Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Whole Body Composition
2.3. Protein Quality Evaluation
2.4. Plasma Analyses
2.5. Protein Synthesis Measurement
2.6. Western-Blot Analysis
2.7. mRNA Analysis
2.8. Mitochondrial Enzymatic Assays
2.9. Statistics
3. Results
3.1. Caloric Intake, Body Composition Evolution, and Final Tissue Weights
3.2. Protein Quality Evaluation
3.3. Plasma Metabolic Parameters and Cytokines
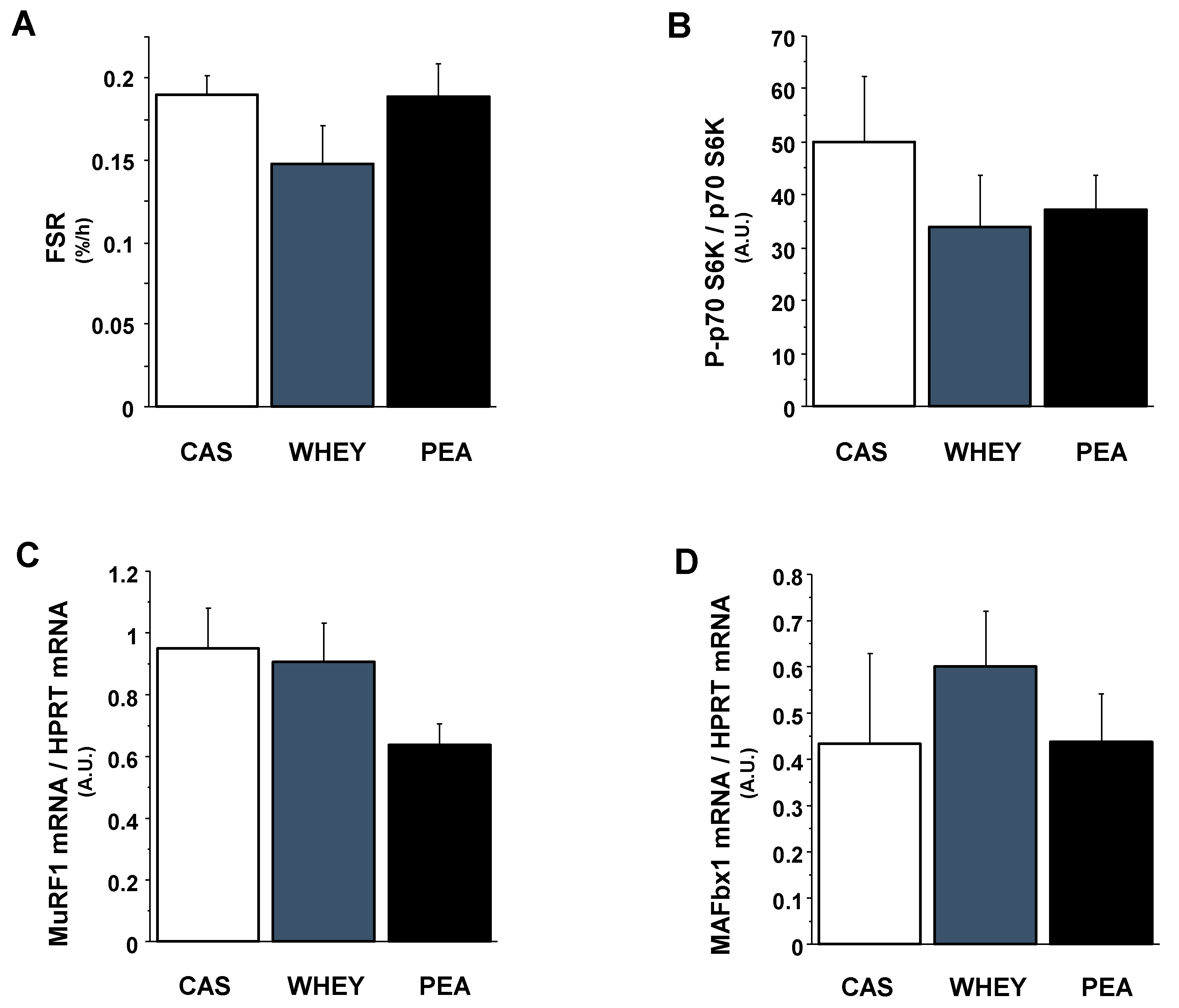
3.4. Markers of Muscle Protein Anabolism and Catabolism
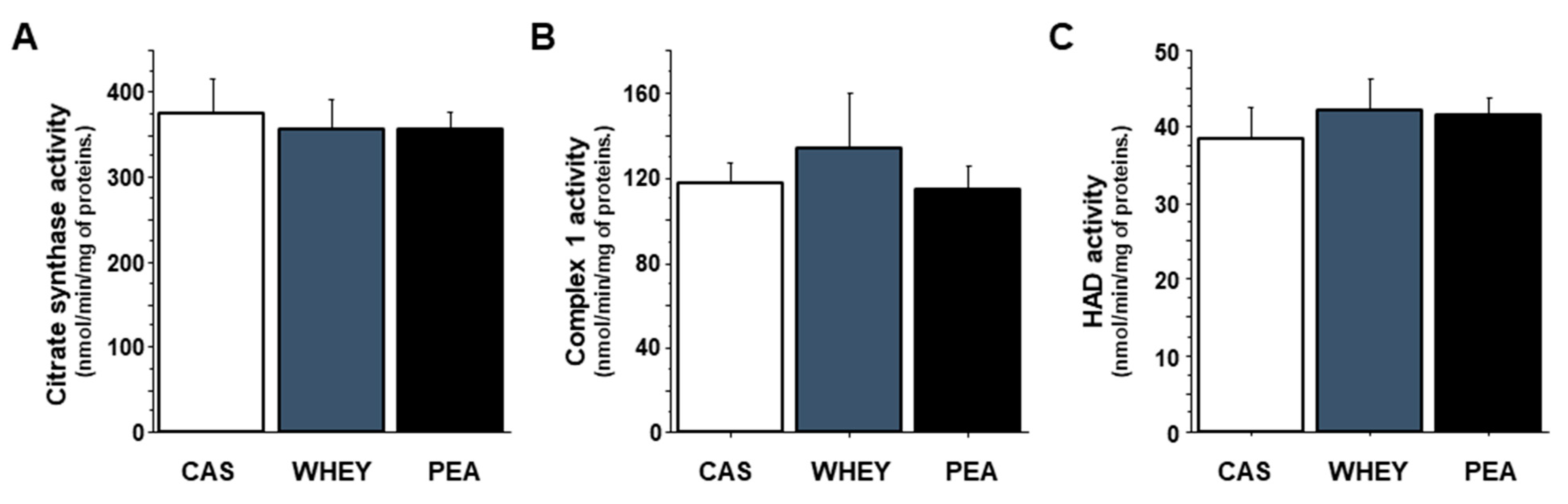
3.5. Muscle Mitochondrial Activity
4. Discussion
4.1. Nitrogen Balance, Digestibility and Rate of Utilization
4.2. Body Composition and Skeletal Muscle Mass
4.3. Mechanisms
4.4. Metabolic Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- New Nutrition Business. 10 Key Trends in Food, Nutrition Health 2020; New Nutrition Business: London, UK, 2020. [Google Scholar]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- WHO; FAO; UNU. Protein and Amino Acid Requirements in Human Nutrition; Report of the Joint FAO/WHO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The effects of whey vs. pea protein on physical adaptations following 8-weeks of high-intensity functional training (HIFT): A pilot study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Babault, N.; Paizis, C.; Deley, G.; Guerin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, placebo-controlled clinical trial vs. whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Cholewa, J.; Shang, H.; Yang, Y.; Ding, X.; Wang, Q.; Su, Q.; Zanchi, N.E.; Xia, Z. Advances in the role of leucine-sensing in the regulation of protein synthesis in aging skeletal muscle. Front. Cell Dev. Biol. 2021, 9, 646482. [Google Scholar] [CrossRef]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidou, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef] [Green Version]
- Overduin, J.; Guerin-Deremaux, L.; Wils, D.; Lambers, T.T. NUTRALYS((R)) pea protein: Characterization of in vitro gastric digestion and in vivo gastrointestinal peptide responses relevant to satiety. Food Nutr. Res. 2015, 59, 25622. [Google Scholar] [CrossRef] [Green Version]
- Dumas, A. Stickstoffbestimmung Nach Dumas. Die Praxis des Org. Chemikers. In N-Determination According to Dumas, 41st ed.; Schrag: Nuremberg, Germany, 1962. [Google Scholar]
- Proll, J.; Petzke, K.J.; Ezeagu, I.E.; Metges, C.C. Low nutritional quality of unconventional tropical crop seeds in rats. J. Nutr. 1998, 128, 2014–2022. [Google Scholar] [CrossRef]
- Zangarelli, A.; Chanseaume, E.; Morio, B.; Brugere, C.; Mosoni, L.; Rousset, P.; Giraudet, C.; Patrac, V.; Gachon, P.; Boirie, Y.; et al. Synergistic effects of caloric restriction with maintained protein intake on skeletal muscle performance in 21-month-old rats: A mitochondria-mediated pathway. FASEB J. 2006, 20, 2439–2450. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Berry, A.; Giraudet, C.; Patrac, V.; Domingues-Faria, C.; Rocher, C.; Guillet, C.; Denis, P.; Pouyet, C.; et al. Fast digestive, leucine-rich, soluble milk proteins improve muscle protein anabolism, and mitochondrial function in undernourished old rats. Mol. Nutr. Food Res. 2017, 61, 1700287. [Google Scholar] [CrossRef] [Green Version]
- Medja, F.; Allouche, S.; Frachon, P.; Jardel, C.; Malgat, M.; Mousson de Camaret, B.; Slama, A.; Lunardi, J.; Mazat, J.P.; Lombes, A. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 2009, 9, 331–339. [Google Scholar] [CrossRef]
- Gutierrez Cortes, N.; Pertuiset, C.; Dumon, E.; Borlin, M.; Hebert-Chatelain, E.; Pierron, D.; Feldmann, D.; Jonard, L.; Marlin, S.; Letellier, T.; et al. Novel mitochondrial DNA mutations responsible for maternally inherited nonsyndromic hearing loss. Hum. Mutat. 2012, 33, 681–689. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Guillet, C.; Tordjman, J.; Reggio, S.; Landrier, J.F.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2alpha activation. Aging Cell 2014, 13, 1001–1011. [Google Scholar] [CrossRef]
- Berrazaga, I.; Salles, J.; Laleg, K.; Guillet, C.; Patrac, V.; Giraudet, C.; Le Bacquer, O.; Gueugneau, M.; Denis, P.; Pouyet, C.; et al. Anabolic properties of mixed wheat-legume pasta products in old rats: Impact on whole-body protein retention and skeletal muscle protein synthesis. Nutrients 2020, 12, 1596. [Google Scholar] [CrossRef]
- Gwin, J.A.; Carbone, J.W.; Rodriguez, N.R.; Pasiakos, S.M. Physiological limitations of protein foods ounce equivalents and the underappreciated role of essential amino acid density in healthy dietary patterns. J. Nutr. 2021, 151, 3276–3283. [Google Scholar] [CrossRef]
- Floret, C.; Monnet, A.F.; Micard, V.; Walrand, S.; Michon, C. Replacement of animal proteins in food: How to take advantage of nutritional and gelling properties of alternative protein sources. Crit. Rev. Food Sci. Nutr. 2021. Published online ahead of print. [Google Scholar] [CrossRef]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Berrazaga, I.; Mession, J.L.; Laleg, K.; Salles, J.; Guillet, C.; Patrac, V.; Giraudet, C.; Le Bacquer, O.; Boirie, Y.; Micard, V.; et al. Formulation, process conditions, and biological evaluation of dairy mixed gels containing fava bean and milk proteins: Effect on protein retention in growing young rats. J. Dairy. Sci. 2019, 102, 1066–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillin, F.M.; Gaudichon, C.; Guerin-Deremaux, L.; Lefranc-Millot, C.; Azzout-Marniche, D.; Khodorova, N.; Calvez, J. Multi-criteria assessment of pea protein quality in rats: A comparison between casein, gluten and pea protein alone or supplemented with methionine. Br. J. Nutr. 2021, 125, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lhoste, E.F.; Mouzon, B.; Andrieux, C.; Gueugneau, A.M.; Fiszlewicz, M.; Corring, T.; Szylit, O. Physiological effects of a pea protein isolate in gnotobiotic rats: Comparison with a soybean isolate and meat. Ann. Nutr. Metab. 1998, 42, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Chamba, M.V.; Hua, Y.; Murekatete, N.; Chen, Y. Effects of synthetic and natural extraction chemicals on yield, composition and protein quality of soy protein isolates extracted from full-fat and defatted flours. J. Food Sci. Technol. 2015, 52, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun-Watherhouse, D.Z.; Zhao, M.; Waterhouse, G.I.N. Protein modification during ingredient preparation and food processing: Approaches to improve food processability and nutrition. Food Bioprocess Technol. 2014, 7, 1853–1893. [Google Scholar] [CrossRef]
- Lohrke, B.; Saggau, E.; Schadereit, R.; Beyer, M.; Bellmann, O.; Kuhla, S.; Hagemeister, H. Activation of skeletal muscle protein breakdown following consumption of soyabean protein in pigs. Br. J. Nutr. 2001, 85, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Laleg, K.; Salles, J.; Berry, A.; Giraudet, C.; Patrac, V.; Guillet, C.; Denis, P.; Tessier, F.J.; Guilbaud, A.; Howsam, M.; et al. Nutritional evaluation of mixed wheat-faba bean pasta in growing rats: Impact of protein source and drying temperature on protein digestibility and retention. Br. J. Nutr. 2019, 121, 496–507. [Google Scholar] [CrossRef] [PubMed]
- NRC National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed.; National Avademic Press: Washington, DC, USA, 1995. [Google Scholar]
- Leterme, P.M.; Monmart, T.; Baudart, E. Amino acid composition of pea (Pisum sativum) proteins and protein profile of pea flour. J. Sci. Food Agric. 1990, 53, 107–110. [Google Scholar] [CrossRef]
- Wroblewska, B.; Juskiewicz, J.; Kroplewski, B.; Jurgonski, A.; Wasilewska, E.; Zlotkowska, D.; Markiewicz, L. The effects of whey and soy proteins on growth performance, gastrointestinal digestion, and selected physiological responses in rats. Food Funct. 2018, 9, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Combe, E.; Pirman, T.; Stekar, J.; Houlier, M.L.; Mirand, P.P. Differential effect of lentil feeding on proteosynthesis rates in the large intestine, liver and muscle of rats. J. Nutr. Biochem. 2004, 15, 12–17. [Google Scholar] [CrossRef]
- Pirman, T.; Combe, E.; Patureau Mirand, P.; Stekar, J.; Oresnik, A. Differential effects of cooked common bean (Phaseolus vulgaris) and lentil (Lens esculenta puyensis) feeding on protein and nucleic acid contents in intestines, liver and muscles in rats. Ann. Nutr. Metab. 2004, 48, 281–287. [Google Scholar] [CrossRef]
- Pirman, T.; Combe, E.; Ribeyre, M.C.; Prugnaud, J.; Stekar, J.; Patureau Mirand, P. Differential effects of cooked beans and cooked lentils on protein metabolism in intestine and muscle in growing rats. Ann. Nutr. Metab. 2006, 50, 197–205. [Google Scholar] [CrossRef]
- Alonso, R.; Grant, G.; Fruhbeck, G.; Marzo, F. Muscle and liver protein metabolism in rats fed raw or heat-treated pea seeds. J. Nutr. Biochem. 2002, 13, 611–618. [Google Scholar] [CrossRef]
- Brown, E.C.; DiSilvestro, R.A.; Babaknia, A.; Devor, S.T. Soy versus whey protein bars: Effects on exercise training impact on lean body mass and antioxidant status. Nutr. J. 2004, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jager, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to find leucine in food and how to feed elderly with sarcopenia in order to counteract loss of muscle mass: Practical advice. Front. Nutr. 2020, 7, 622391. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Silva, M.T.; da Cunha, F.M.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp. Gerontol. 2015, 72, 269–277. [Google Scholar] [CrossRef]
- Combaret, L.; Dardevet, D.; Rieu, I.; Pouch, M.N.; Bechet, D.; Taillandier, D.; Grizard, J.; Attaix, D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J. Physiol. 2005, 569, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.A.; Goena, M.; Santidrian, S.; Larralde, J. Response of muscle, liver and whole-body protein turnover to two different sources of protein in growing rats. Ann. Nutr. Metab. 1987, 31, 146–153. [Google Scholar] [CrossRef]
- Bua, E.A.; McKiernan, S.H.; Wanagat, J.; McKenzie, D.; Aiken, J.M. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J. Appl. Physiol. 2002, 92, 2617–2624. [Google Scholar] [CrossRef]
- Guillet, C.; Masgrau, A.; Walrand, S.; Boirie, Y. Impaired protein metabolism: Interlinks between obesity, insulin resistance and inflammation. Obes. Rev. 2012, 13 (Suppl. 2), 51–57. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Landrier, J.F.; Mothe-Satney, I.; Guillet, C.; Boue-Vaysse, C.; Combaret, L.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; et al. Oleate-enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin. Nutr. 2011, 30, 799–806. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Yamada, K.; Yanagida, S.; Homma, M.; Dairiki, K.; Sasaki, H.; Kawagoshi, A.; Satake, M.; et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012, 106, 1526–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory effects of a pea-derived peptide Leu-Arg-Trp (LRW) on dysfunction of rat aortic vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Boirie, Y. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab. 2005, 31, 5S20–5S26. [Google Scholar] [CrossRef]
- Liu, J.P.; Qian, Y.F.; Qin, G.Y.; Zhao, L.Y.; Chen, G.T. Antidiabetic activities of glycoprotein from pea (Pisum sativum L.) in STZ-induced diabetic mice. Food Funct. 2021, 12, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, H.; Wei, Y.; Cai, M.; Gu, R.; Wang, Y.; Ma, Y.; Chen, L. Pea-derived peptides, VLP, LLP, VA, and LL, improve insulin resistance in HepG2 cells via activating IRS-1/PI3K/AKT and blocking ROS-mediated p38MAPK signaling. J. Food Biochem. 2020, 44, e13454. [Google Scholar] [CrossRef]
| CAS | WHEY | PEA | |
|---|---|---|---|
| Diet composition (g/100 g) | |||
| Protein | |||
| Casein | 14 | ||
| Soluble milk protein | 14 | ||
| Pea protein | 14 | ||
| Fat (soybean oil) | 6 | 6 | 6 |
| Carbohydrates | 68 | 68 | 68 |
| Cellulose | 7.5 | 7.5 | 7.5 |
| Vitamin and mineral mix | 4.5 | 4.5 | 4.5 |
| Calculated energy (kcal/100 g) | 412 | 412 | 412 |
| Amino acid content (g/100 g protein) | |||
| Tryptophan | 1.17 | 2.09 | 0.87 |
| Threonine | 4.18 | 5.09 | 3.79 |
| Aspartic acid | 6.86 | 11.47 | 12.26 |
| Serine | 5.57 | 4.69 | 5.37 |
| Lysine | 7.55 | 9.84 | 7.45 |
| Valine | 6.16 | 5.23 | 5.25 |
| Proline | 10.84 | 4.77 | 4.30 |
| Alanine | 2.87 | 4.93 | 4.34 |
| Phenylalanine | 4.69 | 3.62 | 5.56 |
| Isoleucine | 4.79 | 5.26 | 4.67 |
| Glycine | 1.75 | 1.83 | 4.02 |
| Tyrosine | 4.19 | 2.76 | 3.28 |
| Arginine | 3.11 | 2.54 | 8.12 |
| Leucine | 8.99 | 12.15 | 8.51 |
| Histidine | 2.69 | 2.11 | 2.41 |
| Glutamic acid | 21.47 | 16.86 | 17.70 |
| Methionine | 2.65 | 2.05 | 1.03 |
| Cysteine | 0.49 | 2.70 | 1.07 |
| CASEIN Protein | WHEY Protein | PEA Protein | |
|---|---|---|---|
| Protein (%) | 90.2 | 80.9 | 83.6 |
| Fat (%) | <1 | 4.7 | <1 |
| Carbohydrates (%) | <1 | 4.3 | 5.6 |
| Moisture (%) | 9.0 | 5.6 | 4.4 |
| Ash (%) | <2 | 4.5 | 5.8 |
| Gene Name | Forward and Reverse Primers |
|---|---|
| MAFbx (Muscle atrophy F-box) | For 5’-AGTGAAGACCGGCTACTGTGGAA-3’ Rev 5’-TTGCAAAGCTGCAGGGTGAC-3’ |
| MuRF1 (Muscle RING finger-1) | For 5’-GTGAAGTTGCCCCCTTACAA-3’ Rev 5’-TGGAGATGCAATTGCTCAGT-3’ |
| HPRT (Hypoxanthine-guanine phosphoribosyltransferase) | For 5’-AGTTGAGAGATCATCTCCAC-3’ Rev 5’-TTGCTGACCTGCTGGATTAC-3’ |
| CAS | WHEY | PEA | |
|---|---|---|---|
| Body weight (g) | |||
| Week 0 | 582 ± 23 | 577 ± 14 | 595 ± 28 |
| Week 8 | 585 ± 22 | 607 ± 16 | 612 ± 34 |
| Week 16 | 583 ± 20 | 605 ± 20 | 590 ± 39 |
| Fat mass (g) | |||
| Week 0 | 91 ± 8 | 99 ± 8 | 108 ± 13 |
| Week 8 | 104 ± 7 | 129 ± 18 | 136 ± 21 |
| Week 16 | 99 ± 18 | 127 ± 15 | 131 ± 26 |
| Lean Mass (g) | |||
| Week 0 | 442 ± 18 | 427 ± 15 | 434 ± 15 |
| Week 8 | 431 ± 19 | 424 ± 14 | 420 ± 15 |
| Week 16 | 430 ± 17 | 421 ± 16 | 403 ± 14 |
| CAS | WHEY | PEA | |
|---|---|---|---|
| Plantaris (mg) | 309 ± 34 | 300 ± 23 | 263 ± 0.17 |
| Soleus (mg) | 175 ± 20 | 173 ± 24 | 165 ± 13 |
| Gastrocnemius (g) | 1.52 ± 0.29 | 1.19 ± 0.13 | 1.13 ± 0.06 |
| Quadriceps (g) | 1.94 ± 0.26 | 1.78 ± 0.27 | 1.72 ± 0.24 |
| Hindlimb muscle mass (g) | 8.82 ± 0.51 | 8.01 ± 0.88 | 7.36 ± 0.61 |
| Perirenal adipose tissue (g) | 11.7 ± 2.6 | 15.3 ± 1.4 | 19.7 ± 4.6 |
| Subcutaneous adipose tissue (g) | 11.9 ± 2.3 | 13.3 ± 2.0 | 12.2 ± 2.4 |
| Liver (g) | 13.7 ± 0.9 | 14.3 ± 0.9 | 13.2 ± 1.7 |
| Heart (g) | 1.91 ± 0.05 | 1.88 ± 0.08 | 1.96 ± 0.08 |
| CAS | WHEY | PEA | |
|---|---|---|---|
| Nitrogen intake (g) | 1.47 ± 0.10 | 1.61 ± 0.12 | 1.57 ± 0.11 |
| Fecal nitrogen (g) | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.02 |
| Urinary nitrogen (g) | 0.86 ± 0.07 | 0.88 ± 0.11 | 0.91 ± 0.08 |
| Nitrogen balance (g) | 0.49 ± 0.08 | 0.60 ± 0.20 | 0.61 ± 0.08 |
| Apparent digestibility (%) | 91.6 ± 0.7 | 92.1 ± 0.7 | 91.8 ± 0.8 |
| True digestibility (%) | 99.9 ± 0.5 | 101.2 ± 0.6 | 100.5 ± 0.7 |
| Net protein utilization (%) | 66.3 ± 6.7 | 74.7 ± 6.1 | 81.3 ± 6.8 |
| Biological value (%) | 66.4 ± 6.9 | 73.8 ± 6.0 | 80.8 ± 6.6 |
| CAS | WHEY | PEA | |
|---|---|---|---|
| Insulin sensitivity | |||
| Glucose (g/L) | 0.955 ± 0.106 | 1.010 ± 0.075 | 0.970 ± 0.108 |
| Insulin (ng/mM) | 1.285 ± 0.585 | 0.678 ± 0.213 | 0.553 ± 0.102 |
| HOMA-IR | 6.055 ± 1.884 | 4.232 ± 1.392 | 3.202 ± 0.761 |
| Lipids | |||
| Triglycerides (g/L) | 0.789 ± 0.088 | 0.994 ± 0.364 | 0.604 ± 0.176 |
| Total cholesterol (g/L) | 0.843 ± 0.073 | 0.878 ± 0.067 | 0.833 ± 0.167 |
| Adipokines | |||
| Adiponectin (µg/mL) | 5.145 ± 1.240 | 6.355 ± 0.764 | 8.751 ± 1.109 |
| Leptin (ng/mL) | 4.547 ± 0.416 | 5.172 ± 1.170 | 6.730 ± 2.935 |
| Cytokines | |||
| TNFα (pg/mL) | 11.93 ± 5.90 | 6.28 ± 2.79 | 11.29 ± 2.86 |
| IL-1β (pg/mL) | 155.4 ± 67.9 | 158.1 ± 63.9 | 133.5 ± 29.6 |
| IL-10 (pg/mL) | 58.56 ± 22.95 | 57.26 ± 22.74 | 54.10 ± 10.93 |
| TNFα / IL-10 ratio | 0.264 ± 0.099 | 0.185 ± 0.033 | 0.254 ± 0.071 |
| IL-1β / IL-10 ratio | 2.540 ± 0.090 | 2.580 ± 0.111 | 2.429 ± 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, J.; Guillet, C.; Le Bacquer, O.; Malnero-Fernandez, C.; Giraudet, C.; Patrac, V.; Berry, A.; Denis, P.; Pouyet, C.; Gueugneau, M.; et al. Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats. Nutrients 2021, 13, 4234. https://doi.org/10.3390/nu13124234
Salles J, Guillet C, Le Bacquer O, Malnero-Fernandez C, Giraudet C, Patrac V, Berry A, Denis P, Pouyet C, Gueugneau M, et al. Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats. Nutrients. 2021; 13(12):4234. https://doi.org/10.3390/nu13124234
Chicago/Turabian StyleSalles, Jérôme, Christelle Guillet, Olivier Le Bacquer, Carmen Malnero-Fernandez, Christophe Giraudet, Véronique Patrac, Alexandre Berry, Philippe Denis, Corinne Pouyet, Marine Gueugneau, and et al. 2021. "Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats" Nutrients 13, no. 12: 4234. https://doi.org/10.3390/nu13124234
APA StyleSalles, J., Guillet, C., Le Bacquer, O., Malnero-Fernandez, C., Giraudet, C., Patrac, V., Berry, A., Denis, P., Pouyet, C., Gueugneau, M., Boirie, Y., Jacobs, H., & Walrand, S. (2021). Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats. Nutrients, 13(12), 4234. https://doi.org/10.3390/nu13124234