, a novel oxazolidinone antibiotic: Assessment of monoamine oxidase inhibition using pressor response to oral tyramine. J. Clin. Pharmacol. 2001, 41, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, K.A.; Wattengel, B.A.; Skelly, M.K.; Clark, C.M.; Russo, T.A. Fact versus fiction: A review of the evidence behind alcohol and antibiotic interactions. Antimicrob. Agents Chemother. 2020, 64, 1–17. [Google Scholar] [CrossRef]
- Flanagan, S.; Bartizal, K.; Minassian, S.L.; Fang, E.; Prokocimer, P. In vitro, In Vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob. Agents Chemother. 2013, 57, 3060–3066. [Google Scholar] [CrossRef] [Green Version]
- Scolaro, B.; Nogueira, M.S.; Paiva, A.; Bertolami, A.; Barroso, L.P.; Vaisar, T.; Heffron, S.P.; Fisher, E.A.; Castro, I.A. Statin dose reduction with complementary diet therapy: A pilot study of personalized medicine. Mol. Metab. 2018, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, J.; Pazur, A.; Zachwieja, Z. Statyny i ich interakcje z pożywieniem. Bromat. Chem. Toksykol. 2008, 41, 1023–1029. [Google Scholar]
- Smith, H.T.; Jokubaitis, L.A.; Troendle, A.J.; Hwang, D.S.; Robinson, W.T. Pharmacokinetics of fluvastatin and specific drug interactions. Am. J. Hypertens. 1993, 6, 375–382. [Google Scholar] [CrossRef]
- Won, C.; Oberlies, N.; Paine, M. Mechanisms Underlying Food-Drug Interactions: Inhibition of Intestinal Metabolism and Transport Christina. Pharmacol. Ther. 2012, 13, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Hyrsova, L.; Vanduchova, A.; Dusek, J.; Smutny, T.; Carazo, A.; Maresova, V.; Trejtnar, F.; Barta, P.; Anzenbacher, P.; Dvorak, Z.; et al. Trans-resveratrol, but not other natural stilbenes occurring in food, carries the risk of drug-food interaction via inhibition of cytochrome P450 enzymes or interaction with xenosensor receptors. Toxicol. Lett. 2019, 300, 81–91. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’osso, B.; Galentino, R.; De Michele, S.; Dina, C.Z.; Porta, M.; et al. Food bioactive compounds and their interference in drug pharmacokinetic/pharmacodynamic profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Chemical Compounds | Food Products | |
|---|---|---|
| Dark Chocolate | Red Wine | |
| Water (g) | 0.6 | 89.9 |
| Protein (g) | 6.7 | 0.1 |
| Lipid (g) | 34.3 | 0 |
| Cholesterol (mg) | 0 | 0 |
| Carbohydrate (g) | 56.6 | 0.2 |
| Sugar (g) | 38.3 | 0.2 |
| Total fiber (g) | 1.7 | 1.7 |
| Sodium (µg) | 4000 | 7000 |
| Potassium (µg) | 581,000 | 110,000 |
| Iron (µg) | 21,000 | 1000 |
| Calcium (µg) | 42,000 | 7000 |
| Phosphorum (µg) | 244,000 | 13,000 |
| Thiamin (µg) | 40 | 0 |
| Riboflavin (µg) | 10 | 10 |
| Niacin (µg) | 46 | 46 |
| Vitamin A (µg) | 0 | 0 |
| Energy (kcal/kJ) | 593/2330 | 68/284 |
| Parameter | Chocolate | Wine | ||
|---|---|---|---|---|
| [mg/kg] | [mg/L] | |||
| Total polyphenols ng/GAE/L | 11.7–14.8 | 860.2–2912.0 | ||
| Dihydroflavonols | - | Dihydromyricetin 3-O-rhamnoside 45.0 | ||
| Flavanols | (+)-Catechin (−)-Epicatechin (−)-Epicatechin-(2a-7)(4a-8)- picatechin 3-O-galactoside Cinnamtannin A2 Procyanidin dimer B2 Procyanidin trimer C1 | 107.0–500.0 32.74–125.0 40.0–80.0 290.0–860.0 210.0–540.0 130.0–440.0 | (+)-Catechin (+)-Gallocatechin (−) Epicatechin (−)-Epicatechin 3-O-gallate (−)-Epigallocatechin | 14.0–390.0 0–4.0 0–165.0 0–9.0 6.0 |
| Flavanones | - | Hesperetin Naringenin Naringin | 0.5–0.6 0.4–0.7 7.0–8.0 | |
| Flavonols | Quercetin | 250.0 | Kaempferol Kaempferol 3-O-glucoside Myricetin Quercetin Quercetin 3-O-arabinoside Quercetin 3-O-glucoside Quercetin 3-O-rhamnoside Quercetin 3-O-rutinoside | 0–3.6 6–11.0 0–18.0 0–32.0 4.0–5.0 8.0–23.0 0–18.0 0–32.0 |
| Hydroxybenzoic acids | - | Gallic acid Gallic acid ethyl ester | 0–126.0 14.0–17.0 | |
| Hydroxycinnamic acids | Ferulic acid | 240.0 | 2,5-di-S-Glutathionyl caftaric acid Caffeoyl tartaric acid Caffeic acid Ferulic acid | 11.0–47.0 0 0–77.0 0–10.0 |
| Stilbenes | Resveratrol Resveratrol 3-O-glucoside | 0.4 1.0 | Resveratrol Resveratrol 3-O-glucoside | 0.4 75.0–964.0 |
| Tyrosols | - | Tyrosol Hydroxytyrosol | 5.0 6.0 | |
| Total anthocyanogens | 2.8–4.1 | 21.0–1011.0 | ||
| Antioxidant activity mmol TE/L | 151.7–246.0 | 6.9–15.2 | ||
| Main group | flavan-3-ol | stilbenes | ||
| Investigation | Population | Outcomes | Ref. |
|---|---|---|---|
| FQQ | men & women 32,486/40,009 | I. AF 9978 participants II. CR vs. NCR↑AF ≥34 srv/week 0.96 (95% CI 0.88–1.04) III. HF AF: 0.97 (95% CI 0.94–1.01): 2 srv/week ↑ DCC 0.96 (95% CI 0.90–1.03)—HC vs. LC | [92] |
| FFQ | 55,502 | I. 1–3 srv/month (HR 0.90, 95% CI 0.82 to 0.98) II. 1 srv/week (HR 0.83, 95% CI 0.74 to 0.92) III. 2–6 srv/week (HR 0.80, 95% CI 0.71 to 0.91) IV. ≥1 srv/day (HR 0.84, 95% CI 0.65 to 1.09) men ↔ women | [91] |
| FFQ | men & women 2,157/31,917 | I. 1–3 srv/month (HR 0.88, 95% CI 0.78 to 0.99) II. 1–2 srv/week (HR 0.83, 95% CI 0.72 to 0.94) III. 3–6 srv/week (HR 0.82, 95% CI 0.68 to 0.99) IV. ≥1 srv/day (HR 1.10, 95% CI 0.84 to 1.45) | [90] |
| HEI-2005 | 15,023 adults | ↑HDL-c ↓TG ↓CRP levels, ↓weight | [94] |
| FFQ | men & women 38,182/46,415 | DCC↓risk of stroke (women) | [87] |
| I: 50 g DC II.50 g/mc wash-out period (7 days) | 21 | single dose 50 g DCC ↔ EFP & MFP | [95] |
| FFQ | 590 | ↓ DCC↑risk DM2 | [96] |
| Intervention | Population | Findings | Ref. |
|---|---|---|---|
| CG: 20 g DC (500 mg polyphenols) CC: 20 g placebo DC | 42 | ↔ lipid profile ↓ SBP, DBP ↔ body weight, BMI | [106] |
| 20 mL drink 500 mg theobromine | 44 | I. THB ↔ ABCA1-MCef II. THB↑miR-92a levels III. HFM e ↑PCef and 3 selected miRNAs levels | [65] |
| 50 g DC | 65 | ↑mRNA expression of the AIC IL-10 ↓ intracellular pro-inflammatory stress response AIef | [107] |
| I. 70 g DC (150 mg ECT) + placebo capsules II. pure ECT capsules (2 × 50 mg ECT) + 75 g MW III. placebo capsules + 75 g MC (0 ECT) | 20 | ↑VF after pure ECT ↔ DC | [108] |
| DC (>85% cocoa) | 24 | supplements of DC↑redox state ↓ biomarkers of muscle damage | [109] |
| 85% cocoa DC 1 g/kg or MC | 47 | I. DC↑SBP and DP at rest but buffered the reactivity of DBP, HR, MAP, & DP during MS II. DC buffer cardiovascular reactivity | [72] |
| I. (AD) II. 42.5 g/day ALD III. 18 g/day CPr & 43 g/day of DC IV. all 3 foods (DC + ALD). | 48 | ALD alone or mix DC under controlled-feeding conditions ↓ lipid profiles | [110] |
| 60 g DC + flavan-3-ols & procyanidins (standard DC and MC) | 42 | flavan-3-ol-enriched DC & only DC: I. ↓ UC, L, AA II.↑pyruvate & 4-hydroxyphenylacetate, phenolic compound of bacterial origin. | [65] |
| 20 g LCC »55% cocoa; 20 g HCC; »90% cocoa | 30 | consumption of HCC: ↑VF; ↓ reducing CBAP ↑VR, | [111] |
| 50 g DC & 30 g CPr | 136 | 7-d cocoa-based supplementation↑conditions PL | [112] |
| 40 g DC (cocoa >85%) vs. LC MC (cocoa <35%) polyphenols | 57 | CPC ↑ EF via Nox2 down-regulation in NASH patients. | [113] |
| Study Designed | Population | Findings | Ref. |
|---|---|---|---|
| MORGAM Project | 142,960 | I. AI <10 g/day ~ 11%↓RTM, II. AI >20 g/day—13%↑RTM, III. AI >20 g/day—22%↑RTM, IV. AI <1 drink/day ↓ RTM and CVD, V. AI >2 drinks/d↑RTM | [156] |
| Questionnaires. Phenol-Explorer database | 17,065 | ↑intake flavonoids ~ 47%↓incidence of CVD | [102] |
| RW 100 mL (women) or 200 mL (men) daily (20 weeks) | 108 | Lch↓LDL/HDL ~ 8% (p = 0.0242) and RW ~13% (p = 0.0049) TCh↓6%; (p = 0.0238) and TC↓13%; (p = 0.0361) Lp (a) ↔ any intervention. | [155] |
| Hydroxytyrosol and HVAL in urine samples | 1851 | I. all biomarkers↓CVD risk, II. HVAL strong inverse association, III. HVAL RTM | [157] |
| 1 glass of RW 4–5 day/week (5 years) 28.9 ± 15 g of EtOH/d for 23.4 ± 12.3 years. | 354 | I. drinkers > CAC abstainers, II. drinkers vs. abstainers↑HDL & LDL, ↓CRP III. drinkers vs. abstainers MACE was significantly↓, despite higher CAC. | [147] |
| 150 mL of RW/day women & 300 mL RW (33 g EtOH/d) men | 46 | I.↑HTGC (during 3 months), II. no subject developed hepatic steatosis, III. LDL-ch↓by RW | [158] |
| Questionnaires.: I. abstainers and occasional consumers, II. beer consumers, III. consumers of mixed drinks | 240 | MAI vs. abstainers -↑HDL-c & adiponectin, | [142] |
| WI never/ WI almost never, WI<0.5 L/day, WI >0.5 L/day | 11,248 | I. MWI vs. abstainers at baseline↓risk of CVD II. MWI↓RTM | [152] |
| I. no use II. ≤1 glass/week III. >1 glass/week | 2583 | I. wine/beer (≤1 glass/week)↓vs. abstention, II. <2 g/day EtOH, 2–10, 10–20, >20 g/d—CVD-risk HRs | [148] |
| 150 mL MW, WW or RW (2 years). MedDiet | 224 | RW↑HDL, HDL-C & apolipoprotein(a)1 ↓the total cholesterol–HDL-C | [154] |
| Effects of RW and WW on atherosclerosis | 157 | RW and WW ↔ clinically relevant differences in lipid profile, CRP, blood glucose and other markers of atherosclerosis | [159] |
| FFQ Phenol-Explorer database | 273 | I. 46%↓in risk of CVD of TP intake II. the polyphenols = strongest inverse associations: flavanols, lignans and hydroxybenzoic acids | [127] |
| Questionnaires | 502,616 | ↑RTM compared to participants drinking EtOH↑3–4 days in a week | [153] |
| Intervention | No. of Pts. | Findings | Ref. |
|---|---|---|---|
| 90 day treatment with RSV (300 mg, 1000 mg and placebo) | 65 | sVCAM-1 and tPAI↑ >1000 mg vs. 300 mg vs. placebo groups | [139] |
| RSV—125 mg/day or 500 mg/day or placebo | 66 | RSV ↔ walking performance in people with PAD age >65 | [140] |
| WW (2 drinks/day), WW + TYR (25 mg) and water (control) ad libitum. | 33 | TYR and OHTYR ↑CVD | [54] |
| I. no EtOH, II. 70 mL of tsipouro 38% ABV III. 200 mL RW 13.5% ABV. consumed of EtOH—27 g/day, (8 weeks) | 57 | 8 weeks RWI ↓BCS from peripheral blood cells | [141] |
| RW (150 + 100 mL of water), IPA (250 mL), blonde (250 mL), and free beer (250 mL). | 20 | TYR absorption & endogenous conversion HT | [132] |
| AAW or gin (0.5 g EtOH/kg) | 41 | ↑TLR4, TLR6 and Caspase-1 ↓TLR2, Interleukin-1 receptor, chemokine receptor 3 & inflammasome expression↓chemokine receptor 5↓ | [53] |
| RW or VDK (3 units/day) | 77 | ↑levels of leptin (after RW and VDK), ↑levels of APO A1 (after VDK), ↑adiponectin | [166] |
| (a) fasting +30 g of EtOH from RW, (b) fasting +30 g of EtOH from WW, (c) fasting +30 g of EtOH from VDK, (d) MeDM, (e) HFM, (f) MeDM +30 g of ethanol from RW, (g) MeDM +30 g of ethanol from WW, (h) MeDM +30 g of ethanol from VDK, (i) HFM +30 g of ethanol from RW, (j) HFM +30 g of ethanol from WW, and (k) HFM +30 g of ethanol from VDK | 55 | MAI + nutraceuticals + EtOH + MeDM ↓the risk of atherosclerosis | [167] |
| 150 mL RW, WW or water | 224 | ↓Apo(B)/Apo(A) (no progression in carotid-TPV) | [168] |
| 200 mL/day RW (4 weeks) | 24 | RW ↔ IL-6, TNF-α and CRP overweight subjects ↑adiponectin ↑antioxidant potential of LDL ↑ in paraoxonase & adiponectin | [145] |
| 230 mL/d (∼24g alcohol/d) RW—women, 300 mL/day (∼31g alcohol/d) RW—men or Eq volumes of DRW or water (4 weeks) | 24 | RW↑BP by 2.5 ± 1.2/1.9 ± 0.7mmHg, RW ↔ glycemic or other CRF | [169] |
| gin (100 mL/day, 30 g EtOH), RW (272 mL/day, 30 g EtOH and 798 mg TP), or DRW (272 mL/day, 1.14 g EtOH & 733 mg TP) (4 weeks) | 67 | glucose ↔, plasma insulin & HOMA-IR↓(RW & DRW), HDL, apolipoprotein A-I & A-II ↑ (RW & gin), Lipoprotein↓(RW) | [58] |
| Group of Drug | Name of Drug | Mechanism | Solubility | Liver Metabolism |
|---|---|---|---|---|
| Statins | Atorvastatin Rozuvastatin Fluvastatin Lovastatin Simvastatin Pitavastatin Pravastatin | competitively inhibits 3-hydroxy-3-methylglutaryl-coenzymeA (HMG-CoA) reductase, | Lipophilic Hydrophilic Lipophilic Lipophilic Lipophilic Lipophilic Hydrophilic | CYP450 3A4 CYP450 2C9 and 2C19 CYP450 2C9 (minor) CYP450 3A4 CYP450 3A4 CYP450 2C9 (minor), glucuronidation |
| Fibrate | Fenofibrate Ciprofibrate | Fenofibrate activates peroxisome proliferator activated PPARα, increasing lipolysis, activating lipoprotein lipase, and reducing apoprotein C-III | Lipophilic | weak inhibitors of CYP2C19 and CYP2A6, weak do moderate inhibitors of CYP2C9. |
| Inhibitor PCSK9 | Evolocumab Alirocumab | PCSK9 inhibitors, negative regulation of DLR | Do not induc enzyme | |
| Cholesterol absorption inhibitors | Ezetimibe | Associates with the protein responsible for steroid uptake into the cell—Niemann-Pick C1 like 1 (NPC1L1) in the intestinal mucosal epithelium. | Lipophilic | Does not induce enzyme |
| Antiplatelet drugs | Ticlopidyna Clopidogrel ASA | inhibit platelet activation and aggregation by irreversibly blocking the ADP P2Y12 receptor. inhibition of the enzyme COX-1, inhibiting prostaglandin production, stopping the conversion of AA to TXA2 | Hydrophilic | CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4/5 |
| Direct oral anticoagulants (DOACs) offers | Rivaroxaban Dabigatran Apixaban Edoxaban | Competitively inhibit free and clot bound factor Xa. | Hydrophilic | Although, in theory, food or herbal inhibitors/inducers of CYP3A4 or P-gp might interfere with the pharmacokinetics of DOACs, no direct evidence of such interactions exist |
| PUFA | n-3: EPA’ DHA | EPA i DHA precursors of prostaglandins, thromboxanes and leukotrienes | Lipophilic | (CYP7A1) [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperkowska, B.; Murawska, J.; Przybylska, A.; Gackowski, M.; Kruszewski, S.; Durmowicz, M.; Rutkowska, D. Cardiovascular Effects of Chocolate and Wine—Narrative Review. Nutrients 2021, 13, 4269. https://doi.org/10.3390/nu13124269
Sperkowska B, Murawska J, Przybylska A, Gackowski M, Kruszewski S, Durmowicz M, Rutkowska D. Cardiovascular Effects of Chocolate and Wine—Narrative Review. Nutrients. 2021; 13(12):4269. https://doi.org/10.3390/nu13124269
Chicago/Turabian StyleSperkowska, Beata, Joanna Murawska, Anna Przybylska, Marcin Gackowski, Stefan Kruszewski, Maciej Durmowicz, and Dorota Rutkowska. 2021. "Cardiovascular Effects of Chocolate and Wine—Narrative Review" Nutrients 13, no. 12: 4269. https://doi.org/10.3390/nu13124269
APA StyleSperkowska, B., Murawska, J., Przybylska, A., Gackowski, M., Kruszewski, S., Durmowicz, M., & Rutkowska, D. (2021). Cardiovascular Effects of Chocolate and Wine—Narrative Review. Nutrients, 13(12), 4269. https://doi.org/10.3390/nu13124269