Brow and Masticatory Muscle Activity Senses Subjective Hedonic Experiences during Food Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. Procedure
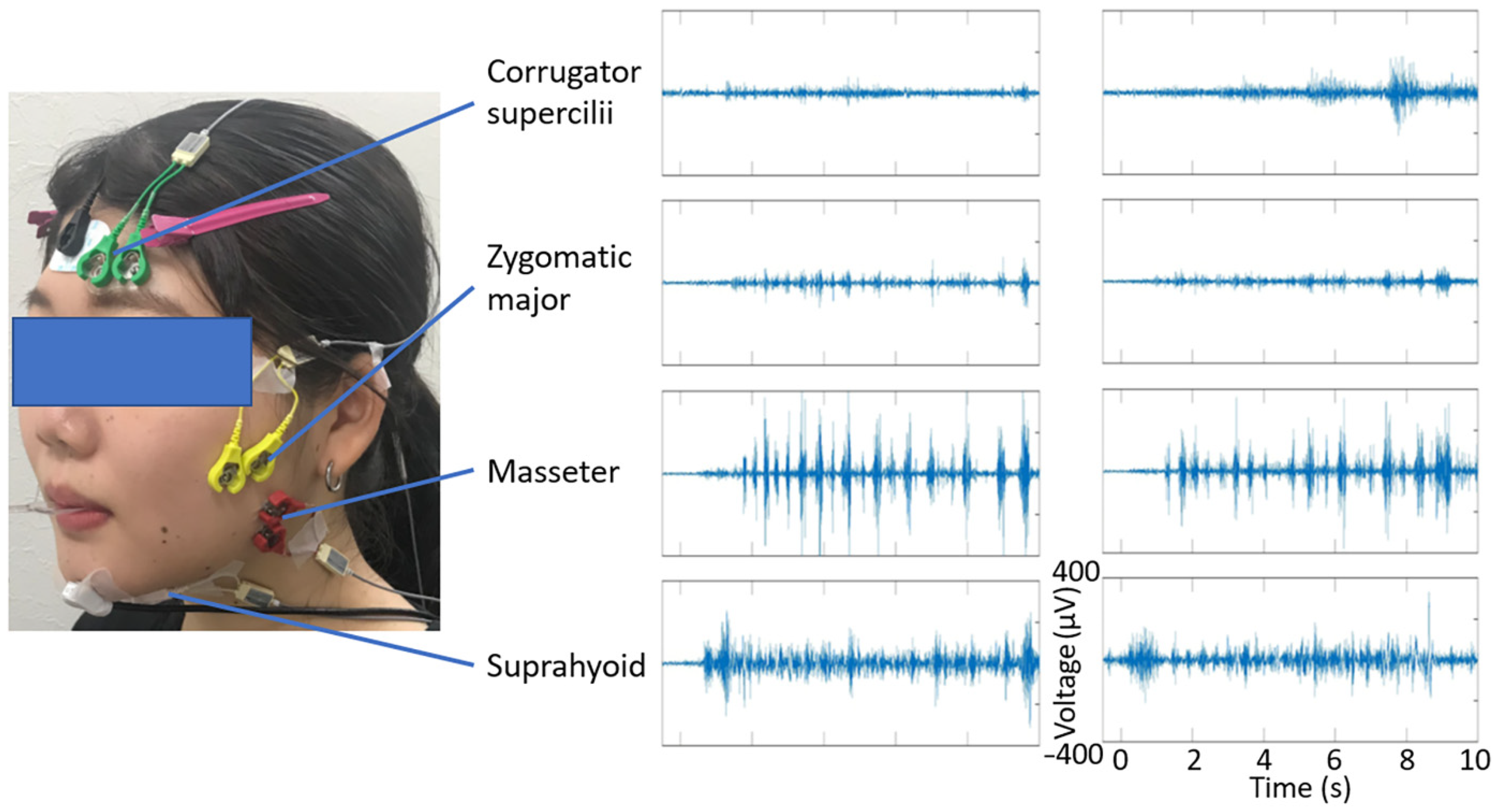
2.4. Physiological Data Recording
2.5. Data Analysis
2.5.1. Preprocessing
2.5.2. Statistical Analysis
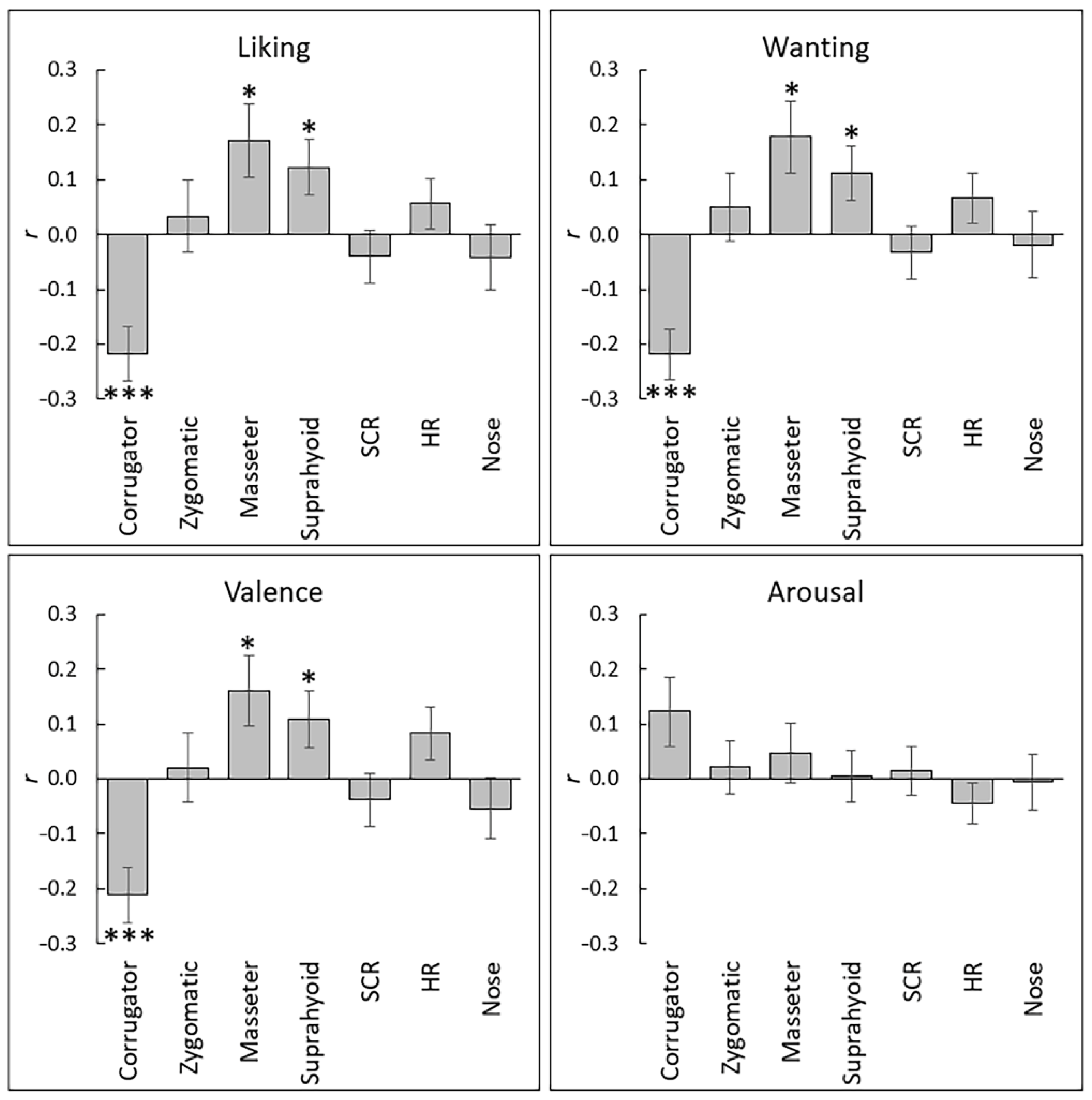
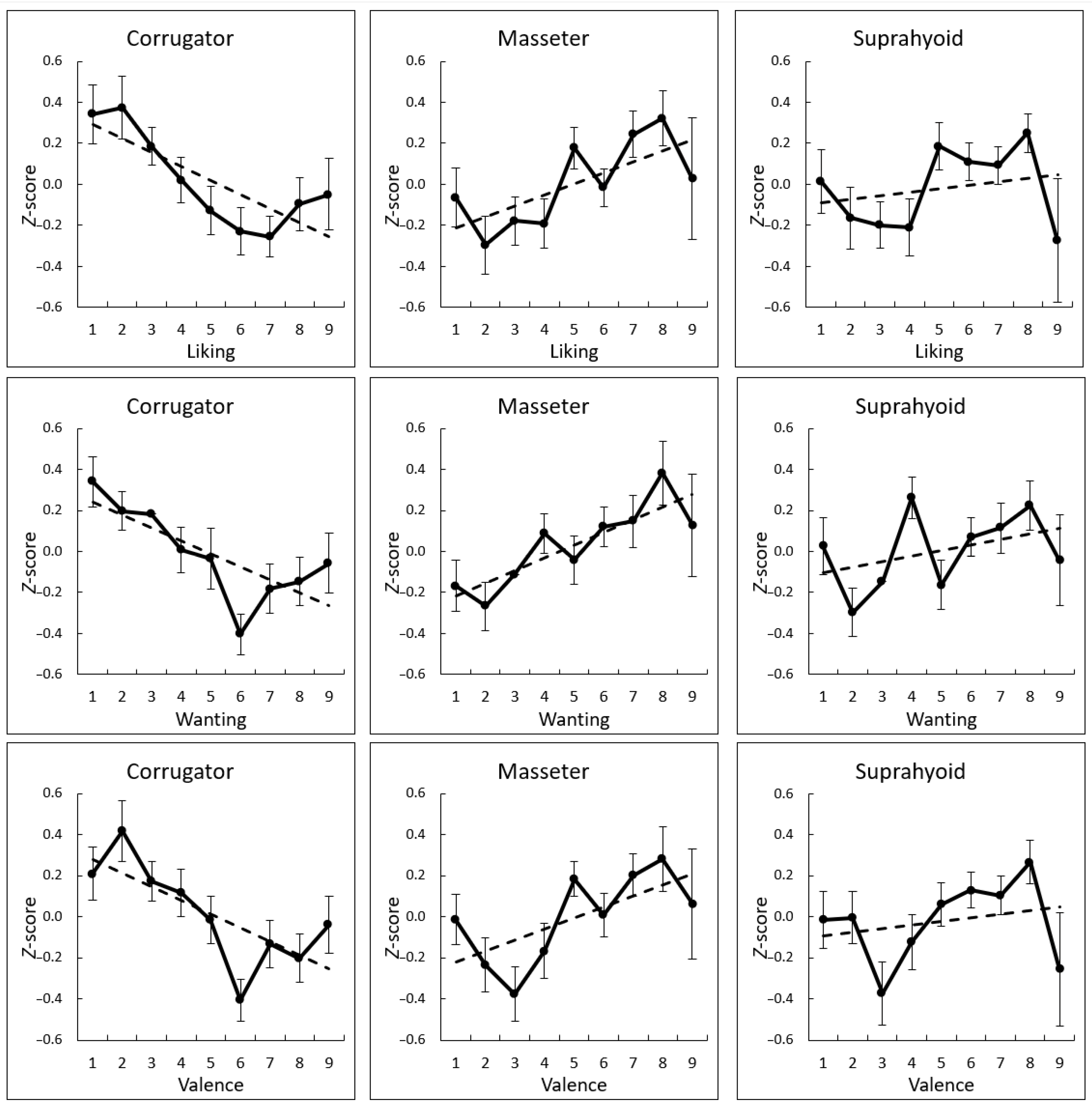
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westenhoefer, J.; Pudel, V. Pleasure from food: Importance for food choice and consequences of deliberate restriction. Appetite 1993, 20, 246–249. [Google Scholar] [CrossRef]
- Macht, M.; Meininger, J.; Roth, J. The pleasures of eating: A qualitative analysis. J. Happiness Stud. 2005, 6, 137–160. [Google Scholar] [CrossRef]
- Schnepper, R.; Georgii, C.; Eichin, K.; Arend, A.K.; Wilhelm, F.H.; Vögele, C.; Lutz, A.P.C.; van Dyck, Z.; Blechert, J. Fight, flight, -or grab a bite! Trait emotional and restrained eating style predicts food cue responding under negative emotions. Front. Behav. Neurosci. 2020, 14, 91. [Google Scholar] [CrossRef]
- Meiselman, H.L. A review of the current state of emotion research in product development. Food Res. Int. 2015, 76, 192–199. [Google Scholar] [CrossRef]
- Cummins, R.A. Measuring happiness and subjective well-being. In Oxford Handbook of Happiness; David, S., Boniwell, I., Conley Ayers, A., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 185–200. [Google Scholar]
- Li, S.; Scott, N.; Walters, G. Current and potential methods for measuring emotion in tourism experiences: A review. Curr. Issues Tour. 2014, 18, 805–827. [Google Scholar] [CrossRef]
- Berridge, K.C. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000, 24, 173–198. [Google Scholar] [CrossRef]
- Sato, W.; Minemoto, K.; Ikegami, A.; Nakauma, M.; Funami, T.; Fushiki, T. Facial EMG correlates of subjective hedonic responses during food consumption. Nutrients 2000, 12, 1174. [Google Scholar] [CrossRef]
- Korb, S.; Massaccesi, C.; Gartus, A.; Lundström, J.N.; Rumiati, R.; Eisenegger, C.; Silani, G. Facial responses of adult humans during the anticipation and consumption of touch and food rewards. Cognition 2020, 194, 104044. [Google Scholar] [CrossRef]
- Korb, S.; Götzendorfer, S.J.; Massaccesi, C.; Sezen, P.; Graf, I.; Willeit, M.; Eisenegger, C.; Silani, G. Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards. eLife 2020, 9, e55797. [Google Scholar] [CrossRef]
- Horio, T. EMG activities of facial and chewing muscles of human adults in response to taste stimuli. Percept. Mot. Skills 2003, 97, 289–298. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Cook, E.W.; Lang, P.J. Affective judgment and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 1989, 3, 51–64. [Google Scholar]
- Lang, P.J.; Greenwald, M.K.; Bradley, M.M.; Hamm, A.O. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology 1993, 30, 261–273. [Google Scholar] [CrossRef]
- Larsen, J.T.; Norris, C.J.; Cacioppo, J.T. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology 2003, 40, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Walter, S.; Scheck, A.; Hrabal, D.; Hoffmann, H.; Kessler, H.; Traue, H.C. Repeatability of facial electromyography (EMG) activity over corrugator supercilii and zygomaticus major on differentiating various emotions. J. Ambient Intell. Humaniz. Comput. 2012, 3, 3–10. [Google Scholar] [CrossRef]
- Sato, W.; Fujimura, T.; Kochiyama, T.; Suzuki, N. Relationships among facial mimicry, emotional experience, and emotion recognition. PLoS ONE 2013, 8, e57889. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Kochiyama, T.; Yoshikawa, S. Physiological correlates of subjective emotional valence and arousal dynamics while viewing films. Biol. Psychol. 2020, 157, 107974. [Google Scholar] [CrossRef]
- Dimberg, U. Facial electromyography and emotional reactions. Psychophysiology 1990, 27, 481–494. [Google Scholar] [CrossRef]
- Ishihara, S.; Nakauma, M.; Funami, T.; Tanaka, T.; Nishinari, K.; Kohyama, K. Electromyography during oral processing in relation to mechanical and sensory properties of soft gels. J. Texture Stud. 2011, 42, 254–267. [Google Scholar] [CrossRef]
- Kohyama, K.; Gao, Z.; Ishihara, S.; Funami, T.; Nishinari, K. Electromyography analysis of natural mastication behavior using varying mouthful quantities of two types of gels. Physiol. Behav. 2016, 161, 174–182. [Google Scholar] [CrossRef]
- Steiner, J.E.; Glaser, D.; Hawilo, M.E.; Berridge, K.C. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001, 25, 53–74. [Google Scholar] [CrossRef]
- Steiner, J.E. The gustofacial response: Observation on normal and anencephalic newborn infants. Symp. Oral Sens. Percept. 1973, 254–278. [Google Scholar]
- Graillon, A.; Barr, R.G.; Young, S.N.; Wright, J.H.; Hendricks, L.A. Differential response to intraoral sucrose, quinine and corn oil in crying human newborns. Physiol. Behav. 1997, 62, 317–325. [Google Scholar] [CrossRef]
- Rochat, P.; Blass, E.M.; Hoffmeyer, L.B. Oropharyngeal control of hand-mouth coordination in newborn infants. Dev. Psychol. 1988, 24, 459–463. [Google Scholar] [CrossRef]
- Ayres, C.; Ferreira, C.F.; Bernardi, J.R.; Marcelino, T.B.; Hirakata, V.N.; Silva, C.H.D.; Goldani, M.Z. A method for the assessment of facial hedonic reactions in newborns. J. Pediatr. 2017, 93, 253–259. [Google Scholar] [CrossRef]
- Rios, J.M.; Miller, A.L.; Lumeng, J.C.; Rosenblum, K.; Appugliese, D.P.; Kaciroti, N.; Gearhardt, A.N. Behavioral responses to sucrose as an indicator of positive hedonic response across the first six months of infancy. Physiol. Behav. 2020, 223, 112914. [Google Scholar] [CrossRef]
- Steiner, J.E.; Lidar-Lifschitz, D.; Perl, E. Taste and odor: Reactivity in depressive disorders a multidisciplinary approach. Percept. Mot. Skills 1993, 77, 1331–1346. [Google Scholar] [CrossRef]
- Weiland, R.; Ellgring, H.; Macht, M. Gustofacial and olfactofacial responses in human adults. Chem. Senses 2010, 35, 841–853. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal Activity; Springer: Tokyo, Japan, 2011. [Google Scholar]
- Cacioppo, J.T.; Berntson, G.G.; Klein, D.J. What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral “illusions”. In Emotion and Social Behavior. Ix; Clark, M.S., Ed.; Sage Publications: Thousand Oaks, CA, USA, 1992; pp. 63–98. [Google Scholar]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biol. Psychiatry 1998, 44, 1248–1263. [Google Scholar] [CrossRef]
- Kosonogov, V.; De Zorzi, L.; Honoré, J.; Martinez-Velazquez, E.S.; Nandrino, J.L.; Martinez-Selva, J.M.; Sequeira, H. Facial thermal variations: A new marker of emotional arousal. PLoS ONE 2017, 12, e0183592. [Google Scholar]
- Danner, L.; Haindl, S.; Joechl, M.; Duerrschmid, K. Facial expressions and autonomous nervous system responses elicited by tasting different juices. Food Res. Int. 2014, 64, 81–90. [Google Scholar] [CrossRef]
- de Wijk, R.A.; Kooijman, V.; Verhoeven, R.H.G.; Holthuysen, N.T.E.; de Graaf, C. Autonomic nervous system responses on and facial expressions to the sight, smell, and taste of liked and disliked foods. Food Qual. Prefer. 2012, 26, 196–203. [Google Scholar] [CrossRef]
- Rousmans, S.; Robin, O.; Dittmar, A.; Vernet-Maury, E. Autonomic nervous system responses associated with primary tastes. Chem. Senses 2000, 25, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Curran, P.J.; Bauer, D.J. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu. Rev. Psychol. 2011, 62, 583–619. [Google Scholar] [CrossRef]
- Epstein, S. The stability of behavior: II. Implications for psychological research. Am. Psychol. 1980, 35, 790–806. [Google Scholar] [CrossRef]
- Ostrof, C. Comparing correlations based on individual-Level and aggregated data. J. Appl. Psychol. 1993, 78, 569–582. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, L.P. Aggregating and testing intra-individual correlations: Methods and comparisons. Multivar. Behav. Res. 2014, 49, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.N.; Hendrie, G.A.; Carty, D. Sensitivity, hedonics and preferences for basic tastes and fat amongst adults and children of differing weight status: A comprehensive review. Food Qual. Prefer. 2016, 48, 359–367. [Google Scholar] [CrossRef]
- Wall, K.M.; Farruggia, M.C.; Perszyk, E.E.; Kanyamibwa, A.; Fromm, S.; Davis, X.S.; Dalenberg, J.R.; DiFeliceantonio, A.G.; Small, D.M. No evidence for an association between obesity and milkshake liking. Int. J. Obes. 2020, 44, 1668–1677. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lohani, M.; Payne, B.R.; Isaacowitz, D.M. Emotional coherence in early and later adulthood during sadness reactivity and regulation. Emotion 2018, 18, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.J.; Svoboda, R.C.; Bae, K.K.; Haase, C.M. Individual differences in sadness coherence: Associations with dispositional affect and age. Emotion 2021, 21, 465–477. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Koç, H.; Çakir, E.; Vinyard, C.J.; Essick, G.; Daubert, C.R.; Drake, M.A.; Osborne, J.; Foegeding, E.A. Adaptation of oral processing to the fracture properties of soft solid. J. Texture Stud. 2014, 45, 47–61. [Google Scholar] [CrossRef]
- Sworn, G. Gellan gum. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2000; pp. 117–135. [Google Scholar]
- Nussinovitch, A. Hydrocolloid Applications: Gum Technology in the Food and Other Industries; Blackie Academic & Professional: London, UK, 1997. [Google Scholar]
- Drewnowski, A. Individual differences in sensory preferences for fat in model sweet dairy products. Acta Psychol. 1993, 84, 103–110. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Fridlund, A.J.; Cacioppo, J.T. Guidelines for human electromyographic research. Psychophysiology 1986, 23, 567–589. [Google Scholar] [CrossRef] [PubMed]
- Schumann, N.P.; Bongers, K.; Guntinas-Lichius, O.; Scholle, H.C. Facial muscle activation patterns in healthy male humans: A multi-channel surface EMG study. J. Neurosci. Methods 2010, 187, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for psychophysiological research ad hoc committee on electrodermal measures. Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar]
- Wilson, F.N.; Johnston, F.D.; Rosenbaum, F.F.; Erlanger, H.; Kossmann, C.E.; Hecht, H.; Cotrim, N.; de Oliveira, R.M.; Scarsi, R.; Barker, P.S. The precordial electrocardiogram. Am. Heart J. 1944, 27, 19–85. [Google Scholar] [CrossRef]
- Thompson, B. Planned versus unplanned and orthogonal versus nonorthogonal contrasts: The neo-classical perspective. In Advances in Social Science Methodology; Thompson, B., Ed.; JAI Press: Greenwich, CT, USA, 1994; Volume 3, pp. 3–27. [Google Scholar]
- Ha, R.R.; Ha, J.C. Integrative Statistics for the Social and Behavioral Sciences; Sage: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Pagano, R.R. Understanding Statistics in the Behavioral Sciences, 10th ed.; Cengage: Boston, MA, USA, 2013. [Google Scholar]
- Holmes, A.P.; Friston, K.J. Generalisability, random effects and population inference. Neuroimage 1998, 7, S754. [Google Scholar] [CrossRef]
- Sato, W.; Yoshikawa, S.; Fushiki, T. Facial EMG activity is associated with hedonic experiences but not nutritional values while viewing food images. Nutrients 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Kergoat, S.; Azais-Braesco, V.; Burton-Freeman, B.; Hetherington, M.M. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiol. Behav. 2015, 151, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Okawa, J.; Hori, K.; Yoshimoto, T.; Salazar, S.E.; Ono, T. Higher masticatory performance and higher number of chewing strokes increase retronasal aroma. Front. Nutr. 2021, 8, 623507. [Google Scholar] [CrossRef] [PubMed]
- Quigley, K.S.; Barrett, L.F. Is there consistency and specificity of autonomic changes during emotional episodes? Guidance from the Conceptual Act Theory and psychophysiology. Biol. Psychol. 2014, 98, 82–94. [Google Scholar] [CrossRef]
- Foster, K.D.; Grigor, J.M.; Cheong, J.N.; Yoo, M.J.; Bronlund, J.E.; Morgenstern, M.P. The role of oral processing in dynamic sensory perception. J. Food Sci. 2011, 76, R49–R61. [Google Scholar] [CrossRef]
- Orne, M.T. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. Am. Psychol. 1962, 17, 776–783. [Google Scholar] [CrossRef]
- Nichols, A.L.; Maner, J.K. The good-subject effect: Investigating participant demand characteristics. J. Gen. Psychol. 2008, 135, 151–165. [Google Scholar] [CrossRef]
- Coles, N.A.; Larsen, J.T.; Lench, H.C. A meta-analysis of the facial feedback literature: Effects of facial feedback on emotional experience are small and variable. Psychol. Bull. 2019, 145, 610–651. [Google Scholar] [CrossRef] [PubMed]
- Finzi, E.; Rosenthal, N.E. Emotional proprioception: Treatment of depression with afferent facial feedback. J. Psychiatr. Res. 2016, 80, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Manda, Y.; Kodama, N.; Maeda, N.; Minagi, S. Effect of food properties and chewing condition on the electromyographic activity of the posterior tongue. J. Oral Rehabil. 2019, 46, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Braud, A.; Boucher, Y. Intra-oral trigeminal-mediated sensations influencing taste perception: A systematic review. J. Oral Rehabil. 2020, 47, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- Schnepper, R.; Richard, A.; Georgii, C.; Arend, A.K.; Naab, S.; Voderholzer, U.; Wilhelm, F.H.; Blechert, J. Bad mood food? Increased versus decreased food cue reactivity in anorexia nervosa and bulimia nervosa during negative emotions. Eur. Eat. Disord. Rev. 2021, 29, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Laeng, B.; Berridge, K.C.; Butter, C.M. Pleasantness of a sweet taste during hunger and satiety: Effects of gender and “sweet tooth”. Appetite 1993, 21, 247–254. [Google Scholar] [CrossRef][Green Version]
- Głuchowski, A.; Czarniecka-Skubina, E.; Kostyra, E.; Wasiak-Zys, G.; Bylinka, K. Sensory features, liking and emotions of consumers towards classical, molecular and note by note foods. Foods 2021, 10, 133. [Google Scholar] [CrossRef]

| Hedonic Quality | Flavor Compound | Concentration (w/w%) | Odor |
|---|---|---|---|
| Negative | Isovaleric acid | 0.0010 | Sweaty |
| (E)-2-nonenal | 0.0005 | Cucumber | |
| Indole | 0.0010 | Fecal | |
| Neutral | Phenethyl alcohol | 0.0100 | Bread |
| Acetoin | 0.0100 | Yogurt | |
| 2,5-dimethylpyrazine | 0.0100 | Roast | |
| Positive | Vanillin | 0.0200 | Vanilla |
| Maltol | 0.0200 | Caramel | |
| Ethyl butyrate | 0.0200 | Pineapple |
| Subjective | Statistic | Physiological | ||||||
|---|---|---|---|---|---|---|---|---|
| Corrugator | Zygomatic | Masseter | Suprahyoid | SCR | HR | Nose | ||
| Liking | t | 4.35 | 0.47 | 2.38 | 2.35 | 0.89 | 1.24 | 1.01 |
| p | <0.001 | 0.642 | 0.024 | 0.026 | 0.38 | 0.224 | 0.885 | |
| d | 0.80 | 0.09 | 0.43 | 0.43 | 0.16 | 0.23 | 0.18 | |
| Wanting | t | 4.47 | 0.78 | 2.62 | 2.19 | 0.69 | 1.43 | 0.97 |
| p | <0.001 | 0.441 | 0.014 | 0.037 | 0.497 | 0.163 | 0.724 | |
| d | 0.82 | 0.14 | 0.48 | 0.40 | 0.13 | 0.26 | 0.18 | |
| Valence | t | 4.05 | 0.35 | 2.32 | 2.06 | 0.80 | 1.76 | 0.42 |
| p | <0.001 | 0.732 | 0.028 | 0.048 | 0.432 | 0.09 | 0.68 | |
| d | 0.74 | 0.06 | 0.42 | 0.38 | 0.15 | 0.32 | 0.08 | |
| Arousal | t | 2.04 | 0.42 | 0.73 | 0.16 | 0.34 | 1.27 | 0.72 |
| p | 0.051 | 0.68 | 0.469 | 0.878 | 0.736 | 0.215 | 0.476 | |
| d | 0.37 | 0.08 | 0.13 | 0.03 | 0.06 | 0.23 | 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, W.; Ikegami, A.; Ishihara, S.; Nakauma, M.; Funami, T.; Yoshikawa, S.; Fushiki, T. Brow and Masticatory Muscle Activity Senses Subjective Hedonic Experiences during Food Consumption. Nutrients 2021, 13, 4216. https://doi.org/10.3390/nu13124216
Sato W, Ikegami A, Ishihara S, Nakauma M, Funami T, Yoshikawa S, Fushiki T. Brow and Masticatory Muscle Activity Senses Subjective Hedonic Experiences during Food Consumption. Nutrients. 2021; 13(12):4216. https://doi.org/10.3390/nu13124216
Chicago/Turabian StyleSato, Wataru, Akira Ikegami, Sayaka Ishihara, Makoto Nakauma, Takahiro Funami, Sakiko Yoshikawa, and Tohru Fushiki. 2021. "Brow and Masticatory Muscle Activity Senses Subjective Hedonic Experiences during Food Consumption" Nutrients 13, no. 12: 4216. https://doi.org/10.3390/nu13124216
APA StyleSato, W., Ikegami, A., Ishihara, S., Nakauma, M., Funami, T., Yoshikawa, S., & Fushiki, T. (2021). Brow and Masticatory Muscle Activity Senses Subjective Hedonic Experiences during Food Consumption. Nutrients, 13(12), 4216. https://doi.org/10.3390/nu13124216







