Bioactivity of Cooked Standard and Enriched Whole Eggs from White Leghorn and Rhode Island Red in Exhibiting In-Vitro Antioxidant and ACE-Inhibitory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Whole Egg Preparation and Simulated Gastrointestinal Digestion
2.3. Measurement of In Vitro Antioxidant Activity through Oxygen Radical Absorbance Capacity Method
2.4. Measurement of In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells
2.5. Measurement of ACE Inhibitory Activity
2.6. Identification of the Peptide Profile through UPLC-MS/MS
2.7. Statistical Analysis
3. Results and Discussion
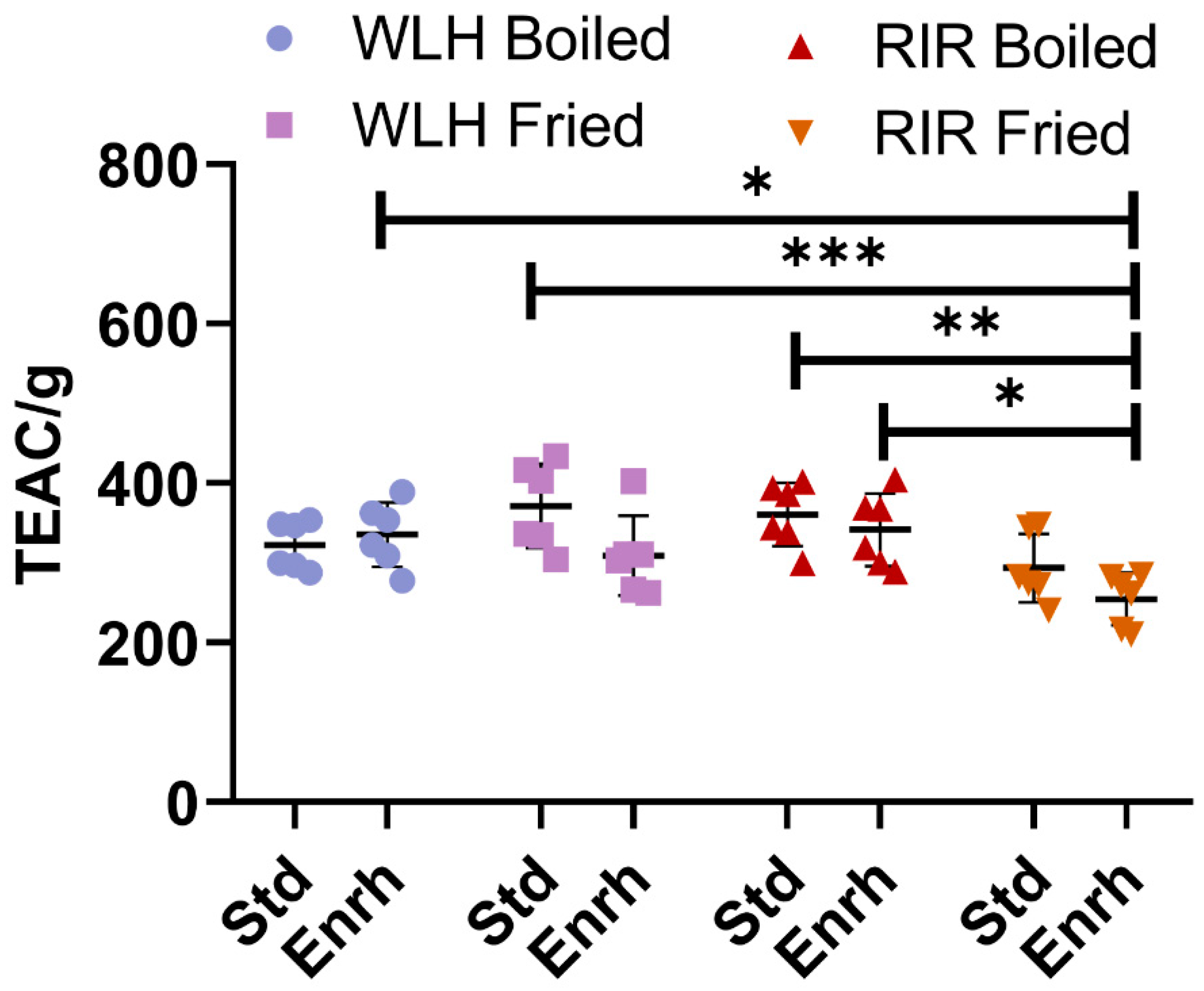
3.1. Antioxidant Capacity of Whole Egg Hydrolysates through Oxygen Radical Absorbance Capacity (ORAC)
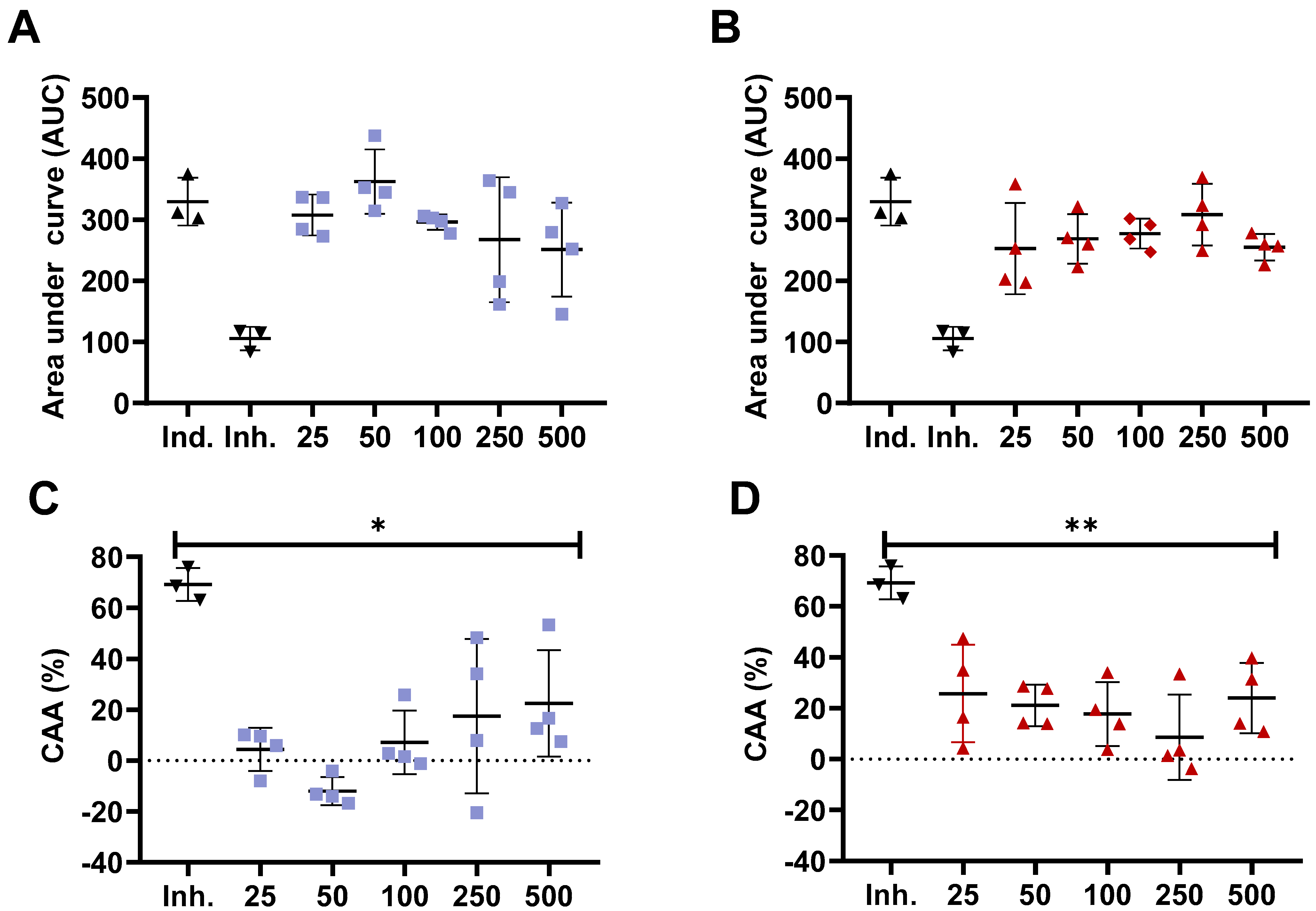
3.2. Measurement of In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells
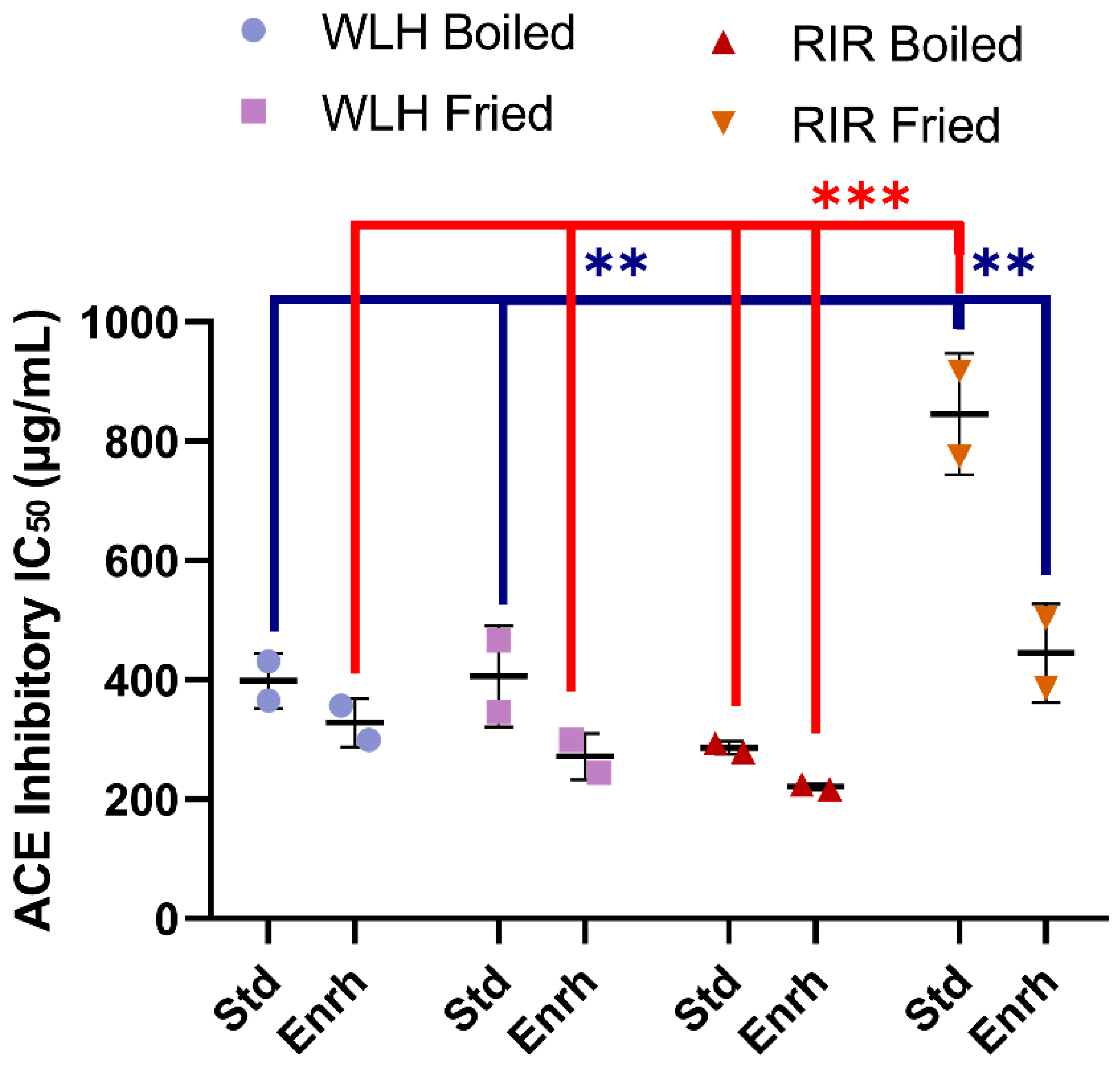
3.3. ACE Inhibition Capacity of Whole Egg Hydrolysates
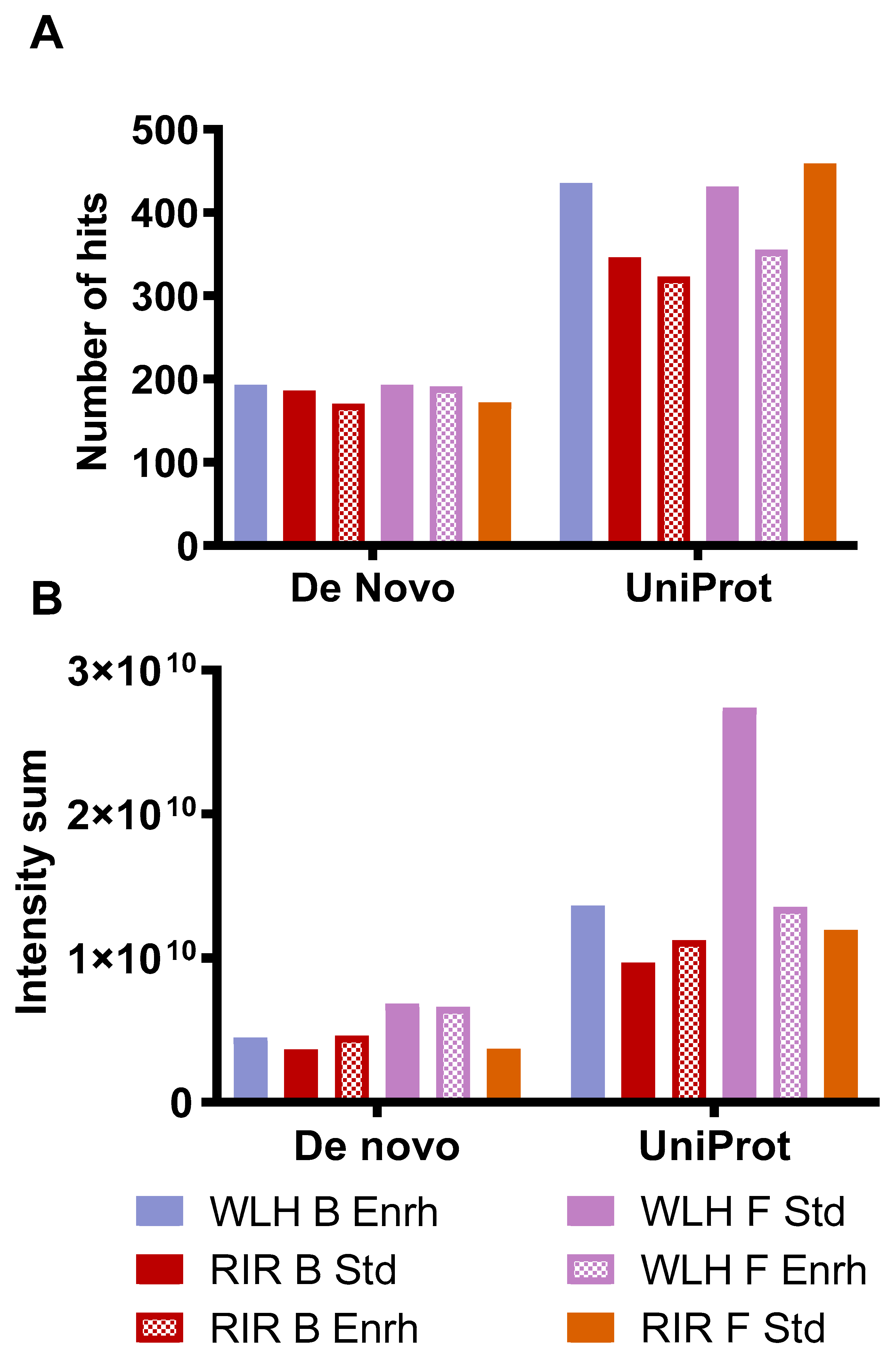
3.4. Whole Egg Hydrolysate LC-MS/MS Peptide Profile and Peptide’s Structure-Function Relationship
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neijat, M.; Zacek, P.; Picklo, M.J.; House, J.D. Lipidomic characterization of omega-3 polyunsaturated fatty acids in phosphatidylcholine and phosphatidylethanolamine species of egg yolk lipid derived from hens fed flaxseed oil and marine algal biomass. Prostagland. Leukot. Essent. Fat. Acids 2020, 161, 102178. [Google Scholar] [CrossRef]
- Panaite, T.D.; Nour, V.; Vlaicu, P.A.; Ropota, M.; Corbu, A.R.; Saracila, M. Flaxseed and dried tomato waste used together in laying hens diet. Arch. Anim. Nutr. 2019, 73, 222–238. [Google Scholar] [CrossRef]
- Shahidi, F. Functional Foods: Their Role in Health Promotion and Disease Prevention. J. Food Sci. 2004, 69, 146–149. [Google Scholar] [CrossRef]
- Yang, W.; Gage, H.; Jackson, D.; Raats, M. The effectiveness and cost-effectiveness of plant sterol or stanol-enriched functional foods as a primary prevention strategy for people with cardiovascular disease risk in England: A modeling study. Eur. J. Health Econ. 2018, 19, 909–922. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, T.; He, K.; Geng, Z.; Wan, X. Dietary green tea powder supplementation enriched egg nutrients and physicochemical property in an indigenous chicken breed. Poult. Sci. 2021, 100, 388–395. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Angiotensin I Converting Enzyme Inhibitory Peptides from Simulated in vitro Gastrointestinal Digestion of Cooked Eggs. J. Agric. Food Chem. 2009, 57, 471–477. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitors from Sour Milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Nolasco, E.; Yang, J.; Ciftci, O.; Vu, D.C.; Alvarez, S.; Purdum, S.; Majumder, K. Evaluating the Effect of Cooking and Gastrointestinal Digestion in Modulating the Bio-Accessibility of Different Bioactive Compounds of Eggs. Food Chem. 2021, 344, 128623. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Jahandideh, F.; Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Beneficial effects of simulated gastro-intestinal digests of fried egg and its fractions on blood pressure, plasma lipids and oxidative stress in spontaneously hypertensive rats. PLoS ONE 2014, 9, e115006. [Google Scholar] [CrossRef] [Green Version]
- Remanan, M.K.; Wu, J. Antioxidant activity in cooked and simulated digested eggs. Food Funct. 2014, 5, 1464–1474. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Savard, P.; Gauthier, S.F.; Schieber, A.; Wu, J. Bioaccessibility and Digestive Stability of Carotenoids in Cooked Eggs Studied Using a Dynamic in Vitro Gastrointestinal Model. J. Agric. Food Chem. 2015, 63, 2956–2962. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Schieber, A.; Wu, J. Effects of storage and cooking on the antioxidant capacity of laying hen eggs. Food Chem. 2016, 194, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Majumder, K.; Panahi, S.; Kaufman, S.; Wu, J. Fried egg digest decreases blood pressure in spontaneous hypertensive rats. J. Funct. Foods 2013, 5, 187–194. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Isolation and identification of peptides from simulated gastrointestinal digestion of preserved egg white and their anti-inflammatory activity in TNF-α-induced Caco-2 cells. J. Nutr. Biochem. 2019, 63, 44–53. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Deng, B.; Long, H.; Tang, T.; Ni, X.; Chen, J.; Yang, G.; Zhang, F.; Cao, R.; Cao, D.; Zeng, M.; et al. Quantitative structure-activity relationship study of antioxidant tripeptides based on model population analysis. Int. J. Mol. Sci. 2019, 20, 995. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure-activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Nowacki, D.; Martynowicz, H.; Skoczyńska, A.; Wojakowska, A.; Turczyn, B.; Bobak, Ł.; Trziszka, T.; Szuba, A. Lecithin derived from ω-3 PUFA fortified eggs decreases blood pressure in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 12373. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Mat, D.J.L.; Le Feunteun, S.; Michon, C.; Souchon, I. In vitro digestion of foods using pH-stat and the INFOGEST protocol: Impact of matrix structure on digestion kinetics of macronutrients, proteins and lipids. Food Res. Int. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Pauls, K.P.; Deng, Z.; Tsao, R. Antioxidant and anti-inflammatory polyphenols and peptides of common bean (Phaseolus vulga L.) milk and yogurt in Caco-2 and HT-29 cell models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Rui, H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Tsao, R. Anthocyanin-rich phenolic extracts of purple root vegetables inhibit pro-inflammatory cytokines induced by H2O2 and enhance antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2016, 22, 363–375. [Google Scholar] [CrossRef]
- Hai Bang, T.; Suhara, H.; Doi, K.; Ishikawa, H.; Fukami, K.; Parajuli, G.P.; Katakura, Y.; Yamashita, S.; Watanabe, K.; Adhikari, M.K.; et al. Wild mushrooms in Nepal: Some potential candidates as antioxidant and ACE-inhibition sources. Evid.-Based Complement. Altern. Med. 2014, 2014, 195305. [Google Scholar] [CrossRef] [Green Version]
- Lam, L.H.; Shimamura, T.; Manabe, S.; Ishiyama, M.; Ukeda, H. Assay of angiotensin I-converting enzyme-inhibiting activity based on the detection of 3-hydroxybutyrate with water-soluble tetrazolium salt. Anal. Sci. 2008, 24, 1057–1060. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Y.L.; Chen, J.; Zhou, L. Synergistic inhibition of lipid oxidation by pea protein hydrolysate coupled with licorice extract in a liposomal model system. J. Agric. Food Chem. 2013, 61, 8452–8461. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Ltd.: Cambridge, UK, 2015; pp. 105–140. ISBN 9781782420972. [Google Scholar]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship modeling of peptides containing 4–10 amino acid residues. QSAR Comb. Sci. 2006, 25, 873–880. [Google Scholar] [CrossRef]
- Rao, P.S.; Nolasco, E.; Handa, A.; Naldrett, M.J.; Alvarez, S.; Majumder, K. Effect of pH and heat treatment on the antioxidant activity of egg white protein-derived peptides after simulated in-vitro gastrointestinal digestion. Antioxidants 2020, 9, 1114. [Google Scholar] [CrossRef]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant Peptides Identified from Ovotransferrin by the ORAC Method Did Not Show Anti-Inflammatory and Antioxidant Activities in Endothelial Cells. J. Agric. Food Chem. 2016, 64, 113–119. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, W.; He, R.; Xiong, F.; Ma, H. Protein breakdown and release of antioxidant peptides during simulated gastrointestinal digestion and the absorption by everted intestinal sac of rapeseed proteins. LWT 2017, 86, 424–429. [Google Scholar] [CrossRef]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial transport of lunasin and derived peptides: Inhibitory effects on the gastrointestinal cancer cells viability. J. Food Compos. Anal. 2018, 68, 101–110. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract-revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, W.; Nimalaratne, C.; Chakrabarti, S.; Wu, J. Purification and characterization of antioxidant peptides from cooked eggs using a dynamic in vitro gastrointestinal model in vascular smooth muscle A7r5 cells. npj Sci. Food 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Jabalera, A.; Cortés-Giraldo, I.; Dávila-Ortíz, G.; Vioque, J.; Alaiz, M.; Girón-Calle, J.; Megías, C.; Jiménez-Martínez, C. Influence of peptides-phenolics interaction on the antioxidant profile of protein hydrolysates from Brassica napus. Food Chem. 2015, 178, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen Radical Absorbance Capacity of Peptides from Egg White Protein Ovotransferrin and their Interaction with Phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yang, Y.J.; Wang, Z.; Xing, C.R.; Yuan, J.; Wang, L.F.; Udenigwe, C.; Ju, X.R. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. npj Sci. Food 2019, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debusca, A.; Tahergorabi, R.; Beamer, S.K.; Partington, S.; Jaczynski, J. Interactions of dietary fibre and omega-3-rich oil with protein in surimi gels developed with salt substitute. Food Chem. 2013, 141, 201–208. [Google Scholar] [CrossRef]
- Henmi, H.; Hata, M.; Takeuchi, M. Studies on the carotenoids in the muscle of salmon-V. Combination of astaxanthin and canthaxanthin with bovine serum albumin and egg albumin. Comp. Biochem. Physiol. Part B Biochem. 1991, 99, 609–612. [Google Scholar] [CrossRef]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Redondo, A.B.; Roque, F.R.; Miguel, M.; López-Fandiño, R.; Salaices, M. Vascular effects of egg white-derived peptides in resistance arteries from rats. Structure-activity relationships. J. Sci. Food Agric. 2010, 90, 1988–1993. [Google Scholar] [CrossRef]
- Suetsuna, K. Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from Allium sativum L (garlic). J. Nutr. Biochem. 1998, 9, 415–419. [Google Scholar] [CrossRef]
- He, R.; Ma, H.; Zhao, W.; Qu, W.; Zhao, J.; Luo, L.; Zhu, W. Modeling the QSAR of ACE-inhibitory peptides with ANN and its applied illustration. Int. J. Pept. 2012, 2012, 620609. [Google Scholar] [CrossRef] [Green Version]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular Link Mechanisms between Inflammation and Cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Wu, J. A New Approach for Identification of Novel Antihypertensive Peptides from Egg Proteins by QSAR and Bioinformatics. Food Res. Int. 2010, 43, 1371–1378. [Google Scholar] [CrossRef]
- Grootaert, C.; Matthijs, B.; Voorspoels, S.; Possemiers, S.; Smagghe, G.; Van Camp, J. Egg-derived bioactive peptides with ACE-inhibitory properties: A literature update. Food Funct. 2017, 8, 3847–3855. [Google Scholar] [CrossRef]
- Miguel, M.; Alvarez, Y.; López-Fandiño, R.; Alonso, M.J.; Salaices, M. Vasodilator effects of peptides derived from egg white proteins. Regul. Pept. 2007, 140, 131–135. [Google Scholar] [CrossRef] [PubMed]

| Peptide Sequence | WLH Boiled Enriched | RIR Boiled Standard | RIR Boiled Enriched | WLH Fried Standard | RIR Fried Standard | WLH Fried Enriched | Source | Enzyme Used | Reference |
|---|---|---|---|---|---|---|---|---|---|
| FF | 5.68E+06 | 5.88E+06 | - | 9.78E+06 | 7.74E+06 | 7.91E+06 | Whole egg | Pepsin-Pancreatin | - |
| YY | - | - | 2.36E+06 | 4.59E+06 | - | 4.51E+06 | Whole egg | Pepsin-Pancreatin | - |
| FY | - | 4.53E+06 | 3.49E+06 | - | 3.16E+06 | - | Whole egg | Pepsin-Pancreatin | - |
| VRFP | - | - | - | - | 1.48E+06 | - | Whole egg | Pepsin-Pancreatin | - |
| LW | 2.01E+07 | 1.43E+07 | - | 1.19E+07 | 1.04E+07 | 1.30E+07 | Ovalbumin | Pepsin | [44] |
| LY | - | - | 6.41E+07 | 7.41E+07 | - | - | Ovotransferrin | Chymotrypsin | [45] |
| NF | - | 3.07E+06 | 5.09E+06 | 2.86E+06 | 3.25E+06 | 2.19E+06 | Ovalbumin | Pepsin-Pancreatin | [46,47] |
| YR | 2.54E+06 | 2.32E+06 | 1.13E+06 | 1.32E+06 | 1.64E+06 | 1.54E+06 | Ovalbumin | Pepsin-Pancreatin | [46,48] |
| AW | - | 1.46E+06 | 1.64E+06 | - | 1.34E+06 | - | Lysozyme | Chymotrypsin-Thermolysin | [46] |
| MPF | 4.69E+07 | 4.14E+07 | 9.30E+07 | 6.43E+07 | 5.12E+07 | 4.48E+07 | Ovotransferrin | Pepsin-Pancreatin | [6] |
| ADHP | - | 3.22E+06 | 7.62E+06 | - | 6.62E+06 | 6.75E+06 | Ovalbumin | Pepsin-Pancreatin | [49] |
| Total | 5.51E+07 | 6.19E+07 | 1.78E+08 | 1.57E+08 | 7.64E+07 | 6.77E+07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolasco, E.; Naldrett, M.; Alvarez, S.; Johnson, P.E.; Majumder, K. Bioactivity of Cooked Standard and Enriched Whole Eggs from White Leghorn and Rhode Island Red in Exhibiting In-Vitro Antioxidant and ACE-Inhibitory Effects. Nutrients 2021, 13, 4232. https://doi.org/10.3390/nu13124232
Nolasco E, Naldrett M, Alvarez S, Johnson PE, Majumder K. Bioactivity of Cooked Standard and Enriched Whole Eggs from White Leghorn and Rhode Island Red in Exhibiting In-Vitro Antioxidant and ACE-Inhibitory Effects. Nutrients. 2021; 13(12):4232. https://doi.org/10.3390/nu13124232
Chicago/Turabian StyleNolasco, Emerson, Mike Naldrett, Sophie Alvarez, Philip E. Johnson, and Kaustav Majumder. 2021. "Bioactivity of Cooked Standard and Enriched Whole Eggs from White Leghorn and Rhode Island Red in Exhibiting In-Vitro Antioxidant and ACE-Inhibitory Effects" Nutrients 13, no. 12: 4232. https://doi.org/10.3390/nu13124232
APA StyleNolasco, E., Naldrett, M., Alvarez, S., Johnson, P. E., & Majumder, K. (2021). Bioactivity of Cooked Standard and Enriched Whole Eggs from White Leghorn and Rhode Island Red in Exhibiting In-Vitro Antioxidant and ACE-Inhibitory Effects. Nutrients, 13(12), 4232. https://doi.org/10.3390/nu13124232







